Introduction
The increasing occurrence of herbicide-resistant weeds threatens global grain production. Growers who have herbicide-resistant weeds or want to prevent their selection and/or spread now spray multiple herbicides sequentially or in a tank-mix in the same field during the same season. Previously, they may have only applied a single mechanism of action by using a single herbicide such as glyphosate. As a result, herbicide use has increased (Green Reference Green2014), and weed biotypes with resistance to multiple herbicide mechanisms of action (up to six in Amaranthus species) have been selected for (Mortensen et al. Reference Mortensen, Egan, Maxwell, Ryan and Smith2012) and reported (Heap Reference Heap2020; Strom et al. Reference Strom, Gonzini, Mitsdarfer, Davis, Riechers and Hager2019). Although common ragweed (Ambrosia artemisiifolia L.) biotypes resistant to more than three mechanisms of action have not yet been reported (Heap Reference Heap2020), this species is also at risk of becoming increasingly problematic as herbicide options are narrowed, particularly in broadleaf crops such as soybean [Glycine max (L.) Merr.].
Once established, A. artemisiifolia populations can produce 3,000 to 62,000 seeds per plant that will disperse and persist in the seedbank, possibly for decades (Bassett and Crompton Reference Bassett and Crompton1975). Managing the seed production and dispersal of uncontrolled populations after their initial detection in the field would limit seed inputs. The use of alternative herbicides or other control options (hand weeding, cultivating, clipping) on weeds that have grown during the critical weed free period will not protect crop yield (Knezevic et al. Reference Knezevic, Evans, Blankenship, Van Acker and Lindquist2002) but can play a significant role in reducing seed inputs into the soil seedbank (Bae et al. Reference Bae, Nurse, Simard and Page2017; Ganie et al. Reference Ganie, Kaur, Jha, Kumar and Jhala2018; Rinella et al. Reference Rinella, Haferkamp, Masters, Muscha, Bellows and Vermeire2010). Seeds can also be destroyed during harvest operations if they are still attached to the weeds and collected by the combine (McCanny and Cavers Reference McCanny and Cavers1988; Shirtliffe and Entz Reference Shirtliffe and Entz2005; Walsh et al. Reference Walsh, Broster, Schwartz-Lazaro, Norsworthy, Davis, Tidemann, Beckie, Lyon, Soni, Neve and Bagavathiannan2018). Solutions to destroy seeds in chaff residue have been tested, promoted, and adopted in Australia. These include chaff collection, burning, or milling (Walsh et al. Reference Walsh, Broster, Schwartz-Lazaro, Norsworthy, Davis, Tidemann, Beckie, Lyon, Soni, Neve and Bagavathiannan2018). A chaff milling solution in particular (the Harrington Seed Destructor) can devitalize high percentages of weed seeds in cereals, peas (Pisum sativum L.), and canola (Brassica napus L.) (Tidemann et al. Reference Tidemann, Hall, Harker and Beckie2017; Walsh et al. Reference Walsh, Harrington and Powles2012, Reference Walsh, Newman and Powles2013). The efficacy of the technique, now incorporated into the back of the harvester, was also efficient in rice (Oryza sativa L.) and soybean (Schwartz-Lazaro et al. Reference Schwartz-Lazaro, Norsworthy, Walsh and Bagavathiannan2017). However, in late-season crops like corn [Zea mays L.] and soybean, some weed species have already shattered high percentages of their seeds before mechanical harvesting of the crop (Davis Reference Davis2008; Forcella et al. Reference Forcella, Peterson and Barbour1996).
Ambrosia artemisiifolia is a monoecious short-day plant. The species is wind pollinated and will start to produce male flowers and pollen (causing allergenic rhinitis) in August in eastern Canada (Bassett et al. Reference Bassett, Holmes and Mac-Kay1961; Deen et al. Reference Deen, Hunt and Swanton1998a, Reference Deen, Hunt and Swanton1998b). The species’ pollen production has been the subject of multiple studies, but female flowering and seed production have not been recorded as often or with the same scrutiny. Knowing to what extent seeds are mature and/or shattered before any late weed control operation is implemented to manage seed dispersal of a weed such as A. artemisiifolia is essential. Therefore, this research aims at assessing the seed-shattering phenology of A. artemisiifolia in spring wheat, soybean, and corn. Based on Davis (Reference Davis2008), we hypothesize that most weed seeds will be shattered before mechanical harvest of corn and soybean crops.
