Introduction
The diagnosis of otitis media with effusion (OME) is based on clinical history, otoscopy and audiometry. Adult patients presenting with persistent unilateral OME and normal findings on ENT examination, including nasopharyngoscopy, usually undergo further medical management or ventilation tube insertion.Reference Seibert and Danner1
In this case report, we describe a patient with a past history of ventilation tube insertion for OME, who presented again with recurrent unilateral hearing loss and apparent OME. He was subsequently found to have a dural arteriovenous fistula associated with an encephalocele and cerebrospinal fluid (CSF) in the middle-ear space. The case illustrates the importance of a high index of suspicion for an uncommon pathology in the presence of any unusual findings, even if the initial clinical picture is suggestive of straightforward recurrence of a previously treated condition.
Case report
A 53-year-old man presented with a 2-year history of right-sided hearing loss and aural fullness. A ventilation tube had been inserted in the right ear for the same symptoms approximately 10 years previously, which resulted in immediate clinical improvement and no complications post-operatively. The symptoms had returned on extrusion of the ventilation tube and had persisted. On closed questioning, the patient admitted to pain in the post-auricular region on the right side, but denied associated tinnitus. There was no history of head trauma.
Otoscopy revealed a thin and atelectatic right tympanic membrane, with straw-coloured middle-ear effusion (Figure 1). Examination of the post-nasal space demonstrated normal findings. Inspection of the post-auricular region showed the area to be pulsatile. Palpation elicited extreme tenderness and confirmed pulsatility. Tympanometry was consistent with right-sided middle-ear effusion, and a pure tone audiogram demonstrated an associated moderate conductive hearing loss.

Fig. 1. Otoscopic view of middle-ear effusion.
In view of the examination findings, imaging was performed. Computed tomography (CT) of the petrous temporal bone (Figure 2) demonstrated numerous punctate and tubular lucencies within the right occipital and mastoid bones (white arrow, Figure 2), resulting in irregularity of the cortex with ill definition of the sigmoid plate (black arrow, Figure 2). There was soft tissue opacification of the right middle ear, with attenuation of the tegmen tympani and the bone adjacent to the proximal tympanic facial nerve canal (Figure 3). Gadolinium-enhanced magnetic resonance angiography demonstrated multiple vascular structures traversing the right occipitomastoid bones (Figure 4). Magnetic resonance imaging (MRI) revealed signal isointense to the brain parenchyma (encephalocele) herniating through the defect in the tegmen tympani and in continuity with the inferior temporal lobe (Figure 5).

Fig. 2. Axial computed tomography scan of the petrous temporal bone, demonstrating punctate and tubular lucencies within the right occipital and mastoid bones (white arrow), and irregularity of the cortex with ill definition of the sigmoid plate (black arrow).

Fig. 3. Coronal computed tomography scan of the petrous temporal bone, showing soft tissue opacification of the right middle ear, with attenuation of the right tegmen tympani and the bone adjacent to the proximal tympanic facial nerve canal.

Fig. 4. Axial gadolinium-enhanced magnetic resonance angiography, demonstrating multiple vascular structures traversing the right occipitomastoid bones (white arrow).

Fig. 5. Coronal magnetic resonance imaging scan demonstrating signal isointense to the brain parenchyma (encephalocele; open arrow), which herniated through the defect in the tegmen tympani.
Following discussion in the neurovascular multidisciplinary team meeting, the patient underwent cerebral angiography, which confirmed a transverse-sigmoid dural arteriovenous fistula, with a patent venous sinus and anterograde flow (Cognard type I). A supra-selective right occipital artery injection (Figure 6) demonstrated the occipital artery (open arrow) feeding the fistulous malformation (black arrow) and early venous drainage to the sigmoid sinus (white arrow).

