Introduction
Temnocephalan species are ectosymbiotic rhabdocoels known mainly from the Neotropical and Australian bioregions. They occur on freshwater hosts, particularly crustaceans, but also molluscs, insects and turtles. Nine valid species of the genus Temnocephala Blanchard, 1849 were recorded on species of Aegla Leach, Reference Leach and Levrault1820 (Crustacea, Anomura, Aeglidae): Temnocephala chilensis (Moquin-Tandon, 1846), Temnocephala axenos Monticelli, 1898, Temnocephala mexicana Vayssière, 1898, Temnocephala talicei Dioni, 1967, Temnocephala cyanoglandula Amato, Amato & Daudt, 2003, Temnocephala mertoni Volonterio, Reference Volonterio2007, Temnocephala dionii Ponce de León, Berón Vera & Volonterio, 2015, Temnocephala grisella Seixas, Dametto & Périco, 2018 and Temnocephala gargantua Ponce de León & Volonterio, 2018 (Ponce de León & Volonterio, Reference Ponce de León and Volonterio2018; Seixas et al., Reference Seixas, Dametto and Périco2018).
Temnocephala axenos is a widespread species, known as an ectosymbiont on several aeglid species (fig. 1; table 1). Monticelli (Reference Monticelli1889) published a brief note on eggs and the embryology of what was supposed to be T. chilensis. These specimens were deposited in the Museum für Naturkunde Berlin (MfN-Berlin) in Germany, labelled as collected in ‘Prov. S. Catherina, Blumenau, Brazil, W. Muller’ (today, the Municipality of Blumenau, Santa Catarina, Brazil), under the number 2736. A few years later, Monticelli (Reference Monticelli1898) first cited T. axenos, assigning the specimens identified as T. chilensis, which the author himself studied in 1889, to this new epibiotic species of an unknown host. Baer (Reference Baer1931) re-examined the specimens studied by Monticelli deposited in MfN-Berlin, as well as the new material collected in ‘Jararaca’, Santa Catarina, Brazil, on Aegla laevis (Latreille, Reference Latreille1818). Baer (Reference Baer1931) also examined specimens of Temnocephala brasiliensis Merton, 1922, synonymizing it as T. axenos. Pérez-González (Reference Pérez-González1949), in the work about physiological aspects of T. axenos and two other species, pointed out the variability of characters in the original description of T. axenos and the re-description made by Baer (Reference Baer1931). Temnocephala bresslaui Pérez-González, 1949 epibiont on Aegla castro Schmitt, 1942 from Paraná, Brazil, was synonymized to T. axenos by Dioni (Reference Dioni1967a). Dioni recorded T. axenos on unidentified species of Aegla and Parastacus Huxley, 1879 from Uruguay and Brazil. In the same year, Dioni (Reference Dioni1967b) recorded T. axenos on the Aegla sp., Aegla uruguayana Schmitt, 1942, Aegla franca Schmitt, 1942 and Aegla humahuaca Schmitt, 1942 from several localities in Argentina. Dioni (Reference Dioni1968) mentioned the presence of T. axenos on Aegla platensis Schmitt, 1942 in Tucumán and Buenos Aires, Argentina, but the first detailed study on Argentinean specimens was done by Damborenea (Reference Damborenea1992). Damborenea (Reference Damborenea1992) and Damborenea et al. (Reference Damborenea, César and Armendáriz1997) studied T. axenos of A. platensis and A. uruguayana from Isla Paulino, Berisso and Isla Martín García, Buenos Aires, Argentina. These same specimens were studied again by Damborenea & Cannon (Reference Damborenea and Cannon2001). Recently, Volonterio (Reference Volonterio2007) updated the description of T. axenos based on Uruguayan specimens in co-occurrence with T. mertoni and T. talicei on A. platensis. Volonterio (Reference Volonterio2007) presented doubts about the validity of T. cyanoglandula for its resemblance to T. axenos and T. bresslaui. Seixas et al. (Reference Seixas, Amato and Amato2015a) complemented the description of the female reproductive system of T. cyanoglandula and confirmed that it was a valid species, but also recognized the need for the review of T. bresslaui, as was proposed by Volonterio (Reference Volonterio2007). Pérez-González (Reference Pérez-González1949) presented very similar illustrations attributed to T. axenos, T. bresslaui and T. chilensis, and, for this reason, Dioni (Reference Dioni1967a), whose studied specimens also showed a huge variation in cirrus size (125–250 μm), believed they were all a single species: T. axenos.