A popular method used to evaluate seed dispersal (shattering) is to place seed traps on the soil surface or at different heights around single or multiple plants to provide an area-based assessment of dispersed seeds (Kollmann and Goetze Reference Kollmann and Goetze1998). An alternative method is to use bags installed on inflorescences like those used to isolate flowers from pollinators (Pickering Reference Pickering1982), allowing the collection of the total seed production (including shattered seeds) of individual plants. Pollination bags were initially made of paper but are now available in plastic fabric made with lightweight apertured films (Schaffert et al. Reference Schaffert, Virk and Senior2016). Placed on plants after the initial flowering period, they are easy to install, inexpensive, and give an individual plant–based evaluation of total seed production. However, seed production could be underestimated if invertebrates that feed on seeds or flower parts are accidently trapped inside the bag or if microclimatic conditions inside the bag alter seed formation. Seed production could also be slightly reduced if successful pollination is inhibited, because A. artemisiifolia is mostly self-incompatible (Friedman and Barrett Reference Friedman and Barrett2008). Any female flowers that are receptive after seed shed has started would be left unfertilized. Therefore, we also tested the effect of the pollen bags on A. artemisiifolia seed production and viability as a follow-up experiment.
Materials and Methods
Trials were set up at Agriculture and Agri-Food Canada’s research and development centers located at Saint-Jean-sur-Richelieu (SJR), QC (45.29°N, 73.35°W) (2014 to 2016), and Harrow (HAR), ON (42.03°N, 82.90°W) (2014 and 2015 only). The trials at SJR were located on different fields of the farm, all on a clay loam (29% to 39% sand, 32% to 36% silt, 30% to 35% clay, depending on field location) with a pH of 5.8 to 6.1 and an organic matter content of 3.6% to 4.2%. Trials at HAR were located in the same field on a loamy fine sand (82% sand, 14% silt, 4% clay) and had a pH of 6.7 and an organic matter content of 1.4%. All fields were plowed in autumn and harrowed in the spring before seeding. For the seed-shattering trials, at each location, three adjacent fields were planted with spring wheat, corn, or soybean. Cultivar, seeding dates, and rates are presented in Table 1. All fields were also fertilized according to standard practices. For the seed bag trial, the evaluation was done at SJR in 2017 in one of the fields used for the seed-shattering trial.
Table 1. Crop seeding information and Ambrosia artemisiifolia seeding and emergence dates at Saint-Jean-sur-Richelieu (SJR) and Harrow (HAR).

At HAR, wheat plots were harvested August 19 or 13 in 2014 and 2015, respectively; soybean was harvested October 27 or 26 in 2014 and 2015, respectively; and corn was harvested November 3 or October 27 in 2014 and 2015, respectively. At SJR, wheat plots were harvested August 28 (2014 and 2015) or 25 (2016); soybean was harvested October 9, 19, or 26 in 2014, 2015, and 2016, respectively; and corn was harvested October 27, October 8, or October 17 in 2014, 2015, and 2016, respectively.
Estimation of Ambrosia artemisiifolia Seed Shattering
The experiment was set as a split-plot design with four replicate blocks that was conducted at two locations and was repeated over 2 yr at HAR and 3 yr at SJR.
Each field of wheat, corn, and soybean was divided into four replicate blocks that included two plots (early vs. late emergence) and multiple subplots (collection dates) with a target A. artemisiifolia density of 5 weeds m−2 seeded on the same date as the crop (early emergence) or when the crop had reached the 2-leaf stage (late emergence). Late emergence corresponded to an average of 18.5 (SE 1.46) d after planting (Table 1).
