INTRODUCTION
The genus Hammondia was erected to accommodate the newly discovered Toxoplasma gondii-like, but obligatory heteroxenous coccidian Hammondia hammondi, using cats as definitive hosts (Frenkel and Dubey, Reference Frenkel and Dubey1975). The oocysts of H. hammondi were morphologically indistinguishable from those of T. gondii of cats, as well as from oocysts of the small race or type of Isospora bigemina, which had originally been reported from dogs by Wenyon and Sheather (Reference Wenyon and Sheather1925) and Wenyon (Reference Wenyon1926). Heydorn (Reference Heydorn1973) and Fayer (Reference Fayer1974) established that the small race of I. bigemina had a two-host life cycle, using dogs as definitive hosts and cattle as intermediate hosts. Other ruminants and coyotes were soon found to act as additional intermediate and definitive hosts, respectively, for this species (e.g., Dubey and Fayer, Reference Dubey and Fayer1976; Dissanaike and Kan, Reference Dissanaike and Kan1977; Dubey and Williams, Reference Dubey and Williams1980; Nassar et al. Reference Nassar, Hilali and Rommel1983; Warrag and Hussain, Reference Warrag and Hussein1983). Isospora bigemina in dogs was renamed Isospora heydorni by Tadros and Laarman (Reference Tadros and Laarman1976) and transferred to the genus Hammondia by Dubey (Reference Dubey and Krier1977); hence the currently used name Hammondia heydorni. In 1998, McAllister et al. reported that dogs also acted as definitive host for the pathogenic species Neospora caninum, which possessed oocysts that were morphologically indistinguishable from those of H. heydorni. From now on, it became epidemiologically important to accurately identify this type of oocyst in dogs, and molecular methods were developed for this purpose (Šlapeta et al. Reference Šlapeta, Koudela, Votýpka, Modrý, Hořejš and Lukeš2002a, Reference Šlapeta, Modrý, Kyselová, Hořejš, Lukeš and Koudelab).
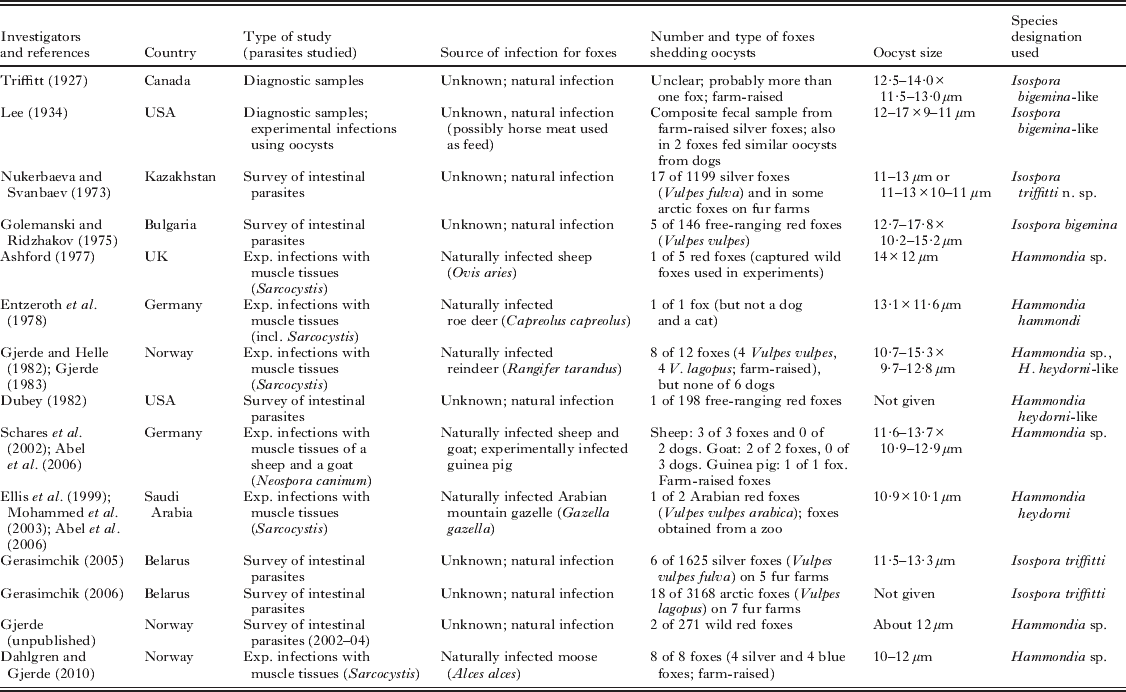
Oocysts similar in size and shape to those of H. heydorni in dogs have also been found in the feces of foxes in several studies, as summarized in Table 1. These studies have included surveys for intestinal parasites of naturally infected free-ranging or farm-raised foxes, as well as experimental infections of foxes with muscle tissues of various hosts, mainly in order to elucidate the life cycle of various Sarcocystis species. Thus, in Norway, Hammondia heydorni-like oocysts were initially detected in foxes used in experiments to determine their role as definitive hosts for Sarcocystis species of reindeer (Gjerde, Reference Gjerde1983), and more recently in foxes fed muscle tissues of Sarcocystis-infected moose (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010). As seen from Table 1, various species designations have been assigned to the H. heydorni-like oocysts in foxes, and most authors have believed them to be conspecific with I. bigemina/H. heydorni of dogs. However, Nukerbaeva and Svanbaev (Reference Nukerbaeva and Svanbaev1973) believed that various Isospora species were host specific, and named the I. bigemina-like oocysts they found in farmed silver and arctic foxes in Kazakhstan, Isospora triffitti n. sp. in recognition of Marjorie J. Triffitt, who had first described such oocysts from foxes (Triffitt, Reference Triffitt1927). This species name was later amended to Isospora triffittae by Levine (Reference Levine1985), since the species had been named after a woman.
Table 1. Overview of previous studies detecting Hammondia-like oocysts in faecal samples from foxes

Some infection experiments have suggested that the Hammondia sp. infecting foxes is not infectious for dogs (Enzeroth et al. Reference Entzeroth, Scholtyseck and Greuel1978; Gjerde, Reference Gjerde1983; Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002). Similarly, molecular comparisons at a few loci between fox-derived Hammondia isolates and H. heydorni isolates from dogs, have indicated that they may represent 2 distinct genetic linages (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002; Mohammed et al. Reference Mohammed, Davies, Hussein, Daszak and Ellis2003; Abel et al. Reference Abel, Schares, Orzeszko, Gasser and Ellis2006). Since molecular and biological data from more Hammondia isolates from foxes might help resolve the question of whether Hammondia sp. in foxes is different from Hammondia heydorni of dogs, the present study was initiated after having been prompted by the unexpected finding of Hammondia oocysts in foxes fed muscle tissues of moose (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010). In addition to this recent Hammondia isolate from foxes, we had available 2 similar, about 25-year-old oocyst isolates from foxes, derived directly, or after passage through sheep, from naturally infected reindeer (Gjerde, Reference Gjerde1983), as well an isolate of H. heydorni-like oocysts from a naturally infected Norwegian dog.
Moreover, a considerable amount of biological and morphological data about the Hammondia sp. of foxes had been gathered during a series of experiments conducted in 1982–1984, subsequent to the initial finding of this coccidian in foxes in Norway in 1981 (Gjerde, Reference Gjerde1983). The major aims of these experiments were to elucidate the biology of this Hammondia sp., and particularly to determine: (1) its development in foxes, including the length of pre-patent and patent periods, the pattern of oocyst shedding and possible variations in oocyst morphology during patency; (2) whether carnivores other than foxes, particularly dogs, might act as definitive hosts; (3) the suitability of foxes, sheep, goats, rabbits and cats as intermediate hosts, including the timing of the formation of the infective stage in these hosts post-infection. The results of most of these studies have, however, not been published before, since it could not be conclusively determined whether the Hammondia sp. of foxes was different from H. heydorni in dogs, which already had been the topic of several similar studies by others. Since current molecular methods have the potential to resolve this crucial question, the major aims of the molecular examination of the 3 fox-derived and 1 dog-derived oocyst isolates were: (1) to PCR-amplify and sequence up to 6 genetic markers from each of the isolates; (2) to compare the new sequences with each other and with corresponding sequences available in GenBank of H. heydorni-like isolates from foxes and dogs in other countries; (3) based on this comparison, and the morphological and biological data obtained from the infection experiments, decide whether Hammondia sp. of foxes should be considered a separate species or a variant of H. heydorni.
For the reasons stated and discussed subsequently, Hammondia sp. of true foxes is considered to represent a separate species, closely related to, but different from, H. heydorni of dogs, but probably identical to I. triffitti of foxes as described by Nukerbaeva and Svanbaev (Reference Nukerbaeva and Svanbaev1973). The name Hammondia triffittae has therefore been used in the following.