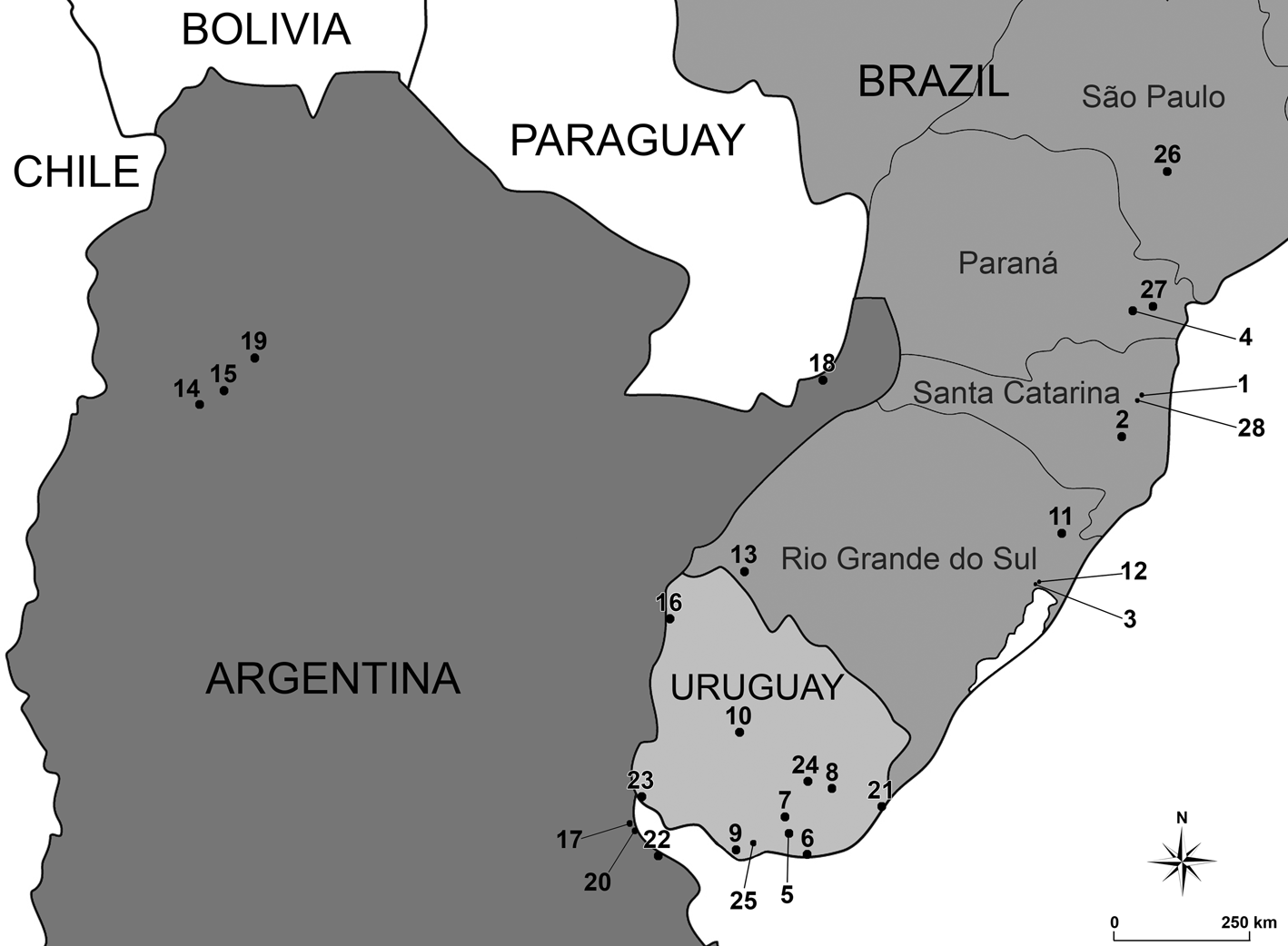
Fig. 1. Partial map of South America showing all the records of Temnocephala axenos to the present time. Full information of the localities and references could be consulted in table 1.
Table 1. Records of Temnocephala axenos to the present time. The data in fig. 1 are a visual summary of this information.

Although T. axenos has been studied by several authors (Baer, Reference Baer1931; Dioni, Reference Dioni1967a, Reference Dioni1968; Damborenea, Reference Damborenea1992; Damborenea et al., Reference Damborenea, César and Armendáriz1997; Volonterio, Reference Volonterio2007), none of their studies showed a search for the type material of the species, a resolution for the misidentification of the type host or the imprecise type locality due to the subsequent geographical division of the municipality cited by Monticelli (Reference Monticelli1889). Furthermore, current species descriptions of Temnocephala are based on characters that were not presented in older descriptions or that are now observed with different techniques. These may, sometimes, jeopardize species characterization in the past, as could be seen in the early publications of T. axenos. Older records of the species lack present important taxonomic characters or present a large variation of these characters, which could be associated to many other species (Monticelli, Reference Monticelli1898; Baer, Reference Baer1931; Dioni, Reference Dioni1967a), making it difficult to identify Temnocephala species on Aegla nowadays. All of these factors lead the species to an uncertain taxonomic status. In these cases, a critical analysis of the original description, a search for the type material, a study of all the records and a description of the species are recommended. The present work aims to answer the following question: what is the current taxonomic characterization of T. axenos?