To evaluate shattering over time, the experiment included up to 12 weekly collection dates (subplots). In each subplot, four weeds were individually bagged using DelNet pollination bags (DelStar Technologies, Austin, TX, USA). These bags were placed over the inflorescence after the general flowering period and before A. artemisiifolia seeds started to shatter (mid- to late August). Bags were custom-made to fit the larger plants using large lengths of material sealed along the edges with an impulse sealer (Emballage Carrousel, Boucherville, QC, Canada). Bags were held upright using a long metal stake along the central stem to which smaller plastic stake sections could be added as the plants grew. Weather data as well as crop and weed stages were recorded throughout the growing season. For each weed collection date, the number of shattered and retained seeds per plant was recorded.
Weed seed viability was tested by taking a subsample (25 to 50 seeds when available) using germination tests (of stratified seeds) at an alternating day/night temperature of 25/10 C using a 16-h photoperiod followed by a standard tetrazolium chloride test at 1.0% for 24 h at 30 to 35 C (AOSA/SCST 2010). Growing degree days (GDD; Tbase = 5 C) were accumulated based on local data using the CIPRA software (Plouffe et al. Reference Plouffe, Bourgeois, Beaudry, Chouinard and Choquette2018) starting from crop planting (crop GDD) and weed planting (weed GDD). Weed GDD were not retained, as they varied from the crop GDD only for the second weed emergence date and did not improve the models based on crop GDD or date.
Mesh Bag Evaluation
The effect of the mesh bags was tested in detail at SJR in 2017 to ensure the bags did not modify A. artemisiifolia seed production or shattering. Corn cultivar, seeding rate, and seeding dates were the same as those for the 2016 trial in SJR (see Table 1). A 40 m by 40 m field was divided into two equally sized plots. Both plots were harrowed and fertilized (based on standard requirements in corn). Plots were either seeded with corn (on May 11) or left as bare soil. Each plot was divided into four subplots (replicate blocks) (3 m by 10 m). Four A. artemisiifolia plants were grown in each subplot. This was achieved by seeding five stratified A. artemisiifolia seeds in six plant locations. Each location was then thinned to obtain one plant (at the 2-leaf stage), and each subplot was thinned to keep only four plants at the flowering stage. Half of these plants were then randomly assigned an open or closed bag. The entire field section was sprayed with glyphosate (1,350 g ae ha−1) and dicamba (192 g ae ha−1) on June 14. All the A. artemisiifolia plants seeded in the trial were protected from the herbicide application using plastic cups. Pollination bags were installed on each A. artemisiifolia plant, except that the top section of the pollen bags was either left open (unsealed) using five stakes or closed using a single central stake along the main stem as in the seed-shattering trial. The bags could also be enlarged (replacing the bag and adding stake sections) as the plants grew. Temperature and relative humidity were measured using HOBO® U23 Pro v2 External Temperature/Relative Humidity data loggers (Onset, Bourne, MA, USA) installed in four open and four closed bags (one per subplot). These probes were installed when the plants started to form seeds (August 25) and recorded temperature and relative humidity once per hour until October 11. When the corn crop reached physiological maturity (October 11), all bagged A. artemisiifolia plants were collected. For each plant, the seeds retained on the plants and shattered in the bags were counted using an Elmor C1 seed counter (Elmor, Schwyz, Switzerland) and weighed (total weight). Seed viability was tested on a subsample of 25 to 50 seeds as described earlier.
Statistical Analyses
Total Seed Production and Seed Viability
The effect of variables on total seed production (last collection date) was evaluated using a split-plot mixed model. Variables included weed seeding period (early vs. late) in a four-block (replicates) design nested within crop type (wheat, corn, or soybean), location (SJR and HAR), and year (2014, both locations; 2015, both locations; and 2016, SJR only). Blocks (replicates) were treated as a random effect. Year and location were not treated as random effects, because the experiment was not repeated every year at every location. Seed viability data were tested using the same analysis but included seed type (retained vs. shed) and collection date as additional fixed effects. ANOVAs were performed using R software (lme4 package; R Development Core Team 2019). Wheat plots were removed from the model due to the absence of weed seed production at time of crop harvest. Means were compared using Tukey’s HSD (honestly significant difference) using R software (multcomp package; R Development Core Team 2019).