MATERIALS AND METHODS
Infection experiments with reindeer-fox isolates of Hammondia triffittae
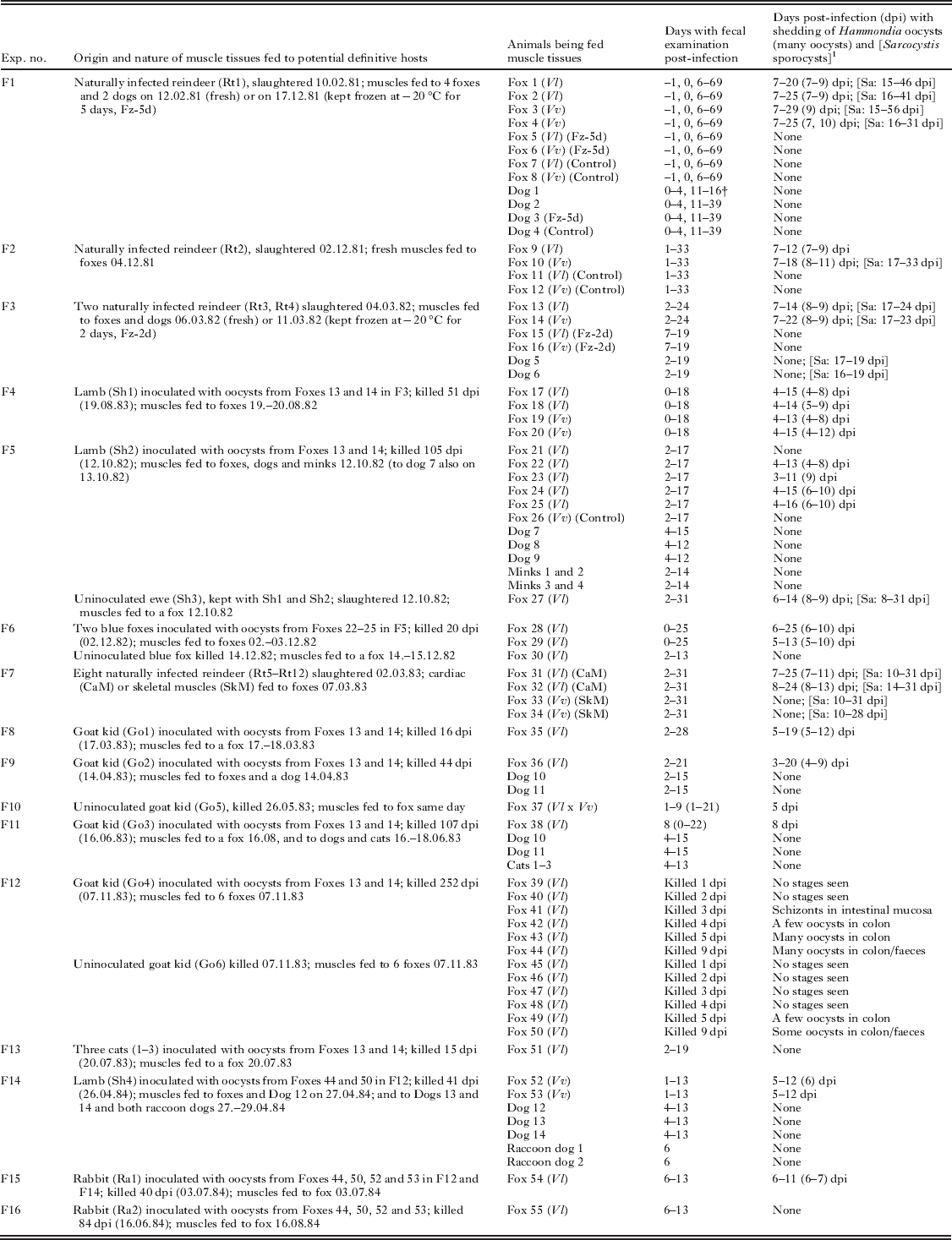
The general design and outcome of experiments F1–F16, which comprised the feeding of foxes and other carnivores with muscle tissues of naturally infected reindeer and potential intermediate hosts experimentally inoculated with Hammondia oocysts, are presented in Table 2. Below are some additional data that were common to all or most of these experiments, as well as some details about the experimental animals used.
Table 2. Design and outcome of experimental infections of foxes (Vv=silver/red foxes; Vl=blue/arctic foxes) and other potential definitive hosts with muscle tissues of animals naturally or experimentally infected with oocysts of Hammondia triffittae
1 The shedding/detection of Sarcocystis sporocysts was often intermittent during the given time period.
† Dog 1 euthanized 16 dpi due to severe diarrhoea caused by parvo virus infection.
Potential definitive hosts fed muscle tissues
All silver foxes (colour mutants of the red fox, Vulpes vulpes), blue foxes (colour mutants of the arctic fox, Vulpes (=Alopex) lagopus), raccoon dogs (Nyctereutes procyonoides), and minks (Mustela vison) had been born and raised at the Dal Research Farm for Fur-bearing Animals, at Heggedal, near Oslo, and were kept at this facility during the experiments. All dogs and cats were kept at different clinics at the Norwegian School of Veterinary Science (NVH), Oslo, during the experiments. None of the experimental animals, except possibly the cats, had eaten raw musculature prior to the experiments. During the experimental periods, the foxes, raccoon dogs and minks were fed the usual fox feed at the Research Farm, consisting mainly of heat-treated slaughterhouse offal, fish offal and cereals. The dogs and cats were fed commercial dry or canned dog and cat feed, respectively. The animals’ ordinary feed was usually withheld on the day of experimental feeding with muscle tissues.
Foxes
A total of 55 foxes were used in 16 separate experiments (F1–F16). Forty-six foxes were fed fresh muscle tissues, 4 foxes were fed muscle tissues that had been kept frozen for 2 or 5 days, and 5 foxes were used as uninfected controls. The foxes were kept individually in wire-mesh cages in open sheds, and fecal samples were usually collected daily from the ground below the cages and placed in labelled plastic bags. In most experiments, both silver and blue foxes were used (Table 2). In total, 39 blue foxes (Vl), 15 silver foxes (Vv), and 1 silver and blue fox hybrid (Vv x Vl) were used. The foxes were of both sexes and 6–24 months old at the time of infection.
Raccoon dogs
Two raccoon dogs (Nyctereutes procyonoides), about 1 year and 3 years of age, respectively, were used in Exp. F14. They were kept individually in 2 cages with wire-mesh floor, and fecal samples were collected from the ground below the cages.
Minks
Four minks (Mustela vison), about 16 months old, were used in Exp. F6. They were kept as pairs in 2 cages with wire-mesh floor, and fecal samples from each pair were collected from the ground below the cages.
Dogs
A total of 14 dogs were used in 6 separate experiments (F1, F5, F9–F11, F14). Twelve dogs were fed fresh muscle tissue, 1 dog was fed frozen and thawed muscle tissues, and 1 dog was an uninfected control. Dogs no. 10 and no. 11 were used in 2 separate experiments (F9 and F11). All dogs were obtained from a commercial breeder, rearing dogs for experimental purposes. They belonged to various breeds and ranged in age from about 6 weeks to about 6 months at the start of the experiments. The dogs were kept in individual cages, except the 6-week-old puppies used in Exp. F1, which were kept together with their mother for the first 10 days after the experimental feeding. Individual fecal samples were collected daily and examined for oocysts.
Cats
Three 6-week-old cats were used in experiment F11. These kittens were obtained from a litter on a farm shortly before the experiment, and were kept together in a single cage. Pooled fecal samples from all the cats were collected daily and examined for oocysts.
Potential intermediate hosts
Naturally infected reindeer and experimentally inoculated sheep, goats, foxes, cats and rabbits were evaluated as intermediate hosts by feeding muscle tissues from them to foxes upon euthanasia.
Reindeer
Muscle tissues used in Exps F1–F3 and F7 were obtained from adult reindeer slaughtered at an abattoir in Kautokeino, Finnmark County, Northern Norway. Muscle tissues (mostly abdominal muscles, diaphragm and heart) were collected from animals with numerous grossly visible sarcocysts in their skeletal muscles, since the initial purpose of these experiments was to study the infectivity of various Sarcocystis species of reindeer for foxes and dogs. The tissues were shipped to Oslo and fed to the experimental animals within 2–3 days after the reindeer had been slaughtered.
Sheep
Three lambs, which had been obtained from NVH's regular sheep flock, were used in 2 separate experiments. The 2 lambs used in Exps F4 and F5 had been born and raised indoors, and were kept in a pen at NVH together with their 3-year-old mother ewe throughout the experimental period. This ewe had been on pasture during 2 grazing seasons before the experiment. The lambs were about 2 months old when inoculated with oocysts. The single, 10-month-old lamb used in Exp. F14, had been kept indoors throughout its entire life, and was kept in a pen at NVH during the experimental period.
Goats
Six 1-week-old goat kids were obtained from Valbjør geiteavlsgard, Vågå (a research farm for goats), and were kept together in a single pen at NVH during the experimental period. Four of the goat kids were inoculated with oocysts when 2½ weeks old, while 2 kids served as inoculated controls. Muscle tissues from these goats were used in Exps F8–F12.
Foxes
Two adult blue foxes from Dal Research Farm were inoculated with oocysts, while a third blue fox from the farm served as an uninoculated control in Exp. F6. Fecal samples from both inoculated foxes were collected on days 6, 7 and 10 post-infection and examined for oocysts, to evaluate a possible direct life cycle.
Cats
The 3 cats fed muscle tissues from a goat kid in Exp. F11, were later inoculated with Hammondia-oocysts and subsequently euthanized. Muscle tissues from the cats were then fed to a fox in Exp. F13.
Rabbits
Two 6-month-old laboratory reared rabbits, kept in individual cages at the Research Animal Unit at NVH, were inoculated with oocysts and used in Exps F15 and F16.
