INTRODUCTION
Within their vertebrate host, malaria parasites (Plasmodium spp.) undergo repeated rounds of asexual replication as haploid meront cells, and eventually produce progeny that develop into the transmission stage, or gametocytes (Valkiunas, Reference Valkiunas, Anwar, Atkinson, Greiner, Paperna and Peirce2005). Each infection could harbour a single clone of genetically uniform meronts and gametocytes, or two or several genotypes of parasites. Many studies, scoring a variety of genetic markers, find that infections of the human malaria parasites P. falciparum, P. malariae, and P. vivax vary in their number of co-existing clones, but multi-clonal infections are common, especially in locations with high transmission (Gupta et al. Reference Gupta, Hill, Kwiatkowski, Greenwood, Greenwood and Day1994; Felger et al. Reference Felger, Smith, Edoh, Kitua, Alonso, Tanner and Beck1999; Tanner et al. Reference Tanner, Beck, Felger and Smith1999; Anderson et al. Reference Anderson, Haubold, Williams, Estrada-Franco, Richardson, Mollinedo, Bockarie, Mokili, Mharakurwa, French, Whitworth, Velez, Brockman, Nosten, Ferreira and Day2000; Leclerc et al. Reference Leclerc, Durand, De Meeus, Robert and Renaud2002; Cui et al. Reference Cui, Mascorro, Fan, Rzomp, Khuntirat, Zhou, Chen, Yan and Sattabongkot2003; Imwong et al. Reference Imwong, Sudimack, Pukrittayakamee, Osorio, Carlton, Day, White and Anderson2006; Bruce et al. Reference Bruce, Macheso, Galinski and Barnwell2007). Very limited data on the Plasmodium of non-human hosts reveal a similar pattern (Vardo and Schall, Reference Vardo and Schall2007). The clonal diversity of Plasmodium infections is important because ecological and evolutionary theory predicts that the number of co-existing genotypes of microparasites will shape the virulence of the infection (Bull, Reference Bull1994; Ewald, Reference Ewald1994, Reference Ewald2004; Nowak and May, Reference Nowak and May1994; May and Nowak, Reference May and Nowak1995; van Baalen and Sabelis, Reference Van Baalen and Sabelis1995; Frank, Reference Frank1996; Read and Taylor, Reference Read and Taylor2001; Schall, Reference Schall, Lewis, Campbell and Sukhdeo2002). This reasoning posits that parasites within single-clone infections face the relatively simple challenge of reproducing at some optimal rate to ensure maximal transmission success of the gametocytes while replacing cells that are removed by the host's immune system. A more complex situation challenges parasites in genetically complex infections because the parasite clones must compete for resources and the opportunity for transmission (de Roode et al. Reference de Roode, Helinski, Anwar and Read2005a). Multi-clonal infections are predicted to be more costly to their host both because of higher parasite density as well as the greater effort expended by the host to fight a diversity of parasite genotypes (Sheldon and Verhulst, Reference Sheldon and Verhulst1996; Taylor et al. Reference Taylor, Mackinnon and Read1998; Schall, Reference Schall, Lewis, Campbell and Sukhdeo2002).