Materials and methods
Specimens of Aegla schmitti Hobbs III, 1978 (fig. 2a–c) were collected at Reservatórios Mananciais da Serra (25°29′S, 048°58′W) (metropolitan area of Curitiba), Municipality of Piraquara, Paraná, PR, Brazil, in May of 2010, and Aegla jarai Bond-Buckup & Buckup, Reference Bond-Buckup and Buckup1994 (fig. 2d, e) were collected at Ribeirão Espingarda (27°01′26.8″S, 049°09′08.5″W), Parque Nacional da Serra do Itajaí, Municipality of Indaial, Santa Catarina, SC, Brazil, in November of 2010 under the Permanent License for the Collection of Zoological Material (SISBio 19.937-1) and License for the Collection at Parque Nacional da Serra do Itajaí (SISBio 23.831-1). The hosts were collected using dip nets and/or large sand sieves, and specimens of A. schmitti were transported live to the Laboratório de Ecologia de Crustacea, UFPR, Curitiba, Brazil, while specimens of A. jarai were carried to the Laboratório de Helmintologia, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, Brazil. The helminths were fixed following the protocols established by Amato et al. (Reference Amato, Seixas and Amato2007) and Seixas et al. (Reference Seixas, Amato and Amato2010). Some specimens were stained in Delafield's haematoxylin or aceto-carmine/fast green, cleared in cedar oil and mounted as permanent slides in Canada balsam. Other specimens were prepared for scanning electron microscopy (SEM) at the Centro de Microscopia Eletrônica, UFRGS. The distribution of rhabditogenic glands, disc glands and paranephrocytes were studied by clearing juvenile specimens in lactophenol. The temnocephalans were studied through a series of techniques focusing especially on the morphology of the female reproductive organs, as well as the morphology of the cirrus structure and the epidermal ‘excretory’ syncytial plates (EPs). Photomicrographs were taken with the microscopes Zeiss Axiolab (Carl Zeiss Microscopy, Jena, Germany) and Leica DMR Hc (Leica Microsystems, Wetzlar, Germany) equipped with Nomarski's differential interference contrast prisms (DIC). The line drawings, photographic images and map were prepared using Adobe Photoshop®, Adobe Illustrator® (Adobe, San Jose, California, United States of America) and Google Earth Web (Google LLC, Mountain View, California, United States of America). Extracted cirri were mounted in Faure's mounting medium (F). The terminology used to describe the male reproductive structures followed Seixas et al. (Reference Seixas, Amato and Amato2010). The images of the EPs were measured, according to Seixas et al. (Reference Seixas, Amato and Amato2015b).

Fig. 2. Aegla schmitti from Paraná, Brazil: (a) dorsal view showing fixated specimen of Temnocephala axenos; (b) mouthparts showing eggs and fixated temnocephalans; (c) ventral view of extended abdomen showing the concentration of T. axenos eggs in the first segments of the abdomen. Aegla jarai from Santa Catarina, Brazil: (d) partial ventral view showing hatched eggs (arrow) on the cheliped; (e) merus part of the cheliped showing unhatched eggs (white arrow) and live specimens of T. axenos (white head arrows) and their red eyes pigmentation (black arrow); (f) grouped eggs of T. axenos cleared in cedar oil and mounted in Canada balsam, showing hatched (white head arrow) and unhatched (arrow) eggs and the detail of one single egg showing the filament (black head arrow). Scale bars: (a) 10 mm; (b, d) 5 mm; (c, e) 2 mm; (f) 250 μm.
Argentinean specimens were studied from the deposited material by Damborenea (Reference Damborenea1992) in the Museo de La Plata (MLP), La Plata, Argentina (catalogue numbers MLP-He 3104–3109, 3114–3116 and 3129–3133). The specimens were stained in chloridric carmine and the measurements were made from in toto specimens mounted on permanent slides in Canada balsam.
Brazilian neotype and additional material were deposited in the Colección de Invertebrados, División Zoología Invertebrados, MLP, La Plata, Argentina. As the studied specimens were collected in Brazil, they should also be deposited in a helminthological collection of reference in the country. However, due to the Covid-19 outbreak, the Brazil–Argentina borders are closed and it was not possible to send the specimens to the Coleção Helmintológica do Instituto Oswaldo Cruz (CHIOC), Rio de Janeiro, RJ, Brazil. We talked directly to the Curator and he suggested that we deposit the material in Argentina and, later, when the pandemic restrictions pass, the material corresponding to CHIOC would be repatriated. Some specimens were deposited in the Coleção Helmintológica do Laboratório de Helmintologia, Departamento de Zoologia, UFRGS, Porto Alegre, RS, Brazil.
Results
Temnocephala axenos Monticelli, 1898
Taxonomic summary
-
Synonyms. Temnocephala chilensis (Moquin-Tandon, 1846) sensu Monticelli (1889); T. brasiliensis Merton, 1922 sensu Baer (1931); T. bresslaui Pérez-Gonzalez, 1949 sensu Dioni (1967a).
-
Type host. Unknown; A. jarai Bond-Buckup & Buckup, Reference Bond-Buckup and Buckup1994 (host of the neotype, present work).
-
Type locality. Santa Catarina, Blumenau, Brazil (Monticelli Reference Monticelli1889, Reference Monticelli1898); Ribeirão Espingarda, Parque Nacional da Serra do Itajaí, Indaial, SC, Brazil (locality of the neotype, present work).
