Worldwide epidemic of obesity
Obesity is defined as excessive fat accumulation that might impair human health and is diagnosed at a body mass index (BMI) ≥ 30 kg/m2.1 The global obesity prevalence has been rising dramatically in recent years, reaching the status of a worldwide epidemic health problem2,Reference Ng, Fleming and Robinson3 that impacts the health public costs and increases the incidence of many metabolic disorders and is a major risk factor for noncommunicable diseases.4,Reference MacMahon, Baigent and Duffy5 The prevalence of obesity increased from 3.2% in 1975 to 11% in 2016 among adult men,Reference Di Cesare, Bentham and Stevens6,7 and there were approximately 281 million adult obese men worldwide in 2016.Reference Bentham, Di Cesare and Bilano8 Among adult women, the prevalence of obesity increased from 6.4% in 1975 to 15% in 2016, and there were approximately 390 million obese adult women in 2016.Reference Di Cesare, Bentham and Stevens6–Reference Bentham, Di Cesare and Bilano8
The elevated intake of energy-dense foods and a decrease in physical activity favor an energy imbalance, and the Western lifestyle is the major factor involved in obesity development.Reference Blüher9 However, there is an individual susceptibility for obesity development, involving behavior, genetic, and epigenetic factors.Reference Blüher9,Reference Hanson and Gluckman10 These factors and the mechanisms that contribute to obesity susceptibility have been exhaustively investigated, and the perinatal environment has demonstrated an important impact on the development of obesity and related disorders throughout life.
Developmental programming
The perinatal period is characterized by intense ontogenetic plasticity due to increased epigenetic machinery activity.Reference Gluckman, Hanson and Buklijas11 Therefore, the exposure to stress during this period may change the epigenome by promoting individual adaptation to early environmental conditions. However, if the environmental conditions are changed throughout life, the subject becomes maladapted and susceptible to the development of obesity and its associated disorders.Reference Hanson and Gluckman10 The concept of the Developmental Origin of Health and Disease (DOHaD) involves the programming of the fetal phenotype by epigenetic changes such as DNA methylation, histone acetylation, and noncoding RNAs.Reference Goyal, Limesand and Goyal12 These changes result in the modification of gene expression patterns by silencing or increasing gene transcription. In response to these epigenetic changes, throughout life, the subject has many adaptive responses, which, all together, are responsible for their health outcomes.Reference Hanson and Gluckman10,Reference Goyal, Limesand and Goyal12 According to the Barker hypothesis of the “fetal origin of adult diseases,”Reference Barker13 in general, this adaptive response involves the thrifty phenotype, which is ultimately responsible for the genesis of the obese phenotype. The pregnancy period is clearly a critical window for developmental programming because of organogenesis. However, the early postnatal period, especially lactation, is sometimes more crucial in increasing the susceptibility to maladaptive metabolic programming.Reference Ellsworth, Harman, Padmanabhan and Gregg14,Reference George, Draycott, Muir, Clifford, Elmes and Langley-Evans15
Rodent models as a useful tool for studying developmental programming
In comparison to other mammals, rodents can be considered as useful tools for the study of early-life events due to some advantages, for instance, short periods of gestation and lactation and large litters. In addition, rodent models can be studied in large numbers and be genetically manipulated.Reference McCarthy, Martin-Fairey and Sojka16,Reference Ratajczak and Muglia17 These characteristics may reduce confounding factors, allowing the identification of direct effects, as well as the molecular mechanisms involved.
Although the main components of pregnancy, birth, and lactation are preserved between rodents and humans, such as the role of progesterone in maintaining pregnancyReference McCarthy, Martin-Fairey and Sojka16 and activation of uterine contraction,Reference Brodt-Eppley and Myatt18,Reference Cook, Zaragoza, Sung and Olson19 there are some differences that deserve to be highlighted. Uterine implantation in rodents is different from humans (many embryos vs one embryo, in general), besides fewer placental hormones acting in this period.Reference Carter20 Before delivery, there is an abrupt withdrawal of progesterone from maternal circulation in mice and rats, while these levels are kept high in humans,Reference Mitchell and Taggart21 with local changes in their metabolism and signaling.Reference Andersson, Minjarez, Yost and Word22,Reference Nadeem, Shynlova, Mesiano and Lye23 There are differences in the stage of maturation of the intestine and brain at birth as well as in the circadian rhythm in rodents and humans.Reference Puiman and Stoll24,Reference Serin and Acar Tek25 In addition, the period in the womb determines the differences in body composition between rodents and humans at birth, which can have an impact on neonatal metabolism. The rodent embryo is born almost immediately after organogenesis, while in the human embryo, many organs grow and develop in the uterus, favoring body weight gain.Reference Xue, Cai and Ma26,Reference Chusyd, Wang, Huffman and Nagy27
Until now, the rodent is the most used animal model to study developmental programming,Reference Nathanielsz28 and most of the knowledge on the topic of early weaning has been studied in rodents. However, we must highlight its limitations and the need to obtain other experimental models, such as swines, sheeps, and nonhuman primates. These mammals may have characteristics more similar to humans, as in relation to the hormonal profile during pregnancy and breastfeeding and adiposity at birth. So far, there are no data in the literature on nonhuman primates and models of early weaning. In addition, there are few studies in lambs that partially corroborate the findings in rodents,Reference Ekiz, Kocak, Yalcintan and Yilmaz29–Reference McCoard, Cristobal-Carballo and Knol32 which reinforces the relevance of rodent models as a useful tool for studying early weaning.