Sporulation of oocysts and administration of oocyst inocula
Fecal samples from foxes, collected on days with high oocyst excretion, were suspended in 2·5% potassium dichromate, sieved through a coarse sieve, and left to sporulate in Petri dishes for several days at room temperature (22–24°C). Sporulated oocysts were subsequently kept refrigerated in 2·5% potassium dichromate until used to infect animals. Before inoculation, oocysts were washed by repeated centrifugation in tap water to remove potassium dichromate. Lambs Sh1 and Sh2 each received about 10 000 sporulated oocysts, whereas the number of oocysts inoculated into other potential hosts was not accurately determined. In all experiments, oocysts from foxes were administered orally. Lambs, foxes and goat kids were inoculated through a stomach tube; rabbits were inoculated into the mouth using a syringe; and cats were given oocysts mixed with their ordinary feed. All animals were fed oocysts once, except lamb Sh4, which received oocysts twice, 3 days apart.
The ability of Hammondia oocysts to sporulate after freezing was evaluated in a small experiment using fecal samples derived from foxes in Exp. F2. The samples were either kept for 3 weeks in the fridge at 4°C; for 1 week in the fridge and 2 weeks in a freezer at −20°C; or for 1 week in the fridge and 2 weeks outdoors at varying temperatures below zero. All samples were then suspended in 2·5% potassium dichromate in Petri dishes at room temperature and examined daily for sporulation of oocysts.
Euthanasia of experimental animals
Lambs, goats kids, cats and rabbits were euthanized with an overdose of pentobarbital (Mebumal 10%) either intravenously or intraperitoneally. The uninoculated control ewe (Sh3; F5) was slaughtered at an abattoir. Foxes used in Exps. F6 and F12 were killed by electrocution according to standard procedures at the research farm. The other foxes, as well as the raccoon dogs, minks and dogs, were only monitored for oocyst shedding, and were not killed.
Preparation and administration of muscle tissue inocula
Muscle tissues from the entire carcass (limbs, trunk, diaphragm and heart) of experimentally inoculated animals were removed, mixed well, cut into smaller pieces and ground. The muscle samples from 12 slaughtered reindeer and 1 slaughtered sheep were treated likewise, but in Exp. F7, the foxes were fed either skeletal or cardiac muscles from reindeer. In a few experiments, the foxes were fed tissues within 3–4 h after the inoculated animal had been killed; in the other experiments the ground muscle tissues were kept refrigerated overnight or for a few days before being fed to foxes, dogs or cats. In the initial experiments using reindeer muscles, some portions were kept frozen at −20°C for 5 days (Exp. F1) or 2 days (Exp. F3) before being thawed and fed to foxes and dogs. About 300–500 g of muscle tissue were usually fed to each fox, raccoon dog or adult dog. Smaller portions were given to cats, minks and dog puppies. The animals were usually monitored for a short while after feeding to ascertain that they ingested most of the muscle tissues provided.
Examination of fecal samples for oocysts
The duration of the fecal examination of all foxes, dogs, raccoon dogs, minks and cats are given in Table 2. The extent of fecal examination varied somewhat between experiments for capacity reasons, but samples from days when the animals were expected to shed maximum numbers of oocysts, if infected, were always examined. Fecal samples were processed and examined for oocysts and sporocysts by microscopy at 200× magnification using a standard flotation technique with ZnCl2/NaCl-solution (specific gravity 1·3) as flotation fluid.
The size of unsporulated and sporulated oocysts and sporocysts was measured with a calibrated ocular screw micrometer. In order to determine whether oocyst size would change during patency, a fairly large number of oocysts from particular foxes were examined daily in some experiments. Thus, from Fox 31 in Exp. F7, 10–60 oocysts were measured from each of days 7–12 post-infection, as well as 20–40 oocysts from Fox 32 from each of days 8–12. Similarly, from Fox 35 in Exp. F8, 30 oocysts from each of days 5–8 post-infection were measured.
Black and white microphotographs of oocysts were recorded on film in connection with some of the experiments in 1981–1984, whereas digital colour photographs were taken in 2009 with a Leica DC480 digital camera of oocysts that had been kept refrigerated for up to 27 years.
Hammondia triffittae oocysts obtained from foxes fed muscle tissues from moose
In October 2007, 4 silver foxes and 4 blue foxes were fed Sarcocystis-infected muscle tissues from moose to determine whether they would act as definitive hosts for some of the Sarcocystis species of this intermediate host. All 8 foxes subsequently harboured or shed Hammondia oocysts and Sarcocystis oocysts/sporocysts as described previously (Exp. 1; Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010). The Hammondia oocysts examined by molecular methods in the present study were collected from the intestinal contents and feces of a silver fox and a blue fox killed 7 days after being fed moose tissues. In both foxes, numerous unsporulated, subspherical Hammondia oocysts were observed in the mucosa along the entire posterior half of the small intestine. The oocysts were set to sporulate in 2·5% potassium dichromate at room temperature, and subsequently kept refrigerated for about a year until concentrated by sucrose flotation for molecular studies. Sporulated oocysts were measured with a calibrated ocular micrometer, and colour photographs of oocysts were recorded on film in 2007 and with a digital camera in 2010. Sporulated oocysts, when examined early in 2010, measured 11·3–13·8× 10·8–12·8 μm (12·6±0·5×11·9±0·4), and their average length to width ratio was 1·06±0·03 (1·00–1·13); n=30.
Hammondia heydorni oocysts obtained from a dog
In August 2000, a veterinary clinic in Eastern Norway submitted a fecal sample from a 5-month-old female Rottweiler dog to the Parasitology lab at NVH for standard coprological examination for parasites, since the dog had suffered from gastrointestinal symptoms, including haemorrhagic diarrhoea, for about 4 weeks. The examination at the lab revealed numerous eggs of the hookworm Uncinaria stenocephala, a moderate number of Cryptosporidium oocysts, as well as a fairly large number of nearly spherical coccidian oocysts, measuring 11–13 μm in diameter (Fig. 1G), consistent with those of H. heydorni and N. caninum of dogs. By request, fecal samples from 4 more days were submitted, 2 of which still contained a considerable number of Hammondia/Neospora-type oocysts. Fecal samples containing oocysts were suspended in 2·5% potassium dichromate, set to sporulate at room temperature, and sporulated oocysts were subsequently kept refrigerated for more than 8 years until used in the present investigation. Sporulated oocysts then measured 11·0–13·3×10·3–12·5 μm (12·2±0·7×11·3± 0·5 μm), and their average length to width ratio was 1·08±(1·00–1·21) (n=30). Photographs of fresh oocysts were recorded on film in 2000, and additional photographs of stored oocysts were taken with a Leica DC480 digital camera in 2009.

Fig. 1. Unsporulated and sporulated oocysts of Hammondia triffittae from foxes (A–F) and Hammondia heydorni from a dog (G–H). (A) Two unsporulated oocysts, presumably of H. triffittae (isolate not examined by molecular methods) from a free-ranging red fox killed in 2002. (B) Two unsporulated oocysts of H. triffittae from foxes fed reindeer meat in Exp. F2 in 1981. (C) A sporulated oocyst of the isolate shown in (B) after 27 years in a fridge. (D) A sporulated oocyst of H. triffittae from foxes fed muscles from an experimentally infected lamb in Exp. F5 in 1982 and stored for nearly 27 years in a fridge. (E–F) An unsporulated and 2 sporulated oocysts of H. triffittae from foxes fed moose musculature in 2007. (G–H) An unsporulated and a sporulated oocyst of H. heydorni from a naturally infected dog diagnosed in 2000; photo of sporulated oocyst after 9 years in a fridge. All photos shown at approximately the same magnification. Scale bar=10 μm for all photos.
Molecular analysis of 4 oocyst isolates: DNA extraction, PCR conditions and sequencing
Oocyst isolates
Hammondia-type oocysts from 4 isolates were genetically characterized by PCR and sequence analysis: (1) oocysts (Fig. 1C) obtained from foxes (Vulpes vulpes and V. lagopus; Vulpes) fed meat from naturally infected reindeer (Rangifer tarandus; Rt) in Exps F2 and F3 in 1981 (isolate HtRtVulpes-1981); (2) oocysts (Fig. 1D) obtained from foxes fed meat from an experimentally inoculated lamb (Ovis aries; Oa) in Exp. F5 in 1982 (isolate HtOaVulpes-1982); (3) oocysts (Fig. 1F) obtained from foxes fed meat from naturally infected moose (Alces alces, Aa) in 2007 (isolate HtAaVulpes-2007); and (4) oocysts (Fig. 1H) isolated in year 2000 from a naturally infected dog, source of infection unknown (isolate HhCanis-2000).
Fecal suspensions of partly to fully sporulated oocysts of the above-mentioned isolates had been stored refrigerated in 2·5% potassium dichromate for 1 to 27 years. Before attempted DNA isolation, oocysts were washed by repeated centrifugation in tap water and then concentrated by flotation in saturated sucrose solution. Oocysts were either harvested directly from the top layer after flotation, or from the sediment following dilution and washing of the floated oocysts by repeated centrifugation in tap water. Isolated oocysts were kept frozen in microcentrifuge tubes at –20°C for a few weeks before DNA extraction.
DNA extraction
The oocyst samples were frozen and thawed either once or thrice. After thawing, DNA was either extracted directly from the oocysts, or after incubation of a mixture of 100 μl of the oocyst suspension and 150 μl of Tris-EDTA buffer at 100 °C for 60 min. In both cases, genomic DNA was extracted from the oocysts using QIAamp® DNA Mini Kit (Qiagen GmbH, Germany) according to the manufacturer's instructions.
Genetic markers and PCR conditions
Six genomic DNA regions were PCR-amplified as detailed below. In addition, genomic DNA extracted from isolate HtAaVulpes-2007 was tested with the N. caninum-specific primer pair Np21/Np6 as described previously (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010).