-
Historical investigation about the possible type locality and type host. Amato et al. (Reference Amato, Amato and Daudt2003) pointed out that the type locality indicated by Monticelli (Reference Monticelli1889), the Municipality of Blumenau, was divided into several regions, so the type material may have been collected in Blumenau or neighbouring places. Before 1890, when the specimens were collected, Blumenau was bigger and less urbanized. Currently, the majority of the niche that could be used by aeglids are probably degraded by human activities. It is known that the rapid advance of the deterioration of water quality is a limiting factor for the population size and subsistence of aeglids in limnic bodies (Bond-Buckup & Buckup, Reference Bond-Buckup and Buckup1994). Baer (Reference Baer1931) received specimens collected in a stream near ‘Jararaca’, Blumenau, Santa Catarina, Brazil (probably Rio Itajaí do Sul, now belonging to the Municipality of Alfredo Wagner, SC) and for the first time pointed a host to the species, stating that the specimens received from Brazil were epibionts of A. laevis. However, at that point (the specimens were collected in Brazil by M. Caullery in September of 1928), the genus Aegla was believed to have only one species. Currently, several other species are recognized, and it is known that A. laevis is not present on the eastern side of the Andes. There are currently 19 species of Aegla recorded in the state of Santa Catarina (Boos et al., Reference Boos, Bond-Buckup, Buckup, Araujo, Magalhães, Almerão, Santos and Mantelatto2012; Santos et al., Reference Santos, Bond-Buckup, Gonçalves, Bartholomei-Santos, Buckup and Jara2017). Aegla jarai and Aegla muelleri Bond-Buckup & Buckup, 2010 in Bond-Buckup et al. (Reference Bond-Buckup, Jara, Buckup, Pérez-Losada, Crandall and Santos2010) co-occur in the Parque Nacional da Serra do Itajaí; however, no specimens of A. muelleri were positive for temnocephalids. Aegla jarai is presented in this work as a probable type host of the species, as it is a restricted taxon of southern Brazil, with wide distribution within the State of Santa Catarina and registered in Ribeirão Caeté (Ferdinand Schadrack Waterfall), Blumenau, SC. Ribeirão Caeté is one of the tributaries of Ribeirão Garcia that originates in the Parque Nacional da Serra do Itajaí and has its mouth in the Rio Itajaí-Açu, in the historic centre of Blumenau, SC. The Parque Nacional da Serra do Itajaí is being presented as a probable type locality, as it is a protected area with a preserved land of approximately 60,000 ha and is 14.7 km from the Municipality of Blumenau, SC (location cited by Monticelli) and 70.1 km from the Municipality of Alfredo Wagner ‘Jararaca’, SC (location cited by Baer).
-
Other hosts and localities. Aegla laevis (Latreille, Reference Latreille1818), ‘Jararaca’, Blumenau, Santa Catarina, Brazil (Baer, Reference Baer1931); Porto Alegre, Rio Grande do Sul, Brazil (Merton, Reference Merton1922); A. castro Schmitt, 1942, Rio Bariguí, south Curitiba, Paraná, Brazil (Pérez-González, Reference Pérez-González1949); Aegla sp. (Crustacea, Anomura, Aeglidae) and Parastacus sp. (Crustacea, Astacidea, Parastacidae), Laguna Del Sauce and Aguas Blancas, Maldonado, Uruguay; Río Santa Lucía and Río Cebollatí, Lavalleja, Uruguay; Río Santa Lucía, Montevideo, Uruguay; Río Negro, Tacuarembó, Uruguay; Arroio Dom Pedro, São Francisco de Paula, Rio Grande do Sul, Brazil; Vila Jardim, Porto Alegre, Rio Grande do Sul, Brazil; Cerro do Jarau, Quaraí, Rio Grande do Sul, Brazil (Dioni, Reference Dioni1967a); Aegla sp., A. uruguayana Schmitt, 1942, A. franca Schmitt, 1942 and A. humahuaca Schmitt, 1942, La Chacra, Andalgalá, Catamarca, Argentina; Río Cochuna, Tucumán, Argentina; Sangrador del Sur, Federación, Entre Ríos, Argentina; Río de la Plata, Vicente López, Buenos Aires, Argentina; Arroyo Santa María (puente), Misiones, Argentina (Dioni, Reference Dioni1967b); A. platensis Schmitt, 1942, Tucumán, Argentina; Buenos Aires, Argentina (Dioni, Reference Dioni1968); Aegla sp., Laguna Negra, Rocha, Uruguay (Ponce de León, Reference Ponce de León1988); A. uruguayana and A. platensis, Berisso, Isla Paulino, Buenos Aires, Argentina (Damborenea, Reference Damborenea1992); A. uruguayana and A. platensis, Isla Martín Garcia, Buenos Aires, Argentina (Damborenea et al., Reference Damborenea, César and Armendáriz1997); A. platensis, Arroyo Molles, Ruta 8, Km 238, Lavalleja, Uruguay; Arroyo Colorado, Ruta 6, Km 35, Canelones, Uruguay (Volonterio, Reference Volonterio2007); A. castro, Itatinga, São Paulo, Brazil (Martínez-Aquino et al., Reference Martínez-Aquino, Brusa and Damborenea2014); A. schmitti Hobbs III, 1978, Reservatórios Mananciais da Serra, Piraquara, Paraná, Brazil (present work).
-
Site of infestation. Branchial chambers and body surface; eggs cemented on the mouthparts, ventral side of the rostrum, chelipeds and ventral abdominal area, being more abundant in the second somite.
-
Other helminth specimens examined. Histological preparations of T. axenos deposited in the collection Vermes at MfN-Berlin: 2736a–c; representative specimens of T. axenos and paratypes of T. grisella deposited in the helminthological collection at MLP: T. axenos – MLP-He 3104–3109, 3114–3116 and 3129–3133, T. grisella – MLP-He 7100–7101; representative specimens of T. cyanoglandula deposited in CHIOC: CHIOC 38214 a–d; representative specimens of T. mexicana deposited in the Colección Nacional de Helmintos, Laboratorio de Helmintologia, Instituto de Biología, Universidade Nacional Autónoma de México (CNH-UNAM): 1311 and 1309.