Percentage of Seeds on Plants during Collection Dates
The effect of collection date on shattered seeds was evaluated using a repeated-measures multivariate split-plot mixed model. Repeated measures were collection dates (up to 12) located in plots randomly assigned to a weed seeding period (early vs. late) in a four-block design nested within crop type (wheat, corn, or soybean), location (SJR and HAR), and year (2014, 2015, and 2016 [SJR only]). Blocks were treated as a random effect. The multivariate ANOVA was performed using R software (R Development Core Team 2019). Year and location were not treated as random effects, because the experiment was repeated 3 yr for only one of the two locations. Wheat plots were removed from the model due to the absence of seed production (see “Results and Discussion”). Percent seed retention (seeds not retained are shattered) was regressed against cumulative GDD (Tbase = 5 C) from crop planting (crop GDD) and Julian day (JD) using one of three models—linear, quadratic, and logistic—using JMP v. 14.0.0 software (SAS Institute, Cary, NC, USA). The logistic model was based on Equation 1:
where y is percentage of seed retained, c is the asymptote, a is the slope, x is GDD or JD, and b is the inflection point.
For the mesh bag evaluation trial, seed production in bags was tested using a factorial mixed ANOVA model. The model included crop (corn vs. bare soil) and bagging (closed vs. open) nested within crop. Block (replicates) was treated as a random variable. Means were compared using Tukey’s HSD. Differences in temperature and relative humidity between open and closed bags were tested using t-tests.
Results and Discussion
Total Seed Production
There was a significant year (F = 60.81, P < 0.001), crop (F = 23.35, P < 0.001), and weed seeding date (emergence period) (F = 38.97, P < 0.001) effect on seed production. Site was not significant (F = 0.03, P = 0.86), but multiple interactions between all variables, including site, were significant (P < 0.05). No A. artemisiifolia seeds were produced or consequently shattered in wheat before crop maturity and harvest in any of the 5 site-year combinations (Table 2). Therefore, unless cut A. artemisiifolia plants are left to grow in wheat stubble after harvest, the inclusion of spring wheat in a rotation will help to reduce A. artemisiifolia seed production and dispersal. On the other hand, there is an ongoing expansion of corn and soybean cropping into the Northern Great Plains, where only short-season crops have typically been grown (Statistics Canada 2019), that could potentially increase the distribution of A. artemisiifolia populations. Ambrosia artemisiifolia seed production was not influenced by year in corn (p > 0.15) (averaging 336.68 [SJE] or 580.86 [HAR] seeds per plant), while production varied (p < 0.05) from 229.88 (SJE 2014) or 378.46 (HAR 2015) to 2,094.69 (SJE 2016) or 1,123.14 (HAR 2014) seeds plant−1 in soybean. These 3- (HAR) to 9-fold (SJR) variations in seed production in soybeans generated differences between corn and soybean, with production being either equivalent (SJR 2014, HAR 2015), higher (up to 6.5-fold) (SJR 2015 and 2016, HAR 2014), or lower (2.2 fold HAR 2015) in soybean compared with corn (Table 2). Higher variability in interrow canopy closure in 76-cm-row soybean can explain variability in competitiveness toward weeds (Datta et al. Reference Datta, Ullah, Tursun, Pornprom, Knezevic and Chauhan2017), as A. artemisiifolia can overtop soybean. Additionally, A. artemisiifolia is often more competitive in soybean than corn (Weaver Reference Weaver2001). As expected, A. artemisiifolia plants that emerged later produced fewer seeds (Deen et al. Reference Deen, Hunt and Swanton1998b; Simard and Benoit Reference Simard and Benoit2012). An 18.5-d delay in emergence reduced seed production by a factor of 2.2 (Table 2). This figure is within expected ranges, as a 4-wk delay can lower total biomass by half (Deen et al. Reference Deen, Hunt and Swanton1998b), and an 18- to 19-d delay has been shown to reduce seed production 3-fold in corn and soybean (Simard and Benoit Reference Simard and Benoit2012).