Exclusive breastfeeding and early weaning
Epidemiological studies
Exclusive breastfeeding during the first 6 months of life is a global recommendation of the World Health Organization (WHO) to reduce infant and childhood morbidity and mortality,33 based on a pivotal Brazilian epidemiological study.Reference Victora, Vaughan and Lombardi34 After this period, the introduction of nutritionally adequate and safe complementary foods is recommended while breastfeeding continues for up to 2 years of age or beyond.35,Reference Kramer and Kakuma36 Therefore, exclusive breastfeeding and continued breastfeeding are the feeding practices for infants and young children defined by the WHO.37
Breast milk is the most adequate food for children in early life. The milk composition is nutritionally adequate for each phase of early life, and it varies between mothers and over the course of lactation to support adequate child growth and development.Reference Dettwyler38 It plays a role in immunological defense, since it has a reduced risk of contamination compared to infant formulas. Human milk contains bioactive nutrients, such as long-chain polyunsaturated fatty acids and indigestible human milk oligosaccharides, which have important roles in immunological defense and microbiota.Reference Thompson39 Moreover, breast milk is an important source of minerals, vitamins, and many hormones, such as leptin, which is crucial for the normal postnatal development of hypothalamic pathways involved in food intake and energy expenditure.Reference Thompson39,Reference Vickers and Sloboda40 Finally, the act of breastfeeding involves many emotional aspects, reinforcing the contact between mother and child. Due to these characteristics, the relationship between mother and child during breastfeeding controls the amount of milk consumed, and the baby learns to self-regulate its energy intake better than formula-fed children during late infancy (second half-year).Reference Singhal and Lanigan41,Reference Li, Fein and Grummer-Strawn42 This mechanism could be involved in the greater weight gain in formula-fed babies compared to breastfed babies.Reference Lucas, Boyes, Bloom and Aynsley-Green43
Breastfeeding plays a protective role against obesity and its disordersReference Horta, Victora and França44 by reducing the odds of overweight and obesity by 13%.Reference Horta, Loret De Mola and Victora45 In a recent epidemiological study involving 22 countries and 100,583 children, the beneficial effect of exclusive breastfeeding in preventing childhood obesity was conclusively confirmed.Reference Rito, Buoncristiano and Spinelli46 This beneficial effect seems to be time dependent because each additional month of breastfeeding was associated with a 4% reduction in the prevalence of overweight.Reference Harder, Bergmann, Kallischnigg and Plagemann47 A longer period of breastfeeding is also associated with a 35% reduction in the incidence of type 2 diabetes, but there is no evidence of a protective effect on blood pressure and total cholesterol.Reference Horta, Loret De Mola and Victora45 Despite the large amount of evidence of its benefits, only 40% of infants in the world were exclusively breastfed for the first 6 months of life between 2006 and 2012.48 Public policies guaranteeing the right to paid maternity leave have improved breastfeeding practices in several countries, increasing the prevalence of exclusive breastfeeding and its durationReference Chai, Nandi and Heymann49; however, the prevalence remains low.Reference Victora, Bahl and Barros50 Therefore, the WHO aimed to increase the rate of exclusive breastfeeding by at least 50% as a global target by 2025.Reference Rito, Buoncristiano and Spinelli46,51
The concern about early weaning is a result of the large amount of evidence from experimental and epidemiological studies about its deleterious impact on the health of progeny of both genders.Reference Baker, Michaelsen, Rasmussen and Sørensen52–Reference Bhargava, Sachdev and Fall57 Early weaning not only deprives offspring of all the beneficial effects of breast milk but can also promote early malnutrition by improperly introducing foods that babies cannot yet consume. On the other hand, commercial infant formulas have higher energy and protein contents than breastmilk,Reference Thompson39 which are involved in the rapid body weight gain of formula-fed babies. In addition, the increased protein content might stimulate insulin release, contributing to increased adiposity.Reference Lucas, Boyes, Bloom and Aynsley-Green43 These mechanisms might be involved in the metabolic programming of early-weaned offspring.
Early weaning is associated with rapid body weight gain during infancy. Infants weaned before 16 weeks of age gained significantly more weight during the first year,Reference Baker, Michaelsen, Rasmussen and Sørensen52 and this rapid weight gain is related to obesity in childhoodReference Ong, Ahmed, Dunger, Emmett and Preece53,Reference Stettler, Zemel, Kumanyika and Stallings54 and adulthood.Reference Stettler, Kumanyika, Katz, Zemel and Stallings55 Children who were breastfed for less than 3 months showed increased obesity rates at 1–7 years of age.Reference Stettler, Kumanyika, Katz, Zemel and Stallings55 Some metabolic disorders are linked to the early rapid growth observed in early-weaned babies, such as elevated blood pressure in adolescence,Reference Adair and Cole56 impaired glucose tolerance in young adults,Reference Bhargava, Sachdev and Fall57 and coronary heart disease.Reference Forsén, Eriksson, Tuomilehto, Osmond and Barker58
Animal models used to study long-term changes caused by early weaning
Epidemiological evidence shows that the increased prevalence of early weaning in humans has an impact on the health of progeny of both sexes throughout life. Therefore, animal models that mimic this phenomenon in different contexts might provide useful information regarding the mechanisms involved in the deleterious effects of early weaning on offspring health. It is interesting to note that most experimental studies in the literature were performed in males.