-
Host specimens deposited. Aegla jarai Bond-Buckup & Buckup, Reference Bond-Buckup and Buckup1994, Coleção de Crustáceos do Departamento de Zoologia, UFRGS 4833–4846.
-
Helminth specimens deposited. Colección de Invertebrados, División Zoología Invertebrados, MLP, La Plata, Argentina. Neotype: MLP-He 7699. Additional material: MLP-He 7700 (T. axenos from A. jarai) and MLP-He 7701 (T. axenos from A. schmitti).
-
Description. Study based on 29 whole mounted adults, six specimens mounted on stubs for SEM, 14 dissected cirri mounted in F, and 16 specimens measured from A. jarai and A. schmitti. Of the 35 specimens from the deposited material by Damborenea (Reference Damborenea1992) in MLP, 20 of those specimens were measured.
-
Diagnosis. Temnocephalid with an elongated body; a medium-sized pedunculated adhesive disc; eyespots with red pigmentation; elliptical–rectangular EPs with marked and less bulged front and rear edges, extend from base of external tentacles to anterior portion of intestine; a small pharynx, wider than long with a larger posterior sphincter; a ‘bow-tie’-shaped intestine with thin wall and well-marked septa; four paranephrocytes, mostly one pair central and the other between the anterior and posterior testes in each side, sometimes all cells central and between the posterior testes; a small vagina with two vaginal sphincters, an asymmetric proximal one and a symmetric distal one; four seminal receptacles; a small and pedunculated egg with a thin subapical filament, opercular plates not observed; large seminal vesicle that opens polarly into a small and elongated prostatic bulb; four round and small testes, smaller anterior testes placed partially behind the posterior portion of the intestine, posterior testes central; cirrus long (175 μm on average) and slightly curved (approximately 17°); small introvert (28 μm on average) with approximately 26 rows of 12 small and sturdy distal spines each.
All the morphometric data of the neotype and Brazilian specimens are presented in table 2, which also shows the comparison of the new data (A. jarai and A. schmitti) and restudied Argentinean specimens from Damborenea (Reference Damborenea1992) with the data provided by Volonterio (Reference Volonterio2007).
Table 2. Morphometric data of the neotype and Brazilian specimens of Temnocephala axenos. Comparison between specimens from the new data (Aegla jarai and Aegla schmitti) and restudied Argentinean specimens from Damborenea (Reference Damborenea1992), with the data provided by Volonterio (Reference Volonterio2007).

SD, standard deviation; A, asymmetric; DVS, distal vaginal sphincter; EPs, epidermal ‘excretory’ syncytial plates; PVS, proximal vaginal sphincter; S, symmetric; VI, vesicula intermedia. All measurements are in micrometres; * indicates measurements in millimetres.
Remarks
The studied specimens of T. axenos from A. jarai (fig. 3b) presented a smaller total body size than the specimens of A. schmitti (fig. 3a); this difference in size can be explained by the greater number of young adults with the reproductive system already formed composing the infrapopulation collected on A. jarai due to the time of collection. The proximal sphincter is also, on average, slightly smaller in the specimens of A. jarai, whereas the distal sphincter is exactly the same in the specimens of both species of Aegla (table 2). In all observed specimens, from both hosts, four seminal receptacles were visualized (fig. 7b, d). The cirrus (fig. 8a, f) has similar measurements on the specimens from both hosts. Introvert length is potentially equal (fig. 8d–e, g–h) (28 μm on average in A. jarai and 27 μm on average in A. schmitti), which can be confirmed by the ratio between the total length of the cirrus and the length of the introvert (6.3:1 on both hosts’ specimens). The EPs (fig. 5a, b) of T. axenos have an elliptical–rectangular shape with marked and less bulged front and rear edges. They were observed only in the specimens of A. schmitti.

Fig. 3. Ventral view of adult specimens, in toto: (a) Temnocephala axenos of Aegla schmitti from Paraná, Brazil; (b) neotype of T. axenos of Aegla jarai from Santa Catarina, Brazil; (c) T. axenos of Damborenea (Reference Damborenea1992) from Argentina. Scale bars: 250 μm.
Restudied Argentinean specimens of Temnocephala axenos
The specimens studied by Damborenea (Reference Damborenea1992) as T. axenos belonged to three different species: T. axenos (fig. 3c) and two other unidentified species. The study of these three species together under the name T. axenos resulted in lower morphometric values presented in Damborenea (Reference Damborenea1992) than those presented in the current study (made exclusively about specimens of T. axenos). All of the studied Argentinean specimens of T. axenos presented a vesicula intermedia (fig. 7e). The other two species found in the studied material from Argentina will be identified and duly recorded in a future paper.