Ssu rRNA gene
The complete ssu rRNA gene was amplified in 3 overlapping fragments, using primer pairs ERIB1/3Hr, S5f/S4r and S3f/Primer B, respectively, as previously described (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2007).
ITS-1
The complete ITS-1 region was amplified using primer pair JB1/JB2 (Barratt et al. 2007). Cycling conditions were: initial Hot Start at 95°C for 15 min, followed by 35 cycles of 94°C for 40 s, 57°C for 45 s, 72°C for 1 min; and final extension at 72°C for 10 min.
ITS-2
The complete ITS-2 region was amplified using primers Tim11F (Payne and Ellis, Reference Payne and Ellis1996) and T13Rmod (5′-CTACAAGTCAACAGCTCA-3′). Cycling conditions were: initial Hot Start at 95°C for 15 min, followed by 35 cycles of 94°C for 40 s, 55°C for 45 s, 72°C for 1 min; and final extension at 72°C for 10 min.
Lsu rRNA gene
The D2/D3 domain of the lsu rRNA gene was amplified using primer pair CR1/CR2 (Ellis et al. Reference Ellis, Morrison, Liddell, Jenkins, Mohammed, Ryce and Dubey1999). Cycling conditions were: initial Hot Start at 95°C for 15 min, followed by 35 cycles of 94°C for 40 s, 55°C for 45 s, 72°C for 1 min; and final extension at 72°C for 10 min.
Alpha tubulin gene
A small fragment (intron 1 region) of the alpha tubulin gene was amplified using primer pair AT264/AT9 (Abel et al. Reference Abel, Schares, Orzeszko, Gasser and Ellis2006). Cycling conditions were: initial Hot Start at 95°C for 15 min, followed by 35 cycles of 94°C for 40 s, 56°C for 45 s, 72°C for 1 min; and final extension at 72°C for 10 min. Since different Sarcocystis species might occur concurrently with Hammondia in foxes, DNA from Sarcocystis alces and Sarcocystis hjorti was included as controls to test whether Sarcocystis DNA was also amplified. DNA amplification was also attempted with primer pair HhGF/HhGR (Abel et al. Reference Abel, Schares, Orzeszko, Gasser and Ellis2006), using several different annealing temperatures. Cycling conditions were: initial Hot Start at 95°C for 15 min, followed by 35 cycles of 94°C for 40 s, 54–60°C for 40 s, 72°C for 45 s; and final extension at 72°C for 10 min. Genomic DNA from isolates HtOaVulpes-1982 and HtAaVulpes-2007 and purified PCR-products from the amplification of isolate HtRtVulpes-1981 with primers AT264/AT9, were used as templates. Since no PCR products could be obtained with this primer pair, amplification with primer pair AT264/HhGR was attempted instead, using the same cycling conditions as for primer pair AT264/AT9. Genomic DNA from isolates HtOaVulpes-1982, HtAaVulpes-2007 and HhCanis-2000, as well as PCR products from the amplification of these isolates with primer pair AT264/AT9, were used as templates for primer pair AT264/HhGR.
HSP70 gene
A portion of the 70 kDa heat shock protein (HSP70) gene was amplified using primer pair HSPF/HSPR (Monteiro et al. Reference Monteiro, Richtzenhain, Pena, Souza, Funada, Gennari, Dubey, Sreekumar, Keid and Soares2007). Cycling conditions were: initial Hot Start at 95°C for 15 min, followed by 35 cycles of 94°C for 40 s, 56°C for 50 s, 72°C for 90 s; and final extension at 72°C for 10 min.
Each PCR reaction mixture contained 3 μl aliquots of the DNA solution, 25 μl of HotStarTaqMaster Mix (Qiagen GmbH, Germany), 20 pmol of each primer, 8 μg bovine serum albumin, and RNase-free water to make a final volume of 50 μl. A negative control was included in each PCR run. All PCR reactions were performed in an iCycler Thermal Cycler (Bio-Rad, USA).
DNA sequence analysis and GenBank submission
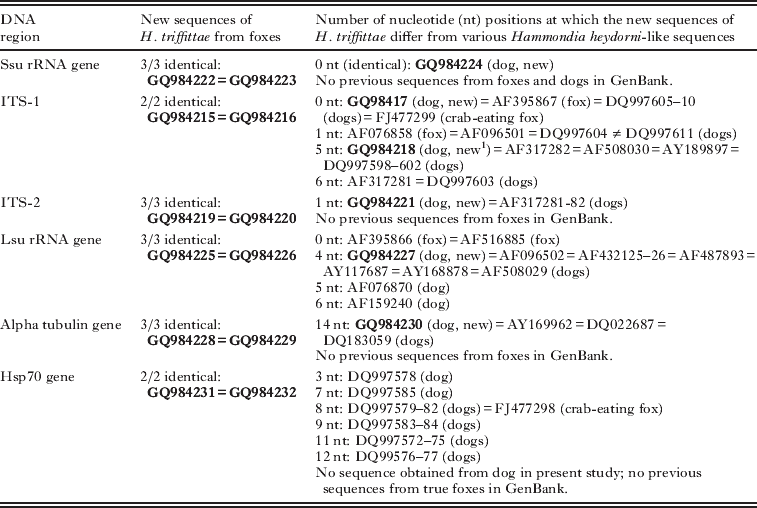
The PCR products were visualized and purified as previously described (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2007). Quantity and purity of the PCR products were evaluated on a NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, USA). The amplified DNA fragments were sent to Eurofins MWG Operon, Germany, for sequencing on both strands, using the same forward and reverse primers as for the PCR. The application Contig Express in Vector NTI® Advance 10 software (Invitrogen) was used to assemble sequences of each DNA region. The non-redundant nucleotide sequence database, maintained by the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/), was searched for sequences similar to those obtained in this study, using the program BlastN. New DNA sequences from all loci were submitted to GenBank and have been issued Accession numbers GQ984215–GQ984232 (Table 3).
Table 3. Comparison of new sequences (in bold) obtained from Hammondia triffittae oocysts from foxes and Hammondia heydorni oocysts from a dog in the present study with other Hammondia heydorni-like sequences available in GenBank (referred to by Accession numbers)
1 Two different ITS-1 sequences were obtained from 2 subsamples of oocysts from the same dog.
RESULTS
Infection experiments with reindeer-fox isolates of Hammondia triffittae
Detailed results of the infection experiments are presented in Table 2. Below, only a brief summary of the major findings is given, as well as oocyst measurements. The results of Exps F1–F3, and the major microscopic findings in foxes nos. 39–44 in Exp. F16, have been briefly published before by Gjerde (Reference Gjerde1983) and Gjerde and Bjerkås (Reference Gjerde and Bjerkås1987), respectively, but have also been included here for the sake of completeness.
Foxes
Thirty foxes (21 blue foxes, 8 silver foxes, and 1 silver and blue fox hybrid) shed typical unsporulated Hammondia oocysts in their feces. In addition, both blue foxes killed 5 days after infection with goat muscles in Exp. F12, had such oocysts in their colon contents. Foxes shed Hammondia oocysts after being fed fresh muscle tissues of either naturally infected reindeer (Exps F1–F3, F7); or experimentally infected lambs (Exps F4, F5, F14), goat kids (Exps F8, F9, F11, F12), blue foxes (Exp. F6) and a rabbit (Exp. F15). In addition, foxes fed tissues of an uninoculated ewe (Exp. F5) and 2 uninoculated goat kids (Exps F10, F12), that had been housed together with inoculated animals, also shed Hammondia-type oocysts. The muscle tissues of inoculated intermediate hosts were infective for foxes at least from 16 days post-infection (p.i.) onwards (Goat 1 in Exp. F8). The single fox fed pooled tissues from 3 inoculated cats (Exp. F13), and a fox fed tissues from 1 of 2 inoculated rabbits (Exp. F16), did not shed any oocysts. Neither did the 2 foxes inoculated with oocysts in Exp. F6. None of the animals inoculated with Hammondia oocysts showed any clinical signs related to the infections; neither did any of the foxes that were fed infectious muscle tissues and subsequently shed oocysts.
Nine of 10 foxes fed fresh muscle tissues of reindeer in Exps F1–F3 and F7, and 1 fox fed tissues of the 3-year-old ewe in Exp. F5, shed Sarcocystis sporocysts in addition to Hammondia oocysts. In contrast to the foxes fed fresh reindeer meat in Exps F1 and F3, none of the 4 foxes fed muscle tissues that had been kept frozen at –20°C for 2 days or 5 days shed any Hammondia oocysts or Sarcocystis sporocysts.
The pre-patent period for shedding of Hammondia oocysts varied between 3 and 7 days. Most foxes shed oocysts in detectable numbers for about 1 week, but some animals shed oocysts for up to 3 weeks. In most foxes, the onset of oocyst shedding was abrupt, with one or a few days of massive oocyst shedding early in patency, followed by several days with fewer oocysts, or intermittent shedding of oocysts (Table 2).
Unsporulated oocysts were spherical to subspherical and had a colourless wall, about 1 μm thick, with no micropyle. The oocysts measured 10·3–15·2×9·3–12·8 μm (12·5±0·7×10·9±0·5 μm), and their average length to width ratio was 1·15±0·07 (1·00–1·49); n=900. Only 5 of 900 oocysts were measured to be perfectly spherical. There was no significant variation in the average size of oocysts shed by individual foxes on different days during early patency (5–12 days p.i.).