Fig. 6. A supra-selective right occipital artery injection demonstrated the occipital artery (open arrow) feeding the fistulous malformation (black arrow) and early venous drainage to the sigmoid sinus (white arrow).
A decision was made to manage the dural arteriovenous fistula conservatively. The patient received Pneumovax®, Meningovax™ and haemophilus vaccinations. Surgical repair of the encephalocele was recommended but declined by the patient.
Discussion
Dural arteriovenous fistulas are acquired lesions that account for less than 10 per cent of intracranial vascular malformations.Reference Satomi and Satoh2 However, this may be an underestimation, as many patients remain asymptomatic and therefore not diagnosed, or the fistulas involute spontaneously.Reference Gupta and Periakaruppan3 Dural arteriovenous fistulas may be found as an isolated lesion or in association with other intracranial vascular pathology, such as cerebral arteriovenous malformations, hereditary haemorrhagic telangiectasia and intracranial aneurysms.Reference Berenstein, Lasjuanias, Lasjaunias and Berenstein4 Racial differences have been reported with regard to the location, with transverse-sigmoid lesions being more abundant in Europe and the USA, and cavernous sinus lesions being more commonly reported in Japan.Reference Satomi and Satoh2
It has been proposed that dural arteriovenous fistula arises from venous hypertension secondary to venous sinus thrombosis, leading to progressive hypertrophy of microvascular dural connections and eventually resulting in arteriovenous shunting. As the fistula increases in size and becomes more diffuse, mechanical obstruction may ensue, resulting in retrograde flow into the cortical veins. Complications may arise with dilatation of the latter, increasing the likelihood of intracranial haemorrhage.Reference Gupta and Periakaruppan3
Most dural arteriovenous fistulas present in the fifth to seventh decades of life. Symptoms can be divided into benign and aggressive; the former are usually secondary to intracranial venous hypertension, and the latter are normally characterised by intracranial haemorrhage or significant neurological deficits (Table 1).Reference Gandhi, Chen, Pearl, Huang, Gemmete and Kathuria5
Table 1. Clinical presentation of symptomatic dural arteriovenous fistula

Clinical presentation depends upon the location of the lesion and the pattern of venous drainage.Reference Hurst, Bagley, Galetta, Glosser, Lieberman and Trojanowski6 Pulsatile tinnitus is the most common otolaryngological manifestation.Reference Cognard, Casasco, Toevi, Houdart, Chiras and Merland7–Reference Satomi, van Dijk, Terbrugge, Willinsky and Wallace9 A case of cribriform plate dural arteriovenous fistula presenting with epistaxis was recently reported.Reference van Dijk, Korsten-Meijer and Mazuri10 In a case report of dural arteriovenous fistula presenting with recurrent otitis externa and pulsatile tinnitus in a diabetic patient,Reference Alatakis, Koulouris and Stuckey11 CT demonstrated middle-ear effusion and transcalvarial channels in the affected temporal bone, as was the case in our patient. To our knowledge, ours is the first reported case of dural arteriovenous fistula presenting with conductive hearing loss, CSF effusion and otalgia.
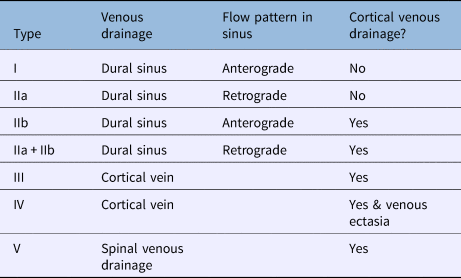
Several classifications exist for grading dural arteriovenous fistula. One of the most commonly used is the Cognard classification (Table 2).Reference Cognard, Gobin, Pierot, Bailly, Houdart and Casasco12 Cognard type I and IIa lesions are associated with a benign and non-aggressive presentation, as these are not associated with cortical venous drainage and therefore the risk of intracranial haemorrhage is low.Reference Davies, Ter Brugge, Willinsky and Wallace13 The other lesion types (IIb–V) are associated with cortical venous drainage, an aggressive feature, and carry a mortality rate of 10.4 per cent, an intracranial haemorrhage risk of 8.1 per cent, and a non-haemorrhagic neurological deficit of 6.9 per cent per annum.Reference van Dijk, terBrugge, Willinsky and Wallace14 Although the risk of conversion of a benign dural arteriovenous fistula to an aggressive type is small (reported in 2 per cent of low-grade lesions),Reference Satomi, van Dijk, Terbrugge, Willinsky and Wallace9 further evaluation may be needed if there is a change in clinical features.
Table 2. Cognard classification for dural arteriovenous fistulaReference Cognard, Gobin, Pierot, Bailly, Houdart and Casasco12