Table 2. Average number of seeds produced by Ambrosia artemisiifolia in different crops during 2 or 3 yr at Harrow (HAR) and Saint-Jean-sur-Richelieu (SJR).a
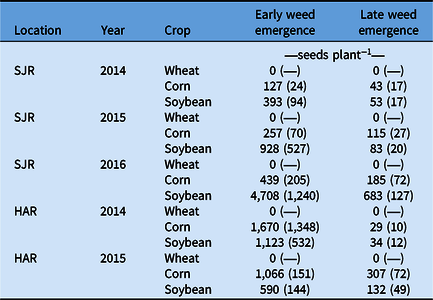
a Standard errors are in parentheses.
Seed Shattering
Seed shattering increased as the season progressed (Figure 1). Ambrosia artemisiifolia planting date (emergence period) did not modify seed shattering in time (P = 0.379) or interact with other variables. This variable was therefore treated as a random variable in all models. Site, year, and crop effects were significant. As for seed production, the wheat data were not included in the model, as no seeds were formed at harvest. The relationship between seed shattering and cumulative GDD since crop planting (crop GDD) or time (JD) was modified by site and year, and the former variable (crop GDD) was also modified by crop. Further investigations revealed that site and year effects were generated by the HAR 2015 data. The HAR 2015 relationship (expressed both in crop GGD and JD) was steeper and linear compared with the logistic model of other site-years (Figure 1). Different relationships at the same location (HAR) suggest differences in weather conditions such as drought and wind modified the seed-shattering phenology. Drought hastens the development of A. artemisiifolia (Allard Reference Allard1945), and we can imagine that wind gusts can hasten seed shed. When analyses were done separately, only the crop effect remained. When regressed against JD, the crop effect was not significant (Figure 1). Although the seed-shattering phenology of other weeds is better explained by GDD than calendar date (Forcella et al. Reference Forcella, Peterson and Barbour1996), A. artemisiifolia seed shattering was better predicted by JD than cumulative degree days from crop planting or from weed emergence. Deen et al. (Reference Deen, Hunt and Swanton1998b) also observed that date of dehiscence was synchronous between early- and late-emerging A. artemisiifolia in the field.

Figure 1. Percentage of seeds retained (seeds not retained are shattered) on Ambrosia artemisiifolia plants as function of growing degree days (GDD) since crop planting (Tbase = 5 C) (left) or Julian day (JD) (right) in corn and soybean. The linear regressions observed at Harrow in 2015 (top graphs) are separated from the rest of the data (Harrow 2014 and Saint-Jean-sur-Richelieu 2014, 2015, 2016) (bottom graphs). The formulas for the general linear (Harrow 2015) and logistic (other site-years) models are indicated. Harvest dates are indicated by arrows (Harrow 2015) or boxes (from first to last for all site-years).
The predominance of calendar dates over thermal time (GDD) as a predictor of seed shattering could be related to the effect of the photoperiod (Allard Reference Allard1945; Deen et al. Reference Deen, Hunt and Swanton1998a, Reference Deen, Hunt and Swanton1998b). Ambrosia artemisiifolia is a short-day plant and will start to produce male flowers when daily photoperiods drop below a certain threshold (e.g., 14.5 h of daylight) (Bassett et al. Reference Bassett, Holmes and Mac-Kay1961; Deen et al. Reference Deen, Hunt and Swanton1998a, Reference Deen, Hunt and Swanton1998b). Female flowering and subsequent seed development are also likely to be modulated by photoperiod. This would explain why synchronous shattering was prevalent (4 out of 5 site-years, both weed emergence periods within site-year and both corn and soybean [seeded 7 to 14 d later than corn] within site-year) in our experiment when expressed in calendar days despite differences in emergence dates and growing conditions. Populations from HAR and SJR should have a different photoperiod threshold if they are locally adapted (Bassett and Crompton Reference Bassett and Crompton1975; Kralemann et al. Reference Kralemann, Scalone, Andersson and Hennig2018; Scalone et al. Reference Scalone, Lemke, Štefanić, Kolseth, Rašić and Andersson2016; Stinson et al. Reference Stinson, Albertine, Hancock, Seidler and Rogers2016, Reference Stinson, Wheeler, Record and Jennings2018). However, studies have shown that most genetic variation is found within rather than among populations in this species (Genton et al. Reference Genton, Shykoff and Giraud2005; Martin et al. Reference Martin, Olsen, Samaniego, Zimmer and Gilbert2016), suggesting that populations are highly admixed due to pollen- and seed-mediated gene flow. If these populations have a similar daylight threshold (±5 d, for example), it would be attained 11 d earlier in HAR compared with SJR (because it is located 3° of latitude north of HAR) (Keisling Reference Keisling1982). As seed collection was done weekly, differences of less than 7 d were undetected.