It is interesting to highlight that the different rodent early weaning models described in this review show conditions that are similar to some human conditions associated with early weaning, which sheds light on the many different aspects involved in early weaning. Although early weaning has many effects on offspring metabolism, the early weaning models have breast milk restriction in common (Fig. 1). This restriction involves not only caloric restriction but also the restriction of nutrients and hormones in maternal breast milk, contributing to the reduced body weight of offspring at PND21. Both body weight and hormonal changes are involved in the imprinting of the thrifty phenotype, promoting changes in the mechanisms involved in energy metabolism control. The resulting energy imbalance, together with the specific adaptive changes in each model that we describe in detail below, is responsible for the susceptibility to obesity and its related metabolic diseases of early-weaned offspring throughout life.

Fig. 1. Early life similarities in rodent early weaning models involved in the offspring metabolic outcome. The importance of breastfeeding is highlighted by rodent early weaning models. Maternal deprivation (MD), pharmacological early weaning (PEW), and nonpharmacological early weaning (NPEW) have milk restriction as a common feature, which reduces the transfer of calories, nutrients, and hormones to the offspring. This early malnutrition reduces the offspring body weight and is involved in the imprinting of the thrifty phenotype. This adaptive change promotes energy imbalance, contributing to the susceptibility to obesity and its related metabolic diseases of early-weaned offspring throughout life.
Maternal deprivation
Weaning in laboratory animals occurs at various time-points depending upon the species/strain and ethical regulations, but frequently in rodents, “standard weaning” will occur on postnatal day (PND) 21.Reference Curley, Jordan, Swaney, Izraelit, Kammel and Champagne59,Reference Sengupta60 At this age, the offspring show a degree of independence and spend more time eating solid food than suckling.Reference Sengupta60 This rodent weaning procedure is performed by separating the dam from her litter. Therefore, the separation of a mother and her litter before PND21 is a model of early weaning.
Early weaning by maternal deprivation (MD) has an impact on the metabolism and behavior of offspring throughout life.Reference Kikusui, Nakamura, Kakuma and Mori61–Reference Nakagaki, Mafra and de Carvalho65 This model involves maternal milk restriction and maternal care restriction, which promotes perinatal stress.Reference Ghizoni, Figueiredo, Moisan, Ogias, Osaki and Gama66 Therefore, the offspring outcomes could be adaptive responses to nutritional changes as well as emotional stress.
The changes in the hippocampus–hypothalamus–pituitary–adrenal (HHPA) axis of offspring after MD highlight the role of perinatal stress in this model. Thus, neonatal changes in corticosterone levels or signaling are involved in many metabolic programming models, and this hormone is a candidate imprinting factor.Reference Macrì67,Reference Weaver, Cervoni and Champagne68 In rodent models, early weaning at PND14–15 increased serum corticosterone 2 d after maternal separationReference Ghizoni, Figueiredo, Moisan, Ogias, Osaki and Gama66 and promoted increased activity and decreased resting behavior over the period from PND15 to 21, revealing stress-induced behavior in both sexes.Reference Kikusui, Isaka and Mori69 In adulthood, early-weaned offspring maintained increased serum corticosterone levels in basal or stressful conditions,Reference Kikusui, Nakamura, Kakuma and Mori61,Reference Kikusui, Takeuchi and Mori70 increased anxiety,Reference Kikusui, Isaka and Mori69 and aggressiveness were exhibited by both sexes.Reference Kikusui, Nakamura, Kakuma and Mori61,Reference Kikusui, Takeuchi and Mori70 In addition, adult female offspring showed decreased maternal behavior with offspring.Reference Kikusui, Isaka and Mori69
In addition to the behavioral changes, the metabolic outcomes in rat offspring were initially exhibited as a reduced body weight, which remained presentReference Crispel, Katz, Ben-Yosef and Hochberg62 or became normal in adulthood (150 d old).Reference dos Santos Oliveira, de Lima, da Silva, da Silva, de Souza and Manhães-de-Castro63,Reference Boueri, Pessanha and Da Costa71 Interestingly, early-weaned animals showed increased glucose tolerance and insulin sensitivity.Reference Crispel, Katz, Ben-Yosef and Hochberg62 However, these animals exhibited changes in the behavioral satiety test compared to late-weaned animals (PND31), suggesting a tendency toward delayed satiety behavior.Reference dos Santos Oliveira, de Lima, da Silva, da Silva, de Souza and Manhães-de-Castro63 In addition, in adult life, the early-weaned rats showed increased hepatic lipogenesis and hepatic cholesterol without changes in glucose tolerance and plasma cholesterol concentrations.Reference Back and Angel64 Interestingly, the hepatic alterations in response to early weaning could be a result of profound changes in the expression of several liver metabolic enzymes, starting with those involved in hepatic metabolic function.Reference Nakagaki, Mafra and de Carvalho65 Early weaning decreases hepatic immune function and accelerates the shift in metabolic functioning during neonatal development, which may affect liver function throughout life.