Discussion
In 2007, we consulted with Dr Birger Neuhaus, curator of the Vermes collection at MfN-Berlin, about the type series or other T. axenos specimens still present in the Museum. There is no information about the type series, and a single specimen of T. axenos was found in the MfN-Berlin as transverse serial cuts distributed on three slides and, according to the curator, this is the only material available of this species and the rest was lost (probably destroyed) during World War II. The slides with seven, six and five series of histological sections were from a whole specimen, which go from the tentacle region to the adhesive disc and showed some organs of the digestive, excretory and reproductive systems. Unfortunately, none of them were taxonomic characters. The only material that remains in the MfN-Berlin fails to characterize or help in the identification of the species. Adding to the lack of identification of its host, a fact evidenced in the species-specific epithet that means ‘unknown host’ and the broadly general type locality, the current taxonomic and nomenclatural status of T. axenos was species inquirenda, meaning a species of doubtful identity needing further investigation (ICZN, 1999). To resolve this situation, a historical investigation was carried out about the possible type locality and type host of the species (see section ‘Historical investigation about the possible type locality and type host’ in Results), and based on Article 75 of the International Code of Zoological Nomenclature (ICZN, 1999) we designated a neotype (MLP-He 7699).
Of the other eight species of Temnocephala epibiont on Aegla, T. chilensis and T. dionii have the most distinct cirri from T. axenos. Both species have a short cirrus (149 μm on average in T. chilensis and 159 μm on average in T. dionii) with a swollen ‘paint-brush like’ introvert being, mostly, differentiated by a groove between the shaft and the introvert presented in T. dionii (Damborenea, Reference Damborenea1992; Ponce de León et al., Reference Ponce de León, Berón Vera and Volonterio2015). Temnocephala talicei and T. mertoni are also species with short cirrus (123 μm on average in T. talicei and 138 μm on average in T. mertoni) (Dioni, Reference Dioni1967a; Damborenea, Reference Damborenea1992; Volonterio, Reference Volonterio2007). Although the cirrus length range of T. mertoni (123–157 μm) coincides with the lowest values of the range of T. axenos (150–200 μm), Volonterio (Reference Volonterio2007) described the cirrus of T. mertoni as ‘straight but with distal portion of shaft sinuous in all specimens’, differentiating it from T. axenos. The author also points out differences in the EPs, number of vaginal sphincters and in other characters. Temnocephala grisella, T. gargantua and T. cyanoglandula present a long and slightly curved cirri. Although similar to T. axenos in shape, the cirrus is much longer in the three species (202 μm on average in T. grisella, 225 μm on average in T. gargantua and 256 μm on average in T. cyanoglandula) (Amato et al., Reference Amato, Amato and Daudt2003; Seixas et al., Reference Seixas, Amato and Amato2015a, Reference Seixas, Dametto and Périco2018; Ponce de León & Volonterio, Reference Ponce de León and Volonterio2018). Temnocephala grisella is very similar to T. axenos, its cirrus length range (195–212 μm) coincides with the largest values of the range of T. axenos and both species have two vaginal sphincters, an asymmetric proximal one and a symmetrical distal one. However, the total diameter of the proximal vaginal sphincter is much larger in T. grisella (83 μm on average in T. grisella and 50 μm on average in T. axenos). Temnocephala grisella has the largest EP in total length among the epibionts of aeglids, but the total body length/EPs length ratio is similar between both species (6.7:1 in T. grisella and 7:1 in T. axenos). However, the shape described as ‘elongate, wider in the area surrounding the excretory pore’ by Seixas et al. (Reference Seixas, Dametto and Périco2018) differs from the elliptical–rectangular shape with marked and less bulged front and rear edges presented in T. axenos. The EPs of T. grisella are also thinner than the EPs of T. axenos, as we can see when comparing the total body width/EPs width ratio (14:1 in T. grisella and 11:1 in T. axenos). Dioni (Reference Dioni1972) did not present morphometric data when recording T. mexicana on indeterminate specimens of Aegla and Parastacus from Bariloche, Argentina. However, Lamothe-Argumedo (Reference Lamothe-Argumedo1968) presented a great cirrus size variability (144–206 μm), while re-describing the species. Although the range was similar to T. axenos, the cirrus of T. mexicana was described as ‘claviforme alargado’, meaning an almost straight shaft and a curved introvert (approximately 26°). The single, big and symmetrical vaginal sphincter of T. mexicana, evidenced by the diagram presented by Lamothe-Argumedo (Reference Lamothe-Argumedo1968) and visualized by the authors in the specimens deposited in CNH-UNAM, also differs from the species T. axenos.
Baer (Reference Baer1931) still had access to specimens of the type series and compared them with a vast amount of material received from Brazil and specimens studied by Merton (Reference Merton1922). Despite criticism about the lack of characters in the original description of the species, the author briefly described the cirrus as ‘a simple chitinous tube surrounded by spines in its distal portion’ and proposed the synonymy of T. brasiliensis. The eggs of T. axenos found in postures on A. jarai (fig. 2d, e) and A. schmitti (fig. 2b, c) were small and pedunculated, with a thin subapical filament (fig. 2d–f). No eggs were observed on the Argentinean specimens. The eggs of the Brazilian and Uruguayan specimens are similar to those presented by Baer (Reference Baer1931) obtained from the material of ‘Jararaca’ and, according to the author, also similar to the eggs studied by Merton (Reference Merton1922). According to Baer (Reference Baer1931), the eggs presented by Monticelli (Reference Monticelli1889) would be ‘monstrosities’ produced by hypersecretion of the shell's secretory glands. Merton (Reference Merton1922) and Baer (Reference Baer1931) described four seminal receptacles in their studies on T. axenos. Volonterio (Reference Volonterio2007) visualized these structures in only 10% of the sample and, in the remaining, observed the vesicula intermedia, a structure with the same function as the seminal receptacles (Damborenea, Reference Damborenea1994; Amato et al., Reference Amato, Amato and Seixas2005). In the present study, four seminal receptacles (fig. 6c) were observed in all studied specimens of A. jarai (fig. 7c, d) and A. schmitti (fig. 7a, b) from Brazil and a vesicula intermedia (fig. 7e) in all restudied Argentinean specimens of Damborenea (Reference Damborenea1992). According to Pérez-González (Reference Pérez-González1949), the seminal receptacles are transitional structures and Volonterio (Reference Volonterio2007) observed the presence of both structures in T. axenos and T. mertoni.