For Plasmodium, evidence bearing on the association of clonal diversity and virulence comes from two sources. First, cross-sectional studies of natural human malaria infections (with no experimental manipulation of clonal diversity) yield equivocal results. Studies on P. falciparum conclude that high clonal diversity may or may not lead to severe symptoms, and that host factors are just as important as parasite effects in determining pathology (Gupta et al. Reference Gupta, Hill, Kwiatkowski, Greenwood, Greenwood and Day1994; Felger et al. Reference Felger, Smith, Edoh, Kitua, Alonso, Tanner and Beck1999; Smith et al. Reference Smith, Felger, Tanner and Beck1999; Tanner et al. Reference Tanner, Beck, Felger and Smith1999; Müller et al. Reference Müller, Charlwood, Felger, Ferreira, do Rosario and Smith2001; Ofosu-Okyere et al. Reference Ofosu-Okyere, Mackinnon, Sowa, Koram, Nkumah, Osei, Hill, Wilson and Arnot2001; Leclerc et al. Reference Leclerc, Durand, De Meeus, Robert and Renaud2002; Cui et al. Reference Cui, Mascorro, Fan, Rzomp, Khuntirat, Zhou, Chen, Yan and Sattabongkot2003). Some authors suggest that complex P. falciparum infections may be asymptomatic, and severe illness results only when novel clones enter an established infection (Felger et al. Reference Felger, Smith, Edoh, Kitua, Alonso, Tanner and Beck1999; Smith et al. Reference Smith, Felger, Tanner and Beck1999). Thus, no general pattern relating clonal diversity to virulence emerges from studies on human malaria parasites. Second, benchmark manipulative experiments using a laboratory rodent malaria model (P. chabaudi in inbred mice) find that genetic diversity leads to competition among clones, changes in the dynamics of infection, and increased virulence (Taylor et al. Reference Taylor, Mackinnon and Read1998; Read and Taylor Reference Read and Taylor2001; Mackinnon et al. Reference Mackinnon, Gaffney and Read2002; de Roode et al. Reference de Roode, Read, Chan and Mackinnon2003, Reference de Roode, Culleton, Cheesman, Carter and Read2004; Mackinnon and Read Reference Mackinnon and Read2004; de Roode et al. Reference de Roode, Helinski, Anwar and Read2005a, Reference de Roode, Pansini, Cheesman, Helinski, Huijben, Wargo, Bell, Chan, Walliker and Readb). Most striking is the finding that complex infections are more virulent for mice even when overall parasitaemia is similar for single-clone and multi-clone infections. The rodent model system allows clean experimental design using well-characterized parasite clones, but lacks the co-evolutionary history of a natural parasite-host association because the parasite in nature infects African thicket rats (Thamnomys), not mice.
The value of the rodent malaria studies prompted us to examine the effects of clonal diversity on the virulence of a lizard malaria parasite, P. mexicanum, in its natural vertebrate host, the fence lizard Sceloporus occidentalis. This parasite-host system has been studied for 3 decades at a site in northern California, USA (Schall, Reference Schall1996, Reference Schall, Lewis, Campbell and Sukhdeo2002). P. mexicanum is virulent for infected lizards; infection is associated with a broad array of haematological, physiological, behavioural, and reproductive deficits (Schall et al. Reference Schall, Bennett and Putnam1982; Schall, Reference Schall1990a, Reference Schallb, Reference Schall1996, Reference Schall, Lewis, Campbell and Sukhdeo2002). However, these costs vary in severity among infections, a finding never explained (Schall, Reference Schall1996, Reference Schall, Lewis, Campbell and Sukhdeo2002). Genetic diversity both within and among P. mexicanum infections is great, with 50–88% of infections (depending on year and site) containing more than a single parasite genotype (Vardo and Schall, Reference Vardo and Schall2007), and experimental infections can be initiated that control the number of parasite clones (Vardo et al. Reference Vardo, Kaufhold and Schall2007; Vardo-Zalik and Schall, Reference Vardo-Zalik and Schall2008). Mixed-clone infections are more variable in their rate of asexual and gametocyte increase and final number of parasites (Vardo-Zalik and Schall, Reference Vardo-Zalik and Schall2008), suggesting that virulence might also be influenced by clonal diversity.
We established replicate experimental infections containing one to many parasite clones (scored using recently characterized variable microsatellite markers for P. mexicanum (Schall and Vardo, Reference Schall and Vardo2007)), and asked if clonal diversity was associated with differences in several measures of virulence relevant for the lizard host: changes in mass, blood haemoglobin and blood glucose concentrations, production of immature erythrocytes, and rapidity of blood clotting. We also asked if virulence was associated with specific parasite genotypes. This study thus provides the second model system that allows manipulation of clonal diversity of a malaria parasite to determine effects on virulence of the infection, and the first study to use a natural, co-evolved Plasmodium-host system for such studies. The results allow comparison of manipulative experiments with a Plasmodium infecting a mammal (P. chabaudi) and a parasite of a reptile to determine the generality of the importance of clonal diversity for the virulence of malaria parasites.