Regardless of the underlying mechanism, based on our models, in 2015 at HAR, 50% and 75% of the seeds were shattered on October 6 and October 24, respectively, and 77% or 79% were shattered at soybean or corn harvest, respectively. In 2014 at HAR and during all 3 yr at SJR, 50% and 75% of the seeds were shattered October 22 and November 7, respectively, and 28% to 69% were shattered at crop harvest (Figure 1).
Seed viability data were analyzed by location due to significant interactions and missing values. At both locations, shed seeds had viability percentages equivalent to retained seeds, and percentages only varied by year and collection date. Viability percentages increased as the season progressed at both locations. At SJR, values averaged 41.91% from first seed shed until late September to reach an average of 78.38% afterward. At HAR, seed viability was tested earlier and averaged 36.50% before first seed shed to reach invariably high values (91.39%) afterward. The viability of seeds during the last collection dates was generally within expected values for A. artemisiifolia and was very high in 2015 at HAR (97.50%) and in 2016 at SJR (92.69%). Seeds collected early undoubtedly had a higher percentage of immature embryos (TeKrony and Egli Reference TeKrony and Egli1997). We expected that shed seeds would be mostly viable, but this was not the case at SJR before late September. We can only surmise that abscission, which is maternally regulated (Roberts et al. Reference Roberts, Elliott and Gonzalez-Carranza2002), took take place whether seeds were mature or not. Forcella et al. (Reference Forcella, Peterson and Barbour1996) observed either constant or erratic weed seed viability in time in corn. We observed constant A. artemisiifolia seed viability for at least a month before soybean or corn harvest.
Effect of Bagging
Although there was a slight reduction in seed viability in the closed bags (by less than 13%, maximum) located in the corn plots, total seed production and biomass were always higher or equivalent in the closed bags (Table 3). The observed slight reduction in viability is not supported by differences in temperature (P = 0.72) or relative humidity (RH) (P = 0.82) as temperature and RH values were equivalent in closed and open bags in corn (19.38 C, 83.73% RH). For bags located in the uncropped (bare) fields, temperature values were equivalent (P = 0.08) (19.07 C), but relative humidity was lower (P < 0.001) in open bags (69.97% RH) compared with closed bags (83.82% RH). Higher seed retrieval (both retained and shattered) in the closed bags in corn plots only is difficult to explain.
Table 3. Average biomass, number, and viability of Ambrosia artemisiifolia seeds on plant (retained, Ret.) and shattered (Shat.) in open and closed pollination bags installed on A. artemisiifolia plants located in corn or in an open (bare) field.a
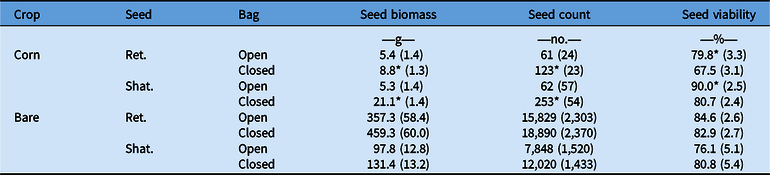
a Standard errors are in parentheses. Asterisks denote significant differences between open and closed bags based on t-tests: *α = 0.05 ; **α = 0.01.