Depending on the clinical presentation, a combination of imaging studies is required to make a formal diagnosis and plan treatment. Intracranial haemorrhage can be investigated with non-contrast CT or MRI imaging. In the presence of dural arteriovenous fistula, both modalities may show: dilated arterioles alongside the dural sinus walls, venous pouches, and signs of venous hypertension in aggressive lesions.Reference Gandhi, Chen, Pearl, Huang, Gemmete and Kathuria5
Additional evaluation with CT angiography or magnetic resonance angiography (MRA) is required if flow void cluster around the dural venous sinus is suspected.Reference Kwon, Han, Kang and Chang15 Recent studies have suggested that high-field (3 Tesla), time-resolved MRA and CT angiography enhanced by four-dimensional rendering may have a role in the screening and evaluation of intracranial dural arteriovenous fistulas, rather than conventional angiography.Reference Farb, Agid, Willinsky, Johnstone and Terbrugge16, Reference Willems, Brouwer, Barfett, terBrugge and Krings17 Of note, normal MRI, MRA or CT angiography findings do not exclude a diagnosis of dural arteriovenous fistula, and, where suspicions persist, formal angiography is required. Conventional angiography remains the ‘gold standard’ for diagnosis and treatment planning, as it also identifies the arterial feeders, the fistula site, direction of the fistula venous drainage, sinus occlusion and circulation time.Reference Gupta and Periakaruppan3
Temporal bone encephaloceles are an acquired or developmental herniation of dura through the underlying cranial base, usually in the region of the tegmen. This may lead to CSF effusion or otorrhoea. The link between dural arteriovenous fistula, encephalocele and CSF effusion, however, is unknown. Thinning of the tegmen may occur due to changes in CSF pressure contributing to encephalocele formation and low-normal pressure fistulas.Reference Kaufman, Yonas, White and Miller18 One previous case report described the potential relationship between dural arteriovenous fistula, venous hypertension, intracranial hypertension and CSF rhinorrhoea.Reference Willems, Willinsky, Segev and Agid19 However, there was no clinical or radiological evidence of raised intracranial pressure in our patient. To the best of our knowledge, there are no previously published cases of CSF middle-ear effusion with an associated dural arteriovenous fistula.
Treatment is dependent on patient factors (e.g. age, co-morbidities), and the location, type and angiographic features of the lesion. All patients should be discussed in a multidisciplinary setting, consisting of interventional neuroradiologists, neurosurgeons, neurologists and radiation oncologists.Reference Gandhi, Chen, Pearl, Huang, Gemmete and Kathuria5 An aggressive dural arteriovenous fistula should be treated early to avoid the risk of haemorrhagic or neurological complications. Although a conservative approach may be more appropriate in benign lesions, treatment should be considered in those patients with significant symptoms resulting in poor quality of life.Reference Gandhi, Chen, Pearl, Huang, Gemmete and Kathuria5
Endovascular therapy is considered the first-line treatment for dural arteriovenous fistulas. It aims to eliminate the arteriovenous shunt completely, utilising a transarterial, transvenous or combined approach. The majority of dural arteriovenous fistulas can be approached via a transarterial route, and embolisation can be achieved with various materials. The transvenous approach is preferable when the diseased segment contributes little to the normal venous outflow and can be occluded completely. Surgery is only indicated if endovascular approaches are not feasible or are unsuccessful.Reference Gandhi, Chen, Pearl, Huang, Gemmete and Kathuria5
• Dural arteriovenous fistula presentation varies depending on location and venous drainage pattern
• The most common otolaryngological presentation is pulsatile tinnitus
• Adult unilateral middle-ear effusions may be a result of cerebrospinal fluid (CSF) leak
• Tegmen thinning may occur due to CSF pressure changes contributing to encephalocele formation and low-normal pressure fistulas
• The link between dural arteriovenous fistula, encephalocele and CSF effusion is unknown
Conclusion
In cases of apparent unilateral otitis media with effusion in an adult, every effort should be made to identify an underlying condition, including a rare but potentially life-threatening pathology.
Competing interests
None declared.