Interestingly, small episodes of maternal separation (4–8 h) before MD at PND17 in the maternal separation and early weaning (MSEW) model show how early-life neglect is reflected in the behavioral changes observed in neglected children, including hyperactivity, anxiety, and attention deficits.Reference Carlyle, Duque and Kitchen72 The MSEW female mice exposed to a high-fat diet (HFD) showed increased body weight, adiposity, and fasting glucose levels.Reference Murphy, Herald, Leachman, Villasante Tezanos, Cohn and Loria73 Moreover, these animals exhibited hyperinsulinemia, hyperleptinemia, and hypertension.Reference Murphy, Herald, Leachman, Villasante Tezanos, Cohn and Loria73 Therefore, MD increased the susceptibility to obesity and metabolic disorders in offspring in response to HFD, especially in female offspring.
Early-life stress is an important factor involved in metabolic programming. Therefore, not only is breast milk deprivation involved in offspring outcomes but also emotional stress resulting from MD. Punctual maternal separation for 24 h in PND10 induces HPA axis hyperactivity, exacerbating the response to stress in adult offspring.Reference Clarke, Cai, Saleh, Buller and Spencer74 Short maternal separation during the lactation period (10 min daily of maternal separation plus stress) also activated the HPA axis at weaning and in adult lifeReference Loizzo, Loizzo and Galietta75 and promoted overweight, hyperglycemia, hyperinsulinemia, hypertriglyceridemia, hypercholesterolemia, and hyperleptinemia in early-weaned offspring in adulthood.Reference Loizzo, Loizzo and Galietta75
Early weaning by MD is an interesting model of early weaning that mimics the real condition of mothers who abandon their child. The repeated lack of contact between mother and litter in rodent models reflects early-life child neglect, which is currently a social health challenge. Therefore, as the restriction of maternal care alone affects offspring development, rodent models of early weaning with no maternal separation could attenuate emotional stress by isolating the impact of breastfeeding restriction on offspring outcomes. For some years, our laboratory has been dedicated to studying the effects on programming of early weaning without MD to better understand the mechanisms underlying the increased risk for the development of obesity and its comorbidities. Fig. 2 depicts some results already published in the pharmacological and nonpharmacological models of early weaning, which are detailed below.

Fig. 2. Similarities and differences in metabolic outcomes between pharmacological early weaning (PEW) and nonpharmacological early weaning (NPEW) adult male offspring. The early weaning models showed some similarities; however, they could promote different offspring outcomes throughout life. The differences between PEW and NPEW adult male offspring are highlighted in red. Legend: brown adipose tissue (BAT), sympathetic nervous system (SNS), uncoupled protein 1 (UCP1).
Pharmacological early weaning
The inhibition of breast milk production using a pharmacological approach is seen as another experimental model of early weaning. Some drugs, such as bromocriptine, a dopamine-2-receptor agonist, are known for their rapid inhibitory effect on prolactin production at the pituitary level,Reference Ben-Jonathan and Hnasko76 promoting the reduction of maternal milk biosynthesis. In a rat model, maternal bromocriptine administration for the last 3 d of lactation showed potent effects in inhibiting prolactin, reducing milk production and, consequently, directly impacting the pup body weight at PND21.Reference Bonomo, Lisboa, Passos, Pazos-Moura, Reis and De Moura77 It is important to note that the pups received less milk but still received chow pellets directly in the cage. Therefore, the reduction of breast milk production by bromocriptine administration in late lactation is considered a pharmacological early weaning model (PEW), which plays a potent role in offspring metabolic programming.
Similar to MD, the PEW model is also a model of early-life stress, highlighted by the increased serum corticosterone level at PND21Reference Fraga, Moura and Silva78 and in adult life.Reference de Moura, Bonomo and Nogueira-Neto79 In addition, these animals exhibited higher catecholamine content in the adrenal gland in adulthood.Reference de Moura, Bonomo and Nogueira-Neto79 These hormonal changes could be involved in behavioral changes during adult life, as indicated by intense anxiety-like behavior and reduced locomotor activity.Reference Fraga, Moura and Silva78
Maternal hypoprolactinemia could alter milk leptin transfer to offspring,Reference Bonomo, Lisboa, Passos, Pazos-Moura, Reis and De Moura77 and this effect could modify hypothalamic circuits involved in satiety and energy expenditureReference Vickers80 during the development of hypothalamic and hippocampal circuits.Reference Coupé, Dutriez-Casteloot and Breton81 At PND21, PEW offspring had higher plasma leptin.33 Despite normal levels at PND22, the PEW offspring showed increased leptin levels at PND30Reference Carvalho, De Oliveira and Peixoto-Silva82 that persisted until adulthood (PND180).Reference Bonomo, Lisboa, Pereira, Cottini, Passos and Gaspar de Moura83 Interestingly, at PND22, the hypothalamus of PEW offspring seemed to be more sensitive to leptin, since leptin receptor (OBR) and signal transducer and activator of transcription 3 (STAT3) protein expression were increased.Reference Carvalho, De Oliveira and Peixoto-Silva82 These leptin changes could be implicated in metabolic disorders promoted by developmental programming by changing energy intake and expenditure control.Reference Vickers80 Indeed, the early weaning model promotes many metabolic disorders, such as overweight, increased visceral adiposity,Reference Bonomo, Lisboa, Pereira, Cottini, Passos and Gaspar de Moura83 hyperglycemia, hypoadiponectinemia, insulin resistance, and dyslipidemia.Reference de Moura, Bonomo and Nogueira-Neto79,Reference Peixoto-Silva, Conceicao and Carvalho84 Our group exhaustively investigated the mechanisms involved in the susceptibility to obesity. Although the adult animals showed resistance to the anorectic effect of leptin under challenge, they did not show food intake changes in basal conditions,Reference Bonomo, Lisboa, Pereira, Cottini, Passos and Gaspar de Moura83 suggesting that hyperphagia is not the mechanism responsible for the elevation in body weight. Additionally, adult PEW offspring did not show alterations in canonical leptin signaling in the hypothalamus,Reference de Moura, Bonomo and Nogueira-Neto79 which does not exclude the participation of other STAT3-independent pathways, such as the phosphoinositide 3-kinase (PI3K) pathway. However, these animals exhibited markers of susceptibility to the disturbance of satiety mechanisms, such as the increased expression of neuropeptide Y (NPY), an orexigenic peptide, in the arcuate nucleus (ARC) and paraventricular nucleus (PVN) of the hypothalamus,Reference Younes-Rapozo, Moura, Manhães, Peixoto-Silva, De Oliveira and Lisboa85 and the presence of astrogliosis, which might indicate hypothalamic inflammation.Reference Younes-Rapozo, Moura, Manhães, Peixoto-Silva, De Oliveira and Lisboa85 These changes are in accordance with leptin resistance in this model. Therefore, in the PEW model, it is possible that the mechanisms of satiety are borderline effective in promoting food intake changes. Perhaps, after a challenge with palatable foods, for example, these animals may develop hyperphagia.