MATERIALS AND METHODS
The experiment was conducted at the University of California Hopland Research and Extension Center, near the town of Hopland, in southern Mendocino County, California, USA. Naturally infected lizards were captured from 5 locations on the 2169 ha field station (all within 1·2 km of 39°00′02″N, 123°04′39″W) to serve as blood donors to initiate experimental infections. Thin blood smears were treated with Giemsa stain and examined under 1000× magnification, and for infected lizards, meront parasitaemia determined by counting parasites in 1000 erythrocytes. Prevalence of P. mexicanum infections was ~6%, and lizards heavily infected with asexually replicating meront stages (>25 meronts per 1000 erythrocytes) were uncommon in early May, so only 5 suitable donor infections containing 1 or 2 parasite clones were identified from 484 lizards sampled. Seventy-eight non-infected adult male lizards with a snout to vent length (svl) >55 mm were collected from 2 locations at the field station (39°02′05″N, 123°05′49″W and 38°59′27″N, 123°05′27″W) where P. mexicanum infection in the lizards has been absent for many years. Examination of blood smears, and a sensitive PCR-based protocol that detects infections subpatent in blood smears confirmed the recipient lizards were not already infected with P. mexicanum (Perkins et al. Reference Perkins, Osgood and Schall1998; Vardo et al. Reference Vardo, Wargo and Schall2005). Infections with P. mexicanum are not readily cleared by the lizard's immune response, therefore any infected lizard, regardless of when the infection occurred, would have detectable parasite concentrations (Bromwich and Schall, Reference Bromwich and Schall1986; Schall and Marghoob, Reference Schall and Marghoob1995). Using these standards, an additional control group of 21 non-infected lizards was maintained throughout the study (below) and none of these lizards converted to infection.
Parasite genotypes for each infected lizard (the naturally infected donors and the experimentally infected animals) were analysed at 3 microsatellite loci (Pmx 306, 747 and 732) using PCR primers and conditions presented by Schall and Vardo (Reference Schall and Vardo2007), and the labelled PCR product run on an ABI Prism instrument and results analysed using GeneMapper software (ABI). Plasmodium parasites in the vertebrate host are haploid cells, so each peak on the resulting pherogram represents a single clone of cells. Preliminary trials using 3 additional markers revealed that clonal diversity was accurately determined by scoring the 3 markers used here (data not shown). Single-clone donor infections were scored as one allele for each locus (2 of the donor infections). One donor infection was scored as 2 clones based on 2 alleles at 1 locus and 1 each at the other 2. Two more donor infections had 2 alleles at more than one locus. Therefore, we estimated the clonal diversity within an infection as the maximum number of alleles observed at any of the 3 microsatellite loci. This estimate represents the minimum number of clones present within an infection and is proposed to be an unbiased estimate of clonal diversity (Anderson et al. Reference Anderson, Haubold, Williams, Estrada-Franco, Richardson, Mollinedo, Bockarie, Mokili, Mharakurwa, French, Whitworth, Velez, Brockman, Nosten, Ferreira and Day2000; Ferreira et al. Reference Ferreira, Nair, Hyunh, Kawamoto and Anderson2002, Reference Ferreira, Karunaweera, Da Silva-Nunes, Da Silva, Wirth and Hartl2007; Bogreau et al. Reference Bogreau, Renaud, Bouchiba, Durand, Assi, Henry, Garnotel, Pradines, Fusai, Wade, Adehossi, Parola, Ali Kamil, Puijalon and Rogier2006; Bruce et al. Reference Bruce, Macheso, Galinski and Barnwell2007; Vardo and Schall, Reference Vardo and Schall2007).
Experimental infections were initiated with blood from 1–4 donors using the protocol of Vardo-Zalik and Schall (Reference Vardo-Zalik and Schall2008). This method results in infection in 100% of lizards inoculated, with a high proportion of clones transferred (Vardo-Zalik and Schall, Reference Vardo-Zalik and Schall2008; Vardo et al. Reference Vardo, Kaufhold and Schall2007; Osgood and Schall, Reference Osgood and Schall2003; Osgood et al. Reference Osgood, Eisen, Wargo and Schall2003; Eisen and Schall, Reference Eisen and Schall2000). Briefly, blood was taken from each donor lizard, and the number of parasites per μl of blood calculated based on counts of parasites per 1000 erythrocytes examined in stained blood smears and erythrocytes/μl of blood estimated using a Hausser counting chamber. Blood from donor lizards was then mixed with phosphate-buffered saline (PBS), and the blood/PBS mixture was diluted to contain approximately 200×103 total meronts. For multiclonal infections initiated with blood from >1 donor, each donor supplied similar numbers of asexual parasites, with the total number of parasites injected held constant (200×103). Infections were started via intraperitoneal injections with 20 μl of the donor blood/PBS mixture.