The Hammondia oocysts generally sporulated within 3 days at room temperature, even after storage in a fridge for up to 3 weeks before attempted sporulation. However, oocysts that had been kept frozen at –20°C for 2 weeks, or oocysts that had been kept at varying temperatures slightly below zero outdoors for 2 weeks, did not sporulate. Sporulated oocysts were spherical to subspherical and measured 11·9–13·2×10·8–12·3 μm (12·7±0·4×11·4±0·4); n=10. The 2 sporocysts within each oocyst were ovoid, thin-walled, and measured 6·5–9·5×5·2–7·2 μm (8·3±0·9×6·3±0·5 μm); n=40. Each sporocyst contained 4 elongate sporozoites.
Dogs, raccoon dogs, cats, and minks
None of the 12 dogs that were fed fresh muscle tissues of infected intermediate hosts, shed any Hammondia oocysts, but 2 dogs fed fresh muscle tissues of reindeer in Exp. F3, shed Sarcocystis sporocysts. The 2 young dogs given fresh muscle tissue in Exp. F1, probably did not ingest any of the infective feed, since they, in contrast to the foxes in that experiment, did not shed any Sarcocystis sporocysts either. One of these puppies developed severe diarrhoea and was euthanized 16 days p.i.; the post-mortem examination revealed that the diarrhoea most likely was due to parvo virus infection. None of the 2 raccoon dogs, 3 cats or 4 minks that were fed fresh muscle tissues of inoculated intermediate hosts, shed any Hammondia oocysts.
Characteristics of nucleotide sequences obtained in the present study and their similarity to related sequences in GenBank
The number of DNA-sequences obtained from different loci in the present study, the similarity between them, as well as their GenBank Accession numbers, are listed in Table 3. When all 3 sequences obtained from fox-derived oocysts were identical, only 2 sequences were submitted; usually 1 each from the original reindeer-fox isolate (HtRtVulpes-1981) and the moose-fox isolate (HtAaVulpes-2007), since isolate HtOaVulpes-1982 probably was identical with HtRtVulpes-1981 (obtained after passage of the latter through sheep). The similarity of the new sequences with sequences retrieved from GenBank of related taxa, has also been summarized in Table 3. Below some additional data are provided.
Ssu rRNA gene
The complete ssu rRNA gene sequences (1745 bp) obtained from all 3 fox isolates and the dog isolate were identical. These sequences differed at 3 nucleotide positions from N. caninum (GenBank nos. AJ271354, U17345 and U17346), at 4 nucleotide positions from T. gondii (GenBank no. EF472967), and at 4 nucleotide positions from H. hammondi (GenBank no. AF096498). No previous sequences of H. heydorni of dogs were available in GenBank for comparison.
ITS-1
The complete ITS-1 related sequences (474 bp, including 400 bp of ITS-1) obtained from 2 of the fox isolates (HtRtVulpes-1982 and HtAaVvulpes-2007) were identical. No attempt was made to amplify the ITS-1 region of fox isolate HtRtVulpes-1981. As for the dog isolate HhCanis-2000, 2 subsamples had been subjected to DNA isolation, PCR-amplification and sequencing. The sequences obtained from each subsample differed from each other by 5 nucleotide substitutions. The sequences from the 2 fox-derived isolates were identical with another isolate from a fox, but also identical with 1 of the 2 sequences obtained from our dog isolate, as well as with 7 previous H. heydorni-isolates, 6 from dogs and 1 from the crab-eating fox (Table 3). In addition, the 2 fox-derived sequences differed at 1 nucleotide position from another isolate presumably from a fox (GenBank no. AF076858), as well as from 3 sequences of H. heydorni from dogs. The second dog-derived sequence, which differed at 5 positions from the fox-derived sequences, was identical with 8 previously sequenced isolates of H. heydorni from dogs (Table 3).
ITS-2
The complete ITS-2 related sequences (505 bp, including 337 bp of ITS-2) obtained from all 3 fox isolates were identical. No previous fox-derived sequences were available in GenBank for comparison. Our dog-derived sequence was identical with 2 previously sequenced isolates of H. heydorni from dogs, and all these sequences differed by a single nucleotide substitution (T/G) from our 3 fox-derived sequences (Table 3). The fox- and dog-derived sequences had only 88% and 87% identities with ITS-2 sequences of N. caninum (GenBank no. L49389) and T. gondii (e.g., GenBank nos. AJ628253–54), respectively.
Lsu rRNA gene
The partial lsu rRNA gene sequences (614 bp) obtained from all 3 fox isolates were identical. They were also identical with 2 previous sequences from foxes (GenBank nos. AF395866 and AF516885). All of these 5 fox-derived sequences differed at the same 4 nucleotide positions from our dog-derived sequence, as well as from 7 previous sequences of H. heydorni from dogs. This difference comprised an insert of 2 nucleotides (GG/--) and 2 substitutions (T/C, A/C). Two additional dog-derived sequences in GenBank also differed at these 4 positions, as well as at 1 or 2 other positions (Table 3).
Alpha tubulin gene
The partial alpha tubulin gene sequences (278 bp) obtained from all 3 fox isolates were identical. These sequences differed from our dog-derived sequence, as well as from 3 previous GenBank sequences of H. heydorni from dogs, at 14 homologous nucleotide positions, comprising 5 nucleotide substitutions and an insert of 9 nucleotides in the fox isolates (Fig. 2 and Table 3). No previous fox-derived sequences of this locus were available in GenBank for comparison, but our 3 fox-derived sequences were identical to a 40 nt long fragment of 2 fox-derived sequences, including a 9 nt long insert relative to the dog-derived sequences, depicted in Fig. 3 reported as by Abel et al. (Reference Abel, Schares, Orzeszko, Gasser and Ellis2006).

Fig. 2. Nucleotide differences (5 substitutions and an insert) between H. triffittae of foxes and H. heydorni of dogs in the intron 1 region of the alpha tubulin gene. Due to a repetitive sequence there are 9 possible alignments of the H. heydorni sequence at the insert, but only 2 (a1 and a2) are depicted here. When aligned as in a2 it is evident that primer HhGR may also bind fairly well to DNA-sequences of H. heydorni, as demonstrated in this study.
Only a small amount of PCR product was obtained from Hammondia-DNA with primers AT264/AT9 under the PCR-conditions used. No PCR products were obtained with primers AT264/AT9 when genomic DNA from either Sarcocystis alces or S. hjorti was used as template. Primer pair HhGF/HhGR did not amplify DNA from any of the 3 fox-derived Hammondia isolates. Primer pair AT264/HhGR, on the other hand, repeatedly yielded PCR products both when genomic DNA from fox-derived (isolates HtOaVulpes-1982, HtAaVulpes-2007) and dog-derived oocysts (isolate HhCanis-2000) were used, and even more so when PCR-products from amplification of the above-mentioned genomic DNA with primers AT264/AT9 were used as templates in the second round of a semi-nested PCR. This was first suggested by similarly sized products on agarose gel, and sequencing of PCR-products from 1 set of such reactions confirmed that amplicons of both H. triffittae from foxes and of H. heydorni from the dog were generated with primer pair AT264/HhGR.
HSP70 gene
The partial HSP70 gene sequences (951 bp) obtained from the 2 fox isolates HtOaVulpes-1982 and HtAaVulpes-2007 were identical. No PCR-products could be obtained for this gene from fox isolate HtRtVulpes-1981, or from the dog isolate. No previous fox-derived sequences were available in GenBank for comparison, but our fox-derived sequences differed at 3–12 nucleotide positions from 14 previous sequences of H. heydorni from dogs, and at 8 positions from a sequence from crab-eating foxes (Table 3). The 3 nucleotide substitutions separating our fox-derived sequences from the most similar sequence of H. heydorni in dogs (GenBank no. DQ997578), were also consistently present in all other sequences from dogs and crab-eating foxes. The resulting amino acid sequences, on the other hand, from our fox-derived DNA sequences and the most similar sequence of H. heydorni from dogs, were identical.
DISCUSSION
Comparisons of molecular data obtained in the present study from Hammondia oocysts from foxes and a dog respectively, showed that they were genetically different at 4 of 6 DNA loci examined, which suggests that they represent 2 separate, but closely related species, using either foxes or dogs as definitive hosts. Since the dog-derived isolate was genetically consistent with H. heydorni of dog origin, characterized by others previously, it follows that the fox-derived isolates were different from this species. Based on our new data, as well as findings reported by others (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002; Mohammed et al. Reference Mohammed, Davies, Hussein, Daszak and Ellis2003; Abel et al. Reference Abel, Schares, Orzeszko, Gasser and Ellis2006), the Hammondia sp. of foxes has been described as a species distinct from H. heydorni of dogs, and has been assigned the name H. triffittae n. comb. In the following sections, various findings leading to this conclusion will be discussed, and the differences and similarities between H. triffittae of foxes and H. heydorni of dog-like canids will be outlined.