Additionally, in the PEW model, disturbance in the energy expenditure mechanisms might be involved in the susceptibility to obesity. In adult life, these offspring showed reduced thyroid hormone, which is an important regulator of energy expenditure.Reference Ortiga-Carvalho, Chiamolera, Pazos-Moura and Wondisford86 Hypothyroidism in this model was characterized by a reduction in serum triiodothyronine (T3), thyroxine (T4), and thyrotropin (TSH, the major regulator of thyroid hormone synthesis), indicating the central disturbance of the thyroid.Reference Bonomo, Lisboa, Passos, Alves, Reis and de Moura87 Hypothyroidism could impair the thermogenic activity of brown adipose tissue (BAT) along with reducing sympathetic nervous system activity in the BAT and reducing adrenergic receptor content (β3-AR) in the PEW offspring.Reference Peixoto, Pietrobon and Bertasso88 Despite these changes, the BAT of PEW offspring did not show a change in uncoupled protein 1 (UCP1) expression but showed reduced peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α) expression.Reference Peixoto, Pietrobon and Bertasso88 Taken together, these changes confirm the presence of hypometabolism in PEW offspring, indicating their susceptibility to obesity. Interestingly, the restoration of serum thyroid hormones and corticosterone levels by chronic low-intensity exercise during life attenuated obesity and its related disorders, such as dyslipidemia and hyperglycemia, in adult male PEW offspring.Reference Boaventura, Casimiro-Lopes, Pazos-Moura, Oliveira, Lisboa and Moura89 Although they showed beneficial responses to chronic exercise, PEW offspring showed increased serum lactate, suggesting an impaired capacity of cell membrane adaptation to chronic exercise load.Reference Boaventura, Casimiro-Lopes, Pazos-Moura, Oliveira, Lisboa and Moura89 These animals did not show changes in physical performance in response to acute exercise.Reference Casimiro-Lopes, Lisboa, Koury, Boaventura, Passos and Moura90
Liver injury is a common finding in metabolic programming models,Reference Miranda, De Almeida and DaRocha91,Reference Franco, Lisboa and Lima92 and due to the hepatic control of carbohydrates and lipid metabolism, this injury is normally involved in associated macronutrient disorders. Interestingly, although the adult offspring exhibited dyslipidemia and glucose intolerance, the liver morphology was conserved in adult PEW offspring, despite the higher hepatic triglyceride levels. The liver in PEW offspring also showed a better redox state than that in control offspring, reaffirming the absence of liver injury at this age.Reference Peixoto-Silva, Conceicao and Carvalho84 This intriguing observation might be a result of the beneficial direct hepatic effects of bromocriptine, as observed in another experimental model.Reference Davis, Pei and Trush93,Reference Popovic, Janicijevic-Hudomal, Kaurinovic, Rasic and Trivic94
We also described the gradual dysfunction of the renal physiology of adult offspring of adult PEW offspring, which occurred without changes in blood pressureReference Passos, Passos and Oliveira95 or bone metabolism.Reference De Albuquerque Maia, Lisboa, De Oliveira, Da Silva Lima, Da Costa and De Moura96,Reference de Albuquerque Maia, Lisboa and de Oliveira97 Although the adult animals showed a reduction in total bone mineral density and mineral content at PND21,Reference De Albuquerque Maia, Lisboa, De Oliveira, Da Silva Lima, Da Costa and De Moura96 they also showed higher total bone mineral density and mineral content along with increased serum 25-hydroxyvitamin D,Reference de Albuquerque Maia, Lisboa and de Oliveira97 which could be related to increased serum leptin levels that have been shown to play a protective role and to exhibit a positive correlation with the metabolism of bone.Reference Steppan, Crawford, Chidsey-Frink, Ke and Swick98
The main clinical indication for bromocriptine is as a therapeutic agent for the treatment of prolactin-secreting tumors.Reference Schlechte99 Exclusive breastfeeding can be successfully established in babies whose mothers are receiving bromocriptine for the treatment of hyperprolactinemia during pregnancy and lactation,Reference Verma, Shah and Faridi100 but it is important to consider its adverse effects. Therefore, the PEW model is able to mimic this early-life exposure to bromocriptine. The offspring outcome from the PEW model could be a result of direct bromocriptine action in offspring and may not only be due to milk restriction. However, offspring that received bromocriptine from PND11 to PND20 of lactation showed a different outcome compared to that of PEW offspring (from mother who received bromocriptine), which showed hyperphagia and hyperthyroidism in adult life.Reference Carvalho, Lisboa and de Oliveira101 Therefore, PEW offspring outcomes throughout life are a result of milk restriction, but we cannot discard the influence of direct bromocriptine action. Therefore, an early weaning model without pharmacological intervention and the maintenance of maternal care could provide clear information about maternal milk restriction at early life by mimicking the common human condition of early weaning.