Because A. artemisiifolia continues to flower after some seeds are shed and plants generally outcross (Friedman and Barrett Reference Friedman and Barrett2008), there could have been a slight reduction in effective pollination leading to empty seeds in the closed bags. Insects might also have been trapped in the bags, generating higher damage rates to shattered seeds. However, because seed production is generally evaluated using seed biomass, pollination bags installed after the flowering period would not underestimate A. artemisiifolia seed production. Bags also allow a plant-based assessment of seed production and dispersal instead of the area-based evaluation generated by seed traps located on the ground.
There is still a possibility that the bags altered seed shed in time, but we advocate that seed traps located on the ground could have underestimated initial seed shed (Forcella et al. Reference Forcella, Peterson and Barbour1996; Kollmann and Goetze Reference Kollmann and Goetze1998). We also recognize that predispersal seed predation is not substantial in A. artemisiifolia (Bassett and Crompton Reference Bassett and Crompton1975). Pollen bags would not be recommended to evaluate seed shed in species that have important pests that feed on flowers and seeds before seed dispersal, such as Amaranthus species predated by micro-moths (Coleophora lineapuvella Chambers) (DeSousa et al. Reference DeSousa, Griffiths and Swanton2003; Nurse et al. Reference Nurse, Booth and Swanton2003), unless treated with an insecticide. Ambrosia artemisiifolia seeds in pollen bags had constant high-viability percentages when collected on mature plants during the month before soybean or corn harvest in our trials. Finally, pollen bags are routinely used on crop plants by breeders, and lightweight nonwoven plastic bags have been shown to be reliable (Schaffert et al. Reference Schaffert, Virk and Senior2016). We recommend the evaluation of the effect of the pollen bags on other weed species.
Implications for Management
In spring wheat, A. artemisiifolia plants did not produce mature seeds before harvest at any location during any growing season. Therefore, harvest weed seed control (Walsh et al. Reference Walsh, Broster, Schwartz-Lazaro, Norsworthy, Davis, Tidemann, Beckie, Lyon, Soni, Neve and Bagavathiannan2018) would not be useful for A. artemisiifolia in this crop, but the inclusion of spring wheat in a rotation should help to lower A. artemisiifolia populations and could explain the low frequency of this species in areas where only short-season crops are grown. In corn and soybean, with a global average of 54.36%, the percentage of shattered seeds at harvest was generally lower than that observed for some other species (waterhemp [Amaranthus tuberculatus (Moq.) Sauer], and redroot pigweed (Amaranthus retroflexus L.) undistinguished, common lambsquarters (Chenopodium album L.), and four grass species) (Davis Reference Davis2008). However, because weeds produce hundreds of seeds, and thresholds that allow seedbank replenishment are low (Davis Reference Davis2008; Longchamps et al. Reference Longchamps, Panneton, Simard and Leroux2014; Simard et al. Reference Simard, Panneton, Longchamps, Lemieux, Légère and Leroux2009), collecting 50% of weed seeds would be largely insufficient to limit recruitment and seedbank replenishment during following seasons, and a delay of a single week in harvest operations would increase this percentage by about 10% (9.7%, linear model; or 11.7%, logistic model). Moreover, because seed shattering was largely a function of calendar date (probably photoperiod driven), integrated management practices such as the stale seedbed or increasing crop density would be unlikely to delay or hasten A. artemisiifolia seed dispersal. Controlling A. artemisiifolia populations that emerge early (e.g., using cultivation) could reduce weed seed inputs by half but, as noted, this would be insufficient. A weed management technique aimed at controlling seed formation, such as the application of phenoxy herbicides at flowering (Bae et al. Reference Bae, Nurse, Simard and Page2017), could be reliable, as late development stages synchronize regardless of emergence date.
Acknowledgments
We thank Manon Bélanger, Kerry Bosveld, Sylvain Fortin, Luc Marchand, Marie-Pier Ricard, Gilles Émond, Aline Philibert, and all the students who collected data. This study was funded by Agriculture and Agri-Food Canada (project no. J-000919).