The new data (Brazilian specimens and restudied Argentinean specimens) were compared to the updated description of the species based on Uruguayan specimens made by Volonterio (Reference Volonterio2007) (table 2) and all had similar total body size. Brazilian and Argentinean specimens were more similar, as the Uruguayan ones generally presented smaller measurements. Even so, the relationship between total body length and cirrus total length is similar between Brazilian (12.2:1 in A. schmitti), Argentinean (12.5:1) and Uruguayan (12.4:1) specimens. Volonterio (Reference Volonterio2007) described T. axenos’ EPs as ‘elliptical, small, extends from base of external tentacles to level of anterior portion of intestine’, and this description confirms what was observed in A. schmitti specimens (fig. 5a, b). We cannot compare the total EPs size or the ratio between total body size and total EPs length (table 2) because the data were not presented by the author, but similar to the material found in the present work, Volonterio (Reference Volonterio2007, Fig. 7) illustrated the EPs as having an elliptical–rectangular shape, even if they were not described in this way. As for the morphological characters of the male reproductive system (figs 6b, d and 8a–h), again the Brazilian and Argentinean specimens showed greater similarity in the total length of the cirrus and introvert. The Uruguayan specimens showed slightly lower values, which can be observed in the ratio between the total cirrus length and the total introvert length (6.3:1 in Brazilian, 6.1:1 in Argentinean and 5.8:1 in Uruguayan specimens). Regarding the width of the base of the shaft, Argentinean and Uruguayan specimens were more similar. Two conspicuous vaginal sphincters (fig. 7a, c) of similar total diameter were observed. The proximal sphincter is asymmetrical and has an anterior portion smaller than the posterior portion, whereas the distal sphincter is symmetrical (figs 6c and 7b, d, e). Brazilian and Argentinean specimens had similar total diameter of both sphincters and, as occurred in other characters, Uruguayan specimens had lower values. The vagina is short, and its length had a smaller variation in the Brazilian specimens (of A. schmitti) than in the Argentinean and Uruguayan ones. Despite the wider variation, the measurements on average are similar, lower in Uruguayan specimens. As described for the Uruguayan specimens, the Brazilian and Argentinean specimens presented four paranephrocytes. In most specimens, one pair is central and the other is shifted between the anterior and posterior testes on both sides of the body (fig. 4a), sometimes the four cells are central and located between the posterior testes, near the disc glands (fig. 7f). Due to fixation and staining, they were best observed in Brazilian specimens. The pair of paranephrocytes that shifted between the anterior and posterior testes had already been visualized and erroneously described as a cement gland in Temnocephala trapeziformis Amato, Amato & Seixas, 2006 (Amato et al., Reference Amato, Amato and Seixas2006).

Fig. 4. Juveniles specimens cleared in lactophenol, showing details of the glands of Temnocephala axenos of Aegla jarai from Santa Catarina, Brazil. (a) In toto specimen in ventral view, showing cirrus (c), oesophageal glands (eg), paranephrocytes (arrows), pharynx (p) and rhabditogenic gland ducts entering tentacles (head arrows). (b) Dorsal view showing the anterior and posterior limit of the rhabditogenic glands (white arrows), the anterior and posterior limit of the disc glands (black head arrows) and central disc glands (asterisk). Scale bars: (a) 250 μm; (b) 100 μm.

Fig. 5. Temnocephala axenos of Aegla schmitti from Paraná, Brazil, observed with SEM, showing details of the epidermal ‘excretory’ syncytial plates (EPs). (a) In toto specimen in dorsal view, showing the highlighted EPs, their limits (head arrows) and nephridiopore (n). (b) EPs and nephridiopore (n). Scale bars: (a) 200 μm; (b) 100 μm.

Fig. 6. Diagrams of neotype of Temnocephala axenos of Aegla jarai from Santa Catarina, Brazil. (a) Adult specimen, ventral view, showing adhesive disc (ad), anterior testes (at), excretory vesicle (ev), Haswell glands (hg), intestine (i), mouth (m), pharynx (p), posterior testis (pt), reproductive system (rs), tentacles (t) and vitelline glands (vg). Partial diagrams of the reproductive system: (b) male, showing cirrus (c), deferent vessels (dd), prostatic bulb (pb), prostatic cells (pc), prostatic secretions (ps) and seminal vesicle (sv); (c) female, showing anterior portion of the proximal vaginal sphincter (apvs), distal vaginal sphincter (dvs), genital atrium (ga), genital pore (gp), ovary (ov), posterior portion of the proximal vaginal sphincter (ppvs), vagina (va), vitelline duct (vd), seminal receptacles (sr) and vesicula resorbens (vr); (d) cirrus showing the proximal limit of the introvert (arrow). Scale bars: (a) 250 μm; (b, c) 50 μm; (d) 15 μm.