Nonpharmacological early weaning
The nonpharmacological early weaning (NPEW) model is closer to common human early weaning conditions and is without confounding factors, such as high stress or drug side effects. The NPEW model is performed in the last 3 d of lactation by introducing a physical barrier using a breast bandage to interrupt nipple suction.Reference Lima, de Moura and Passos102 In contrast to the other early weaning models, this model did not promote acute stress in offspring, since the basal serum corticosterone level at PND21 and in adults was unchanged,Reference Lima, Moura and Franco103 reinforcing the isolated impact of maternal milk restriction during late lactation on offspring outcome. Indeed, we did not observe behavioral changes in adulthood.Reference Fraga, de Moura and da Silva Lima104
Although they were not exposed to early-life stress, the NPEW offspring showed decreased body weight and length, adiposity, and serum glucose and serum insulin levels at PND21, which are changes related to milk deprivation.Reference Lima, de Moura and Passos102 At this age, the animals showed hypoleptinemia, hypothyroidism (low T3 syndrome), and unaltered adrenal catecholamine content.Reference Lima, Moura and Franco103 The T3 reduction could be a strategy to reduce metabolism and, therefore, energy expenditure during the period of energy restriction. In adult life, the NPEW offspring initially exhibited a normal body weight at PND120.Reference Nobre, Lisboa and Lima105 However, the body weight was increased throughout life, and the animals were overweight and showed increased total and visceral adiposity at PND180.Reference Lima, de Moura and Passos102 In addition, at PND180, the NPEW offspring exhibited metabolic disorders such as hyperglycemia, insulin resistance, hypertriglyceridemia,Reference Lima, de Moura and Passos102 and hypertension.Reference Franco, Lisboa and Lima92 The serum thyroid hormones and corticosterone levels were normal in adult NPEW offspring, but they showed increased adrenal catecholamine content and increased adrenergic β-3 receptor (β3-AR) expression in adipose tissue, suggesting the presence of reduced levels of catecholamines in serum and reduced tissue effects.Reference Lima, Moura and Franco103
This model has an important impact on hypothalamic circuits involved in the control of satiety. The adult PEW offspring showed increased serum leptin and hypothalamic leptin resistance,Reference Lima, de Moura and Passos102 as indicated by increased NPY and decreased cocaine- and amphetamine-regulated transcript (CART) expression, particularly in the PVN.Reference Younes-Rapozo, De Moura and Da Silva Lima106,Reference Lima, Franco and Peixoto-Silva107 In addition, these animals showed hypothalamic inflammation, which was highlighted by the increased tumor necrosis factor alpha (TNF-α) expression in the ARC nucleus.Reference Lima, De Oliveira, Da Silva, Maia, De Moura and Lisboa108 These central changes explain the basal hyperphagic behaviorReference Lima, de Moura and Passos102 and the disturbance in the anorexigenic response after leptin challenge.Reference Lima, De Oliveira, Da Silva, Maia, De Moura and Lisboa108 Interestingly, milk restriction altered the hypothalamic circuitry not only in the long term but also in the short term, as observed by increased hypothalamic NPY expression after PND21.Reference Younes-Rapozo, De Moura and Da Silva Lima106
Another satiety mechanism disrupted in NPEW offspring in adulthood is the expected increase in serum glucagon-like peptide 1 (GLP-1) levels after a meal, which stimulates anorexigenic neurons.Reference Quitete, Nobre, Peixoto-Silva, de Moura, Lisboa and De Oliveira109 This change might contribute to the hyperphagic phenotype. Early changes in this satiety mechanism were also observed, and it seems to be involved in the adaptation to malnutrition early in life. At PND21, the increased GLP-1 activity in the hypothalamus and adipose tissue plays an adaptive role by imprinting a thrifty phenotype.Reference Quitete, Nobre, Peixoto-Silva, de Moura, Lisboa and De Oliveira109 Interestingly, these changes and other metabolic features were reversed by chronic calcium supplementation in adulthood without an impact on hyperphagia,Reference Nobre, Lisboa and Lima105,Reference Quitete, Nobre, Peixoto-Silva, de Moura, Lisboa and De Oliveira109 revealing the contribution of other mechanisms in this programming model, such as hypothalamic leptin resistance.Reference Lima, de Moura and Passos102,Reference Lima, De Oliveira, Da Silva, Maia, De Moura and Lisboa108
In addition to hyperphagic behavior, changes in energy expenditure might contribute to obesity in NPEW adult offspring. Although serum thyroid hormone levels were normal in adulthood, the thermogenic activity of BAT might be disturbed in NPEW offspring. We observed reduced sympathetic nervous system activity in BAT, followed by reduced UCP1 and PGC1-α expression.Reference Peixoto, Pietrobon and Bertasso88 BAT function also seemed to be compromised by a reduction in the pAMPK/AMPK ratioReference Peixoto, Pietrobon and Bertasso88; taken together, the BAT changes could promote hypometabolism, contributing to the obesity phenotype.