Molecular characteristics of the isolates examined at 6 genetic loci
The ssu rRNA gene has been extensively used to differentiate between various apicomplexans and to infer their phylogenetic relationships (Šlapeta et al. Reference Šlapeta, Modrý, Votýpka, Jirků, Lukeš and Koudela2003; Morrison et al. Reference Morrison, Bornstein, Thebo, Wernery, Kinne and Mattsson2004). However, there are only minor differences between various members of the Toxoplasmatinae (Toxoplasma gondii, Hammondia hammondi, Neospora caninum, Neospora hughesi) at this gene (Jenkins et al. Reference Jenkins, Ellis, Liddell, Ryce, Munday, Morrison and Dubey1999; Marsh et al. Reference Marsh, Barr, Packham and Conrad1998; Morrison et al. Reference Morrison, Bornstein, Thebo, Wernery, Kinne and Mattsson2004), which was further substantiated in this study by the finding of identical sequences for H. heydorni and H. triffittae. Similarly, Marsh et al. (Reference Marsh, Barr, Packham and Conrad1998) found no difference at this gene between N. hughesi and N. caninum, but nevertheless described N. hughesi as a new species different from N. caninum on the basis of a small difference between their ITS-1 sequences, as well as in some morphological features. Subsequent studies have revealed further differences between these 2 species at other DNA loci, and thus supported their initial proposition (Marsh et al. Reference Marsh, Howe, Wang, Barr, Cannon and Conrad1999; Walsh et al. Reference Walsh, Vemulapalli, Sriranganathan, Zajac, Jenkins and Lindsay2001).
It has been estimated that a common ancestor of the domestic dog and the red fox and arctic fox existed approximately 10 million years ago (Graphodatsky et al. Reference Graphodatsky, Perelman, Sokolovskaya, Beklemisheva, Serdukova, Dobigny, O'Brien, Ferguson-Smith and Yang2008). Based on ssu rRNA gene sequence data, Su et al. (Reference Su, Evans, Cole, Kissinger, Ajioka and Sibley2003) have suggested that the divergence of T. gondii from N. caninum and H. hammondi happened about 12 million years ago, and that these taxa diverged on the basis of their choice of definitive hosts. An even more recent split between the dog and fox lineages of Hammondia concurrently with the separation of ancient foxes from dogs, might therefore explain the absence of ssu rRNA gene sequence differences between H. heydorni and H. triffittae.
This study confirmed previous findings that fox-derived ITS-1 sequences might be identical to sequences of H. heydorni from dogs (Ellis et al. Reference Ellis, Morrison, Liddell, Jenkins, Mohammed, Ryce and Dubey1999; Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002; Monteiro et al. Reference Monteiro, Richtzenhain, Pena, Souza, Funada, Gennari, Dubey, Sreekumar, Keid and Soares2007) and crab-eating foxes (Soares et al. Reference Soares, Cortez, Gennari, Sercundes, Keid and Pena2009). Hence, H. triffittae and H. heydorni cannot be differentiated on the basis of this region of the genome. Moreover, the H. heydorni-specific primer JS5 designed by Šlapeta et al. (Reference Šlapeta, Koudela, Votýpka, Modrý, Hořejš and Lukeš2002a) also matches the fox-derived ITS-1 sequences and will therefore presumably also yield a PCR-product for H. triffittae.
Two variants of the ITS-1 sequence were obtained from the H. heydorni oocysts collected within a few days from the single dog in this study. In contrast, Monteiro et al. (Reference Monteiro, Richtzenhain, Pena, Souza, Funada, Gennari, Dubey, Sreekumar, Keid and Soares2007) examined DNA from H. heydorni oocysts derived from 12 dogs at both the ITS-1 and HSP70 gene locus, but found no evidence of mixed infections with multiple variants in individual dogs. Neither have other studies examining a single or a few dog-derived isolates reported such variation within individual isolates (e.g., Ellis et al. Reference Ellis, Morrison, Liddell, Jenkins, Mohammed, Ryce and Dubey1999; Abel et al. 2006). It remains to be determined whether such variation in oocyst-derived DNA is due to ingestion of intermediate host tissues infected with 2 separate lineages of H. heydorni, or whether individual zoites may harbour different ITS-1variants in different copies of their rRNA genes. The absence of previous reports about such variation in individual oocyst isolates from either foxes or dogs, favours the first explanation.
In this study, the ITS-2 region of a Hammondia sp. from foxes was sequenced for the first time, which revealed a difference at this locus of 1 nucleotide substitution compared with H. heydorni from dogs. However, this difference is so small that the ITS-2 region might not be a good marker for differentiating between H. triffittae and H. heydorni. The D2/D3 domain of the lsu rRNA gene, on the other hand, seems to constitute a more well-suited genetic marker for this purpose, as our results confirmed previous observations (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002; Mohammed et al. Reference Mohammed, Davies, Hussein, Daszak and Ellis2003) that fox- and dog-derived Hammondia isolates differ at 4 particular nucleotide positions in this region of the lsu rRNA gene.
The intron 1 region of the alpha tubulin gene also seems to be a good genetic marker for distinguishing between H. triffittae and H. heydorni. Thus, our fox-derived Hammondia isolates differed at 14 nucleotide positions from all known dog-derived sequences of this gene (present study; Siverajah et al. Reference Siverajah, Ryce, Morrison and Ellis2003; Abel et al. Reference Abel, Schares, Orzeszko, Gasser and Ellis2006). There were, however, no fox-derived sequences of this gene fragment in GenBank available for comparison, even though Abel et al. (Reference Abel, Schares, Orzeszko, Gasser and Ellis2006) apparently sequenced 2 Hammondia-isolates from foxes along with 2 H. heydorni isolates from dogs. However, in Fig. 3 of their paper (Abel et al. Reference Abel, Schares, Orzeszko, Gasser and Ellis2006), short fragments of both fox- and dog-derived sequences are displayed, but these sequences are actually complementary and reverse to the customary sense strands shown in nucleotide sequence databases. When this is accounted for, it is evident that the 3 fox-derived sequences from our study are identical with their 2 fox-derived sequences in the 40-nt-long region shown, including an insert of 9 nt relative to the dog-derived sequences. Thus, our study confirms the presence of this particular difference between Hammondia from foxes and dogs. However, we found an additional difference between fox- and dog-derived Hammondia-isolates in this region of the alpha tubulin gene, comprising 5 nucleotide substitutions, which was not mentioned by Abel et al. (Reference Abel, Schares, Orzeszko, Gasser and Ellis2006), either because they were absent from their fox isolates, not recognized, or their existence simply not recorded in their paper. Anyway, the 9-nt-long insert/deletion by itself seems to be sufficient to distinguish between H. triffittae from foxes and H. heydorni from dogs, and the intron 1 region of the alpha tubulin gene therefore seems to constitute a well-suited marker for discrimination between these 2 species.
The small amount of PCR product obtained with primers AT264/AT9 was probably due to the fact that there are only a few copies of the alpha tubulin gene in lower eukaryotes (Siverajah et al. 2003). Primer pair HhGF/HhGR, which was designed by Abel et al. (Reference Abel, Schares, Orzeszko, Gasser and Ellis2006) to specifically amplify a short sequence of the alpha tubulin gene of Hammondia from foxes only, since the reverse primer HhGR allegedly spanned the region of the above-mentioned insert, did not produce any PCR products for any of the fox isolates in this study. As the nucleotide sequence of primer HhGF could not be found within any of the sequenced isolates from either foxes or dogs, or within the 1500 bp long alpha tubulin gene sequence of an isolate of H. heydorni available in GenBank (AY169962), it appears that this primer sequence was incorrectly reproduced by Abel et al. (Reference Abel, Schares, Orzeszko, Gasser and Ellis2006). Reverse primer HhGR, on the other hand, worked well in combination with forward primer AT264, both with fox- and dog-derived Hammondia DNA, even though Abel et al. (Reference Abel, Schares, Orzeszko, Gasser and Ellis2006) claimed that primer HhGR was specific for fox-derived Hammondia DNA. However, as can be seen from Fig. 2 in this paper, there is only a minor mismatch between primer HhGR and the H. heydorni sequence at the very 5′-end of the primer, and this mismatch was obviously not sufficient to prevent amplification of H. heydorni-DNA.
The sequence of the HSP70 coding gene also seems to differ between H. triffittae and H. heydorni. Thus, our 2 fox-derived sequences differed at 3–12 nucleotide positions from all previously sequenced isolates of H. heydorni from dogs (Monteiro et al. Reference Monteiro, Richtzenhain, Pena, Souza, Funada, Gennari, Dubey, Sreekumar, Keid and Soares2007) and the crab-eating fox (Soares et al. Reference Soares, Cortez, Gennari, Sercundes, Keid and Pena2009), including a consistent difference at 3 particular nucleotide positions. However, due to the apparent sequence variation of H. heydorni at the HSP70 gene (Soares et al. Reference Soares, Cortez, Gennari, Sercundes, Keid and Pena2009), and the fact that no other Hammondia isolates from foxes so far have been examined at this locus, additional Hammondia isolates from both foxes and dogs should be sequenced to determine whether H. heydorni and H. triffittae are consistently different at this gene, and thus can be unambiguously identified on the basis of this locus.
In summary, the genetic differences that seem to be consistently present between fox- and dog-derived Hammondia isolates at the lsu rRNA gene and the alpha tubulin gene, and apparently also at the ITS-2 locus and the HSP70 coding gene, strongly suggest that these lineages should be regarded as separate species.
Biological characteristics and differences
Definitive hosts
The series of infection experiments reported here for the first time (Exps F4–F16), as well as the recently reported infection of foxes with tissues from moose (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010), confirmed the initial observations by Gjerde (Reference Gjerde1983) that the red fox (Vulpes vulpes) and the arctic fox (Vulpes (=Alopex) lagopus) are equally well suited as definitive hosts for H. triffittae. There seem to be no differences between these 2 fox species as regards susceptibility to infection, pattern and timing of oocyst shedding, or the size and shape of the oocysts shed. Similarly, the other Hammondia isolates that seem to belong to H. triffittae on the basis of their identical lsu rRNA gene and partial alpha tubulin gene sequences, have been found in different subspecies of the red fox (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002; Mohammed et al. Reference Mohammed, Davies, Hussein, Daszak and Ellis2003).