Fig. 7. Female partial reproductive system of an adult specimen of Temnocephala axenos: (a, b) T. axenos of Aegla schmitti from Paraná, Brazil. (c, d) Neotype of T. axenos of Aegla jarai from Santa Catarina, Brazil. (e) Temnocephala axenos from Damborenea (Reference Damborenea1992), Argentina. (f) Partial reproductive and glandular system of a juvenile specimen of T. axenos of A. jarai from Santa Catarina, Brazil. Abbreviations: apvs, anterior portion of the proximal vaginal sphincter; c, cirrus; dvs, distal vaginal sphincter; ga, genital atrium; gp, genital pore; ov, ovary; pb, prostatic bulb; ppvs, posterior portion of the proximal vaginal sphincter; pvs, proximal vaginal sphincter; sv, seminal vesicle; va, vagina; vi, vesicula intermedia; vr, vesicula resorbens. Head arrows, paranephrocytes; asterisk, seminal receptacles. Scale bars: (a–d) 50 μm; (e, f) 100 μm.

Fig. 8. Temnocephala axenos, male partial reproductive system observed with Nomarski's DIC microscopy. (a–e) Temnocephala axenos of Aegla schmitti from Paraná, Brazil: (a) cirrus (c), deferent vessels (arrows), genital atrium (ga) and the insertion (head arrow) of the seminal vesicle (sv) in the prostatic bulb (pb); (b) entire cirrus (lateral view), showing the evident curvature, the proximal limit of the introvert (head arrow) and the shaft rim (arrow), typical of adult worms; (c) entire cirrus (dorsal or ventral view), genital atrium (ga), prostatic bulb (pb), the proximal limit of the introvert (head arrow) and the shaft rim (arrow); (d–e) introvert seen in different focusing planes, showing the proximal limit of the introvert (head arrow). (f–h) Temnocephala axenos of Aegla jarai from Santa Catarina, Brazil: (f) cirrus (c), distal vaginal sphincter (arrows), prostatic bulb (pb), proximal vaginal sphincter (head arrows), seminal receptacles (asterisks), seminal vesicle (sv) and vagina (va); (g, h) introvert seen in different focusing planes, showing the proximal limit of the introvert (white head arrow). Scale bars: (a) 100 μm; (b, c, f) 50 μm; (d) 10 μm; (g, h) 20 μm.
Although Volonterio (Reference Volonterio2007) has made a detailed study of the Uruguayan specimens of T. axenos, thus updating the species description, the specimens were not even geographically close to the type locality and a neotype was not designed to validate the species’ taxonomic status again. The ‘possible’ validity of T. bresslaui was cited in Volonterio (Reference Volonterio2007), Seixas et al. (Reference Seixas, Amato and Amato2015a) and Ponce de León & Volonterio (Reference Ponce de León and Volonterio2018). This proposal was based on the length of the cirrus, and table 2 shows that there is great variation in this character within T. axenos. This argument reinforces the need for this work, so that there is a clear characterization of T. axenos to, in the future, compare and define what happens with T. bresslaui. The historical background and the discussion about geographical origins and hosts of the species, as well as a designation of a neotype, allow comparative material of the type locality and type host to exist, eliminating doubts about the identification of T. axenos.
Acknowledgements
Our thanks to CONICET for the post-doctoral scholarship awarded to S.A.S.; to CAPES (Coordenação de Aperfeiçoamento do Pessoal de Nível Superior) for the doctoral scholarship awarded to S.A.S. (2008–2013); to Dr Jorge Ernesto de Araújo Mariath and Dr Rinaldo Pires dos Santos, Laboratório de Anatomia Vegetal (UFRGS), for allowing the use of the Leica DMR Hc microscope to make the DIC photomicrographs; to the staff of the Centro de Microscopia Eletrônica (UFRGS) for SEM operation; to Dr Ludwig Buckup for his help contacting and translating the letters to the Museum für Naturkunde Berlin; to Dr Birger Neuhaus, curator of the Vermes collection (MfN-Berlin), for the loan of the T. axenos representative specimen and for the precious information about the type series; to Dr Laura Utz and Dr Sean Cooney for enabling the acquisition of the original description of T. axenos from the Smithsonian Institution; to Dr Setuko Masunari, Dr André Trevisan and Dr Murilo Zanetti Marochi from the Laboratório de Ecologia de Crustacea (UFPR) for the collection and identification of the specimens of A. schmitti; to Dr Harry Boos Jr from the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) for his invaluable help in the field and for making access and collection in the Parque Nacional da Serra do Itajaí possible; to Dr Georgina Bond-Buckup for the identification of the specimens of A. jarai; to Bibiana S. Oliveira Fam, Débora N. Souza and Lucas Casagrande for their help in the laboratory; and to the biologists and English translators Joyce Baptista and Dr Philip J. Scholl for kindly reviewing the English in several versions of the manuscript.
Financial support
This work was supported by the Agencia Nacional de Promoción Científica y Técnica (grant number PICT 2016-3290) and Universidad Nacional de La Plata (grant number 11/N884).
Conflicts of interest
None.
Ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed.