The NPEW model also exhibited dysfunction in organs involved in metabolic disorders. In addition, white adipose tissue is affected by early weaning. In adult life, the white adipose tissue of NPEW offspring exhibited hypertrophic adipocytes, the increased expression of adipogenesis markers, such as CCAAT/enhancer-binding protein beta (C/EBPB) and peroxisome proliferator-activated receptor gamma (PPAR-γ), and the increased expression of inflammatory markers, such as interleukin 6 (IL-6), TNF-α and monocyte chemotactic protein 1 (MCP1).Reference Lima, De Oliveira, Da Silva, Maia, De Moura and Lisboa108,Reference Nobre, Lisboa and Peixoto-Silva110 We also observed markers of reduced vitamin D signalingReference Nobre, Lisboa and Peixoto-Silva110 and increased glucocorticoid signalingReference Miranda, Pietrobon and Bertasso111 in the visceral compartment; however, we also observed normal serum corticosterone levels,Reference Lima, Moura and Franco103 which could contribute to increased adipogenesis and lipogenesis in this tissue.Reference Nobre, Lisboa and Peixoto-Silva110,Reference Miranda, Pietrobon and Bertasso111 Adipose tissue dysfunction could be involved in the differential expression of adipocytokines via decreased adiponectin and increased leptin contentReference Lima, Moura and Franco103 and thereby impact serum hormone levels.Reference Lima, de Moura and Passos102 The white adipose tissue of NPEW offspring also showed increased expression of β3-AR, and its upregulation could be due to decreased serum catecholamines, which could be involved in decreased lipolysis in these animals.Reference Lima, Moura and Franco103
Interestingly, although they showed reduced bone mineral density at PND21,Reference De Albuquerque Maia, Lisboa, De Oliveira, Da Silva Lima, Da Costa and De Moura96 the adult NPEW offspring showed beneficial changes in bone structure and metabolism. They exhibited increased total bone mineral density and mineral content, including improved bone microarchitecture, and these changes improved the bone biomechanical properties.Reference de Albuquerque Maia, Lisboa and de Oliveira97
In adult life, the liver of NPEW offspring showed increased levels of markers of protein oxidation and lipid peroxidation and decreased antioxidant activity of glutathione peroxidase (GPx)Reference Franco, Lisboa and Lima92 and superoxide dismutase (SOD),Reference de Oliveira, Lima, Conceição, Peixoto-Silva, Moura and Lisboa112 revealing an imbalance in the redox state. Indeed, the liver morphology revealed steatosis, which was reinforced by increased levels of hepatic triglycerides.Reference Franco, Lisboa and Lima92 Liver dysfunction is likely to be involved in hypertriglyceridemia and hyperglycemia in NPEW offspring in adulthood.Reference Lima, de Moura and Passos102 These hepatic alterations (liver oxidative stress and microsteatosis) are in contrast to the observed PEW offspring phenotype, suggesting the protective effects of early bromocriptine exposure on the metabolic programming of liver function. Indeed, the NPEW offspring treated with bromocriptine during the last 3 d of lactation were protected from steatosis and glucose intolerance.Reference Peixoto-Silva, Moura and Carvalho113 The beneficial effects of bromocriptine on the liver were described before in adult animals.Reference Davis, Pei and Trush93,Reference Popovic, Janicijevic-Hudomal, Kaurinovic, Rasic and Trivic94 In addition, early bromocriptine exposure attenuated hyperphagia and increased adiposity and hyperleptinemia in NPEW offspring.Reference Peixoto-Silva, Moura and Carvalho113 These observations highlight the deleterious impact of isolated breast milk restriction on liver metabolism, reinforcing the effects of early weaning on the hepatic gene expression profile and function throughout life.Reference Nakagaki, Mafra and de Carvalho65
Although these models mimic the human conditions of early weaning by promoting early life breast milk restriction and the thrifty phenotype throughout life, the hepatic outcome is not the only difference between adult PEW and NPEW offspring. In Fig. 2, we show the similarities and differences between these rodent early weaning models in terms of the function of metabolic tissues and outcomes. Despite similar outcomes for overweight and adiposity, the outcomes for metabolic disorders and hormonal changes were different in their effects and intensity.