In this study, a total of 12 dogs were fed fresh muscle tissues of intermediate hosts (reindeer, sheep, goats) that contained infectious stages of H. triffittae as evidenced by the subsequent shedding of H. triffittae oocysts by foxes fed concurrently with the dogs. However, the 2 young dogs used in Exp. F1 may not have ingested any muscle tissues, since they, in contrast to the foxes in that experiment, did not shed any Sarcocystis sporocysts. Still, at least 10 dogs ingesting infectious stages of H. triffittae in muscle tissues of intermediate hosts, did not become infected, whereas nearly all foxes fed such tissues became infected and shed oocysts, as did all 8 foxes fed muscle tissues of moose in 2007 (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010). Consequently, the stages of H. triffittae in the muscle tissues of the intermediate hosts seem to be highly infectious for foxes, but non-infectious for dogs. The same seems to be true for the Hammondia sp. of foxes in Germany. Thus, Schares et al. (Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002) could not identify any Hammondia oocysts in the faeces of 5 dogs fed in parallel with the 6 foxes that got infected with Hammondia sp. Similarly, Entzeroth et al. (Reference Entzeroth, Scholtyseck and Greuel1978) fed to both a fox and a dog muscle tissues of naturally infected roe deer, but observed Hammondia-like oocysts in the faeces of the fox only, whereas both animals shed Sarcocystis sporocysts, which also would indicate that this Hammondia sp. was infectious for foxes only.
In addition to the apparent specificity of H. triffittae for foxes as observed in experimental infections, none of the H. heydorni-like oocyst isolates from naturally infected dogs have ever been found to be identical with H. triffittae at the D2/D3 domain of the lsu rRNA gene or at the alpha tubulin gene. Wapenaar et al. (Reference Wapenaar, Jenkins, O'Handley and Barkema2006) found Hammondia-like oocysts in the feces of 2 of 271 free-ranging red foxes in Canada, which were further examined in PCR assays using primers specific for either H. heydorni or N. caninum. Both isolates were negative for H. heydorni, but positive for N. caninum with primers Np21/Np6. In a previous study, primers Np21/Np6 did not yield a PCR product for the HtAaVulpes-2007 isolate of H. triffittae (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010), and thus it would appear that the Canadian foxes indeed had N. caninum oocysts in their feces, but these oocysts might have been ingested with the feed, since foxes experimentally infected with tissue stages of N. caninum have not shed any oocysts of this species (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002). The Hammondia-like oocysts detected in the feces of 2 of 271 Norwegian red foxes surveyed in 2001 and 2002 (Table 1; Gjerde, unpublished observations), were unfortunately not kept for molecular analysis, but presumably also belonged to H. triffittae.
The South American crab-eating fox (Cerdocyon thous) has recently been reported to be another definitive host for H. heydorni, since an oocyst isolate from this canid had 100% sequence identity at the ITS-1 region and the HSP70 gene with H. heydorni-isolates from dogs (Soares et al. Reference Soares, Cortez, Gennari, Sercundes, Keid and Pena2009). However, it is important to note that the crab-eating fox, together with the other South American foxes, form a separate clade within the family Canidae, and that this clade is more distantly related to the clade of red fox-like canids (‘true foxes’), which includes the red fox and arctic fox, than to the clade of wolf-like canids, which includes the grey wolf, the dog and the coyote (Bardeleben et al. Reference Bardeleben, Moore and Wayne2005; Graphodatsky et al. Reference Graphodatsky, Perelman, Sokolovskaya, Beklemisheva, Serdukova, Dobigny, O'Brien, Ferguson-Smith and Yang2008). It has been estimated that the ancestors of the South American canids and the wolf-like canids diverged from the red fox-like canids about 9–10 million years ago, and that the South American canids and wolf-like canids diverged about 6–7·4 million years ago (Graphodatsky et al. Reference Graphodatsky, Perelman, Sokolovskaya, Beklemisheva, Serdukova, Dobigny, O'Brien, Ferguson-Smith and Yang2008). Thus, it is possible that H. triffittae separated from H. heydorni when the red fox-like canids separated from the other canids and has since co-evolved with the true foxes (genera Vulpes and Fennecus). This would imply that South American foxes might be unsuitable as definitive hosts for H. triffittae.
The placement of the raccoon dog within the family Canidae is somewhat uncertain, but recent phylogenies either place it within the clade of red fox-like canids, or as a sister taxon to this clade (Bardeleben et al. Reference Bardeleben, Moore and Wayne2005; Graphodatsky et al. Reference Graphodatsky, Perelman, Sokolovskaya, Beklemisheva, Serdukova, Dobigny, O'Brien, Ferguson-Smith and Yang2008). This would suggest that the raccoon dog might still be a possible definitive host of H. triffittae in spite of the seemingly negative outcome of the infection experiment (Exp. F14), which might have been due to examination of too few fecal samples (from day 6 p.i. only).
Intermediate hosts
Four separate experiments (Exps F1–F3, F7), of which the first 3 were described by Gjerde (Reference Gjerde1983), established that reindeer were natural intermediate hosts for H. triffittae. Similarly, the experiment in 2007 proved that moose was another natural intermediate host for this species in Norway (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010). Exps F4–F6 and F8–F15 further established that sheep, goats, blue (arctic) foxes and a rabbit, but not cats, were experimental intermediate hosts for H. triffittae. If we assume that the Hammondia-like oocysts shed by foxes fed muscle tissues of various hosts in other studies, also belong to H. triffittae, then the guinea pig is another experimental intermediate host (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002), while sheep (Ashford, Reference Ashford1977; Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002), goats (Schares et al. Reference Schares, Heydorn, Cüppers, Mehlhorn, Geue, Peters and Conraths2002), roe deer (Entzeroth et al. Reference Entzeroth, Scholtyseck and Greuel1978), the Arabian mountain gazelle (Mohammed et al. Reference Mohammed, Davies, Hussein, Daszak and Ellis2003), and possibly also horses (Lee, Reference Lee1934) may act as additional natural intermediate hosts for this species. As for horses, the foxes and dogs used in Lee's (Reference Lee1934) transmission experiments were apparently fed fresh horse meat, and the shedding of I. bigemina-like oocysts by 2 foxes and several dogs inoculated orally with such oocysts in his experiments, was probably the result of ingestion of infective tissue stages contained in the fresh horse meat used as feed, as also suggested by Heydorn (Reference Heydorn1973). The fact that foxes may also act as intermediate hosts for H. triffittae, implies that transmission to foxes may not only occur through predation and scavenging, but also through cannibalism.
The intermediate host range of H. triffittae seems to be quite similar to that of H. heydorni in that both species use various bovids and cervids for this purpose. The fact that fewer animal species have so far been determined to be intermediate hosts for H. triffittae than for H. heydorni, is probably mostly due to the fact that relatively few studies have involved feeding of foxes with fresh muscle tissues of various hosts, compared with the number of such studies conducted with dogs or coyotes. For H. heydorni, cattle (Heydorn, Reference Heydorn1973; Fayer, Reference Fayer1974), sheep and goats (Dubey and Williams, Reference Dubey and Williams1980), water buffalos (Dissanaike and Kan, Reference Dissanaike and Kan1977; Nassar et al. Reference Nassar, Hilali and Rommel1983), camels (Nassar et al. Reference Nassar, Hilali and Rommel1983; Warrag and Hussain, Reference Warrag and Hussein1983), wapiti (Margolin and Jolley, Reference Margolin and Jolley1979), moose (Dubey and Williams, Reference Dubey and Williams1980), and white-tailed deer (Dubey et al. Reference Dubey, Sreekumar, Miska, Hill, Vianna and Lindsay2004) seem to be natural intermediate hosts, of which only sheep, goats and moose so far have been recorded as such for H. triffittae. Similar to foxes as regards H. triffittae, dogs may act as both definitive and intermediate hosts for H. heydorni (Heydorn, Reference Heydorn1973; Dubey and Fayer, Reference Dubey and Fayer1976; Matsui et al. Reference Matsui, Morii, Iijima, Ito, Tsunoda, Correa and Fujino1981), whereas cats do not seem to act either as definitive or intermediate host for any of these species (present study; Heydorn, Reference Heydorn1973). From what is presently known, H. triffittae might differ from H. heydorni in its ability to use rabbits as intermediate hosts. Thus, the single fox fed muscle tissues of an inoculated rabbit in Exp. F15 shed oocysts, whereas none of 5 dogs fed musculature of 3 rabbits inoculated with large numbers of H. heydorni oocysts became infected (Heydorn, Reference Heydorn1973), and neither did a dog fed tissues of a rabbit that had been inoculated with a moderate number of such oocysts (Matsui et al. Reference Matsui, Morii, Iijima, Ito, Tsunoda, Correa and Fujino1981). However, more experiments are necessary to confirm these findings.