Interestingly, we have shown that these metabolic disorders could be attenuated by chronic nutritional interventions during the adult life of NPEW offspring. Hepatic injury was reversed by resveratrol supplementation in adulthoodReference Franco, Lisboa and Lima92 and by treatment with Ilex paraguariensis (yerba mate),Reference de Oliveira, Lima, Conceição, Peixoto-Silva, Moura and Lisboa112 while adipose tissue dysfunction was prevented by calcium supplementation.Reference Nobre, Lisboa and Peixoto-Silva110
Currently, the major reason for a short breastfeeding duration is the early return to work,Reference Ogbuanu, Glover, Probst, Liu and Hussey114 which makes an important contribution to the increased prevalence of early weaningReference Victora, Bahl and Barros50 due the increased number of women in the workforce and plays an important financial role in the family.Reference Chai, Nandi and Heymann49 In this situation, breast milk restriction is the major imprinting factor involved, and its isolated impact on offspring metabolism is well represented in the NPEW model, highlighting the importance of exploring the observed mechanisms involved in metabolic programming.
Sex-related differences
As previously mentioned, the majority of data available concerning early weaning have been reported from male animals. However, recently, female offspring in early weaning models were investigated, and, in general, the findings were different, suggesting a sex dimorphism for some outcomes.
Tables 1 and 2 depict the findings in early-weaned female offspring compared to the findings in males. Female offspring in both pharmacological and NPEW models exhibited higher adiposity and hyperphagia, despite having normal body weight. A sex-dependent mechanism seems to be involved in this phenotype because the females showed differences in hormonal profiles,Reference Pietrobon, Bertasso and Silva115 BAT thermogenic capacity,Reference Peixoto, Pietrobon and Bertasso88 fat deposit distribution, and glucocorticoid status.Reference Miranda, Pietrobon and Bertasso111
Table 1. Different metabolic parameters in offspring of both sexes in a pharmacological early weaning (PEW) model
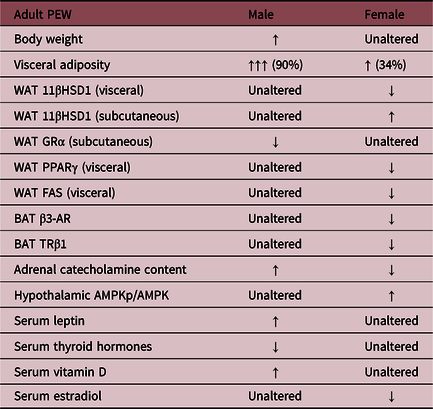
These data were described by Miranda et al., 2019; Pietrobon et al., 2019; and Peixoto et al., 2019. Legend: White adipose tissue (WAT); 11β-hydroxysteroid dehydrogenase (11β-HSD1); glucocorticoid receptor (GRα); peroxisome proliferator-activated receptor gamma (PPARγ); fatty acid synthase (FAS); brown adipose tissue (BAT); β3-adrenergic receptor (β3-AR); thyroid hormone receptor β1 (TRβ1).
Table 2. Different metabolic parameters of offspring of both sexes in a nonpharmacological early weaning (NPEW) model
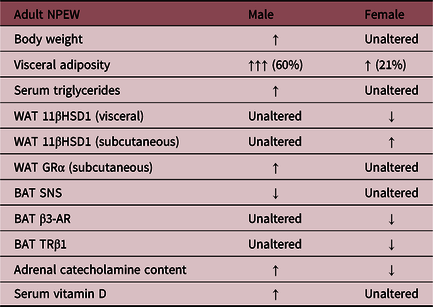
These data were described by Miranda et al., 2019; Pietrobon et al., 2019; and Peixoto et al., 2019. Legend: White adipose tissue (WAT); 11β-hydroxysteroid dehydrogenase (11β-HSD1); glucocorticoid receptor alpha (GRα); brown adipose tissue (BAT); sympathetic nervous system (SNS); β3-adrenergic receptor (β3-AR); thyroid hormone receptor β1 (TRβ1).
Perspectives
Based on epidemiological studies and on the early weaning models in rodents described in this review, the importance of breast milk in energy homeostasis and in the behavior of offspring is evident. Although the MD, PEW, and NPEW models share similarities that suggest potential targets for epigenetic changes, the precise mechanism involved in developmental programming has not yet been fully elucidated. Therefore, several aspects must be investigated, emphasizing the need for further studies in this area, for example, addressing the transgenerational effect and using different animal models, including nonhuman primates.
The sex-related differences have been described in some programming models during pregnancy, and, interestingly, female protection is reported on some outcomes related to the placenta, such as lower risk for placental inflammation compared to male fetus,Reference Kim, Young, Grattan and Jasoni116 differences in the pattern of placental gene expression,Reference Sood, Zehnder, Druzin and Brown117 and in response to perinatal insult.Reference Mao, Zhang, Sieli, Falduto, Torres and Rosenfeld118 In the early weaning model (without the placental influence), the strategies for growth and adaptation to the maternal environmentReference Clifton119–Reference Eriksson, Kajantie, Osmond, Thornburg and Barker121 may be different between sexes, which deserves further study.
Thus, rodent models are important tools to clarify the mechanisms involved in the developmental programming by early weaning. This knowledge can help generate new public health policies to reinforce the role of exclusive breastfeeding for up to 6 months in promoting health and reducing overweight and obesity in childhood and adulthood.
Acknowledgments
The authors thank the students of the Laboratory of Endocrine Physiology of the State University of Rio de Janeiro for all the scientific contribution that inspired the writing of this review.
Financial Support
LLS, EGM, and PCL are researchers from the State University of Rio de Janeiro, which have research projects supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, 001).
Conflicts of Interest
The authors declare no conflict of interest.