Development in the definitive host
The development of H. triffittae in the intestine of foxes as reported by Gjerde and Bjerkås (Reference Gjerde and Bjerkås1987) seems to be quite similar to that reported for H. heydorni in dogs (Heydorn et al. Reference Heydorn, Gestrich and Ipczynski1975; Dubey and Fayer, Reference Dubey and Fayer1976). Thus, using light and transmission electron microscopy, Gjerde and Bjerkås (Reference Gjerde and Bjerkås1987) examined the intestinal mucosa of 6 foxes killed 1–5 and 9 days after being fed tissues of an experimentally infected goat (foxes 39–44 and goat Go4 in Exp. F12 in Table 2 in present paper). In the fox killed 5 days p.i., developmental stages were very numerous, and occurred throughout the small intestine, except the duodenum. Schizonts were the predominant stage in the jejunum, while gamonts were numerous in the ileum. All developmental stages in all foxes were almost exclusively localized at the distal end of the villi, mostly at their tips. However, in spite of the large number of developmental stages present, there was only minor damage to the intestinal mucosa (Gjerde and Bjerkås, Reference Gjerde and Bjerkås1987). The development of oocysts in the posterior portion of the small intestine only, was confirmed in the 2 Hammondia-infected foxes killed 7 days after being fed tissues of naturally infected moose (Dahlgren and Gjerde, Reference Dahlgren and Gjerde2010). In that study Hammondia oocysts were only seen in mucosal scrapings from the posterior half of the small intestine, whereas Sarcocystis oocysts occurred throughout the entire small intestine. Moreover, the pre-patent and patent period, pattern of oocyst shedding, and oocyst morphology are also closely similar between H. triffittae and H. heydorni. Hence, these species cannot be distinguished on the basis of oocyst morphology, and molecular methods using appropriate genetic markers are necessary to accomplish this, as demonstrated in this study.
Development in the intermediate host
So far, there is only scant knowledge about the development of H. triffittae in its intermediate hosts, and about the nature of its infectious tissue stage. However, many haematoxylin and eosin-stained histological sections were examined from various muscles of the 2 lambs (Sh1 and Sh2) inoculated with oocysts and fed to foxes in Exps. F4 and F5. Only 7 cyst-like structures were found within striated muscle cells, each measuring 10–20 μm in greatest diameter and containing a small number of zoites (Gjerde, unpublished observations). Whether these ‘cysts’ had been derived from the inoculation of the lambs with oocysts of H. triffittae, or from other infections, e.g., T. gondii, could not be conclusively determined. Nevertheless, these small cysts are consistent with ‘cysts’ of Hammondia sp. of foxes observed in tissue culture by Schares et al. (Reference Schares, Meyer, Bärwald, Conraths, Riebe, Bohne, Rohn and Peters2003). Similarly, H. heydorni of dogs also seems to form very small tissue cysts. Thus, Matsui (Reference Matsui1991) found only 2 small cysts, about 10–12 μm in diameter, in the brain of 2 guinea pigs killed 77 and 189 days, respectively, after infection with a high number of oocysts from dogs.
In the present study, infectious stages of H. triffittae seemed to be present in the muscle tissues of intermediate hosts at least by 16–20 days p.i., since the ingestion of such tissues by foxes resulted in oocyst shedding. This is in agreement with the in vitro development of Hammondia sp. of foxes reported by Schares et al. (Reference Schares, Meyer, Bärwald, Conraths, Riebe, Bohne, Rohn and Peters2003), who found that all parasitophorous vacuoles present in culture cells 19 days p.i. seemed to represent cyst stages. Similarly, for H. heydorni from dogs, Matsui et al. (Reference Matsui, Morii, Iijima, Ito, Tsunoda, Correa and Fujino1981) found that tissues of inoculated guinea pigs and dogs had become infectious for dogs by 20 days p.i., but not by 10 days p.i., whereas Matsui (Reference Matsui1991) found that tissues of infected guinea pigs were infectious for dogs 21 days p.i. and later, but not on days 5, 9 or 14 after oocyst inoculation. Thus, H. triffittae and H. heydorni seem to develop in a closely similar manner in their intermediate hosts.
Relationship between H. triffittae and previous Hammondia-like oocyst isolates from foxes
It is reasonable to believe that the Isospora bigemina- or Hammondia-like oocysts found in foxes in various other studies summarized in Table 1, also belong to H. triffittae, although indistinguishable oocysts of other species might occasionally occur as spurious parasites, as suggested by the possible finding of N. caninum oocysts in 2 foxes by Wapenaar et al. (Reference Wapenaar, Jenkins, O'Handley and Barkema2006). In particular, the size and shape of the oocysts originally designated Isospora triffitti by Nukerbaeva and Svanbaev (Reference Nukerbaeva and Svanbaev1973) are fully consistent with the oocysts of H. triffittae as described in the present paper. The same is true for the oocysts found in foxes in Belarus and designated I. triffitti by Gerasimchik (Reference Gerasimchik2005). Moreover, no studies have so far indicated that foxes may be the natural definitive hosts of a valid Isospora species with oocysts indistinguishable from those of H. triffittae; all true Isospora species of foxes having distinctly larger oocysts (Nukerbaeva and Svanbaev, Reference Nukerbaeva and Svanbaev1973; Golemanski and Ridzhakov, Reference Golemanski and Ridzhakov1975).
No molecular data accompanied the original description of Isospora triffitti by Nukerbaeva and Svanbaev (Reference Nukerbaeva and Svanbaev1973), and those oocysts are probably no longer available for molecular studies. Similarly, no molecular data were presented by Gerasimchik (Reference Gerasimchik2005, Reference Gerasimchik2006) when the same species name was applied to some of the oocysts identified in foxes in Belarus. Hence, the exact relationship of these oocysts to the presently examined Hammondia sp. of foxes will probably never be revealed. However, in order to avoid any further confusion, the amended species designation (Levine, Reference Levine1985) has been retained for the Hammondia sp. of foxes described in the present paper, but the former species I. triffitti/I. triffittae has been transferred to the genus Hammondia (family: Sarcocystidae) because of its heteroxenous life cycle and close relationship to Hammondia heydorni of dogs.
TAXONOMIC SUMMARY
Name: Hammondia triffittae (syn. Isospora triffitti, I. triffittae)
Description: Oocysts (Figs 1 B–F) spherical to subspherical, with wall about 1 μm thick, colourless; micropyle and oocyst residuum absent; unsporulated oocysts 12·5±0·7×10·9±0·5 μm (10·3–15·2×9·3–12·8 μm, n=900), with L:W ratio 1·15±0·07 (1·00–1·49, n=900); sporulated oocysts 12·7±0·4×11·4± 0·4 μm (11·9–13·2×10·8–12·3 μm, n=10); two sporocysts, each with 4 sporozoites; sporocysts ovoid 8·3±0·9×6·3±0·5 μm (6·5–9·5×5·2–7·2 μm, n=40); sporozoites elongate.
Type definitive hosts: The red fox (Vulpes vulpes) and the arctic fox (Vulpes lagopus). Life cycle obligatory heteroxenous.
Type intermediate hosts: Reindeer (Rangifer tarandus) and moose (Alces alces).
Other intermediate hosts: Sheep, goats, rabbits and arctic foxes (experimental infections).
Type locality: Norway.
Site of infection in definitive hosts: Schizogony mainly in jejunum; gamogony mainly in posterior jejunum and ileum. Development mainly at tips of intestinal villi (Gjerde and Bjerkås, Reference Gjerde and Bjerkås1987).
Pre-patent period: 3–7 days, usually 6–7 days.
Patent period: About 3 weeks; high oocyst excretion usually only during first week of patency.
Sporulation time: About 3 days at room temperature (22–24°C).
Site of infection in intermediate hosts: Not conclusively determined, but probably very small, thin-walled cysts (10–20 μm in diameter) formed in striated muscle cells; each cyst containing only a few zoites.
Material deposited: Oocysts and phototypes of oocysts of H. triffittae from foxes of isolates HtRtVulpes-1981 (reindeer/fox) and HtAaVulpes-2007 (moose/fox), as well as oocysts of H. heydorni from a dog (isolate HhCanis-2000) have been deposited at the National History Museum, Oslo, Norway, and have been issued collection numbers NHMO-Prot00012, NHMO-Prot00013 and NHMO-Prot00014, respectively. Nucleotide sequences of H. triffittae from 6 genetic markers have been submitted to GenBank, and have been issued Accession numbers GQ984215–16 (ITS-1), GQ98422–23 (ssu rRNA gene), GQ984219–20 (ITS-2), GQ944225–26 (lsu rRNA gene), GQ944228–29 (alpha tubulin gene), and GQ944231–32 (HSP70 gene).
Etymology: Nukerbaeva and Svanbaev (Reference Nukerbaeva and Svanbaev1973) named an Isospora species of foxes Isospora triffitti in recognition of parasitologist Marjorie J. Triffitt, who first described similar oocysts from foxes. Levine (Reference Levine1985) amended this name to I. triffittae, since M. J. Triffitt was a woman.
Remarks: The oocysts of Hammondia triffittae in foxes are morphologically indistinguishable from oocysts of Hammondia heydorni in dogs and other wolf-like canids, and can only be unambiguously differentiated from the latter by molecular methods using the appropriate genetic markers. However, true foxes do not seem to act as definitive hosts for H. heydorni, and neither do dogs for H. triffittae.
ACKNOWLEDGEMENTS
The first author (Bjørn Gjerde) conducted the initial series of trials in 1981–1984, and isolated the H. heydorni oocysts from a dog in 2000; both authors participated in the isolation of H. triffittae from foxes fed moose tissues in 2007; whereas the second author (Stina S. Dahlgren) performed the major part of the molecular characterization of all oocyst isolates. The authors would like to thank all those who assisted in the care, handling and sampling of experimental animals at different facilities in 1981–1984 and 2007. Special thanks are due from the first author to Professor Emeritus Oddvar Helle for his encouragement and support during the initial studies of Hammondia of foxes in 1981–1984.
FINANCIAL SUPPORT
The major part of this study was funded by internal money from the Parasitology Laboratory, NVH. The molecular characterization of the Hammondia-isolates was supported by a small grant from the Norwegian Research Council.