Using the minimum number of clones rule described above, 4 treatment groups received blood containing 1, 2, 3, or >3 clones (4–6 clones). This range in clonal diversity is similar to that seen within natural infections of P. mexicanum (Vardo and Schall, Reference Vardo and Schall2007). Each experimental infection was genotyped on day 30 when infections become patent in the blood and day 80 at the end of the experiment. Not all introduced clones became established (as observed previously in such experiments with P. mexicanum; Vardo et al. Reference Vardo, Kaufhold and Schall2007). Three lizards receiving blood from a 2-clone donor established a single clone, 5 recipients from a 4-clone donor lost 1 of those clones, and one receiving 4 clones lost 2 of those clones (total losing 1 or more clones=9/78 infections).
Two sources of error could have confounded estimation of the true number of clones per experimental infection. First, very low level clones in the donors (cryptic clones) could have been undetected, but passed to the recipients. In the 78 recipients here, and approximately 200 other experimental infections (for example, Vardo et al. Reference Vardo, Kaufhold and Schall2007), no novel genotypes were ever seen in recipient lizards. Also, experiments feeding vectors on infected lizards, then genotyping the parasite as oocysts, never detected any novel parasite genotype (unpublished observations). Vectors of Plasmodium tend to amplify low-level cryptic clones (Nwakanma et al. Reference Nwakanma, Kheir, Sowa, Dunyo, Jawara, Pinder, Milligan, Walliker and Babiker2008). We conclude that such cryptic clones were absent or rare in the donor infections. Second, the apparent loss of some clones when transferring to the recipients (9 of 78 infections) may be spurious. Genotyping infections twice (at days 30 and 80) found no evidence of this error, and only 4 experimental infections would have been placed into an incorrect treatment group if we missed 1 or more clones (below). Although cryptic clones seem unlikely in the experimental infections, use of a natural system such as P. mexicanum requires caution when examining results.
Three treatment groups were established. Three replicates of Treatment Group 1 (single-clone recipient infections) received blood from donors I (N=8), II (N=8), or III (N=3) (Table 1). Donors I and II harboured single-clone infections, and donor III a 2-clone infection, but only one of those was detected in the recipients. Four replicates of Treatment Group 2 (2-clone infections) received blood from donors I+II (N=8), III (N=5), IV (N=8) and III+IV+V (N=5). Two replicates of Treatment Group 3 (3-clone infections) received blood from donors III+IV (N=1) and donors III+IV+V (N=4). Again, assignment of lizards to Treatment Groups 2 or 3 was based on the number of clones that became established. Three replicates of Treatment Group >3 (with 4 or more clones) received blood from donors III+IV (N=7), donors I+II+III+IV (N=10), and donors III+IV+V (N=11). Thus, a total of 78 infections were initiated into treatments with 1 (N=19 total), 2 (N=26), 3 (N=5), and >3 (N=28) clones. The control group of 21 lizards, judged not infected by the criteria given above, was used to monitor changes in virulence measures due to captivity.
Table 1. Donor infections used to initiate replicate recipient infections of the malaria parasite Plasmodium mexicanum in the natural lizard host, Sceloporus occidentalis
(Three microsatellite loci were genotyped to score the number of clones present. Alleles for each locus are labelled from smallest (shortest repeat) to longest (A–D for Locus Pmx306, 1–6 for Locus Pmx732, and a–c for Locus Pmx747; loci described by Schall and Vardo (Reference Schall and Vardo2007) ). Number of clones present (N clones) is reported as the largest number of alleles seen for any locus.)

For the 3-month experimental period, lizards were housed outdoors in 12 vector-proof cages, with lizards housed by replicate to insure that parasite clones could not be passed by means other than vector bites among the treatments (Schall and Smith, Reference Schall and Smith2006). The cages were hung from overhead wire in a 5×2 m area, and each week were randomly moved within that array. Drops of blood were drawn from toe clips to make a blood smear and a dried/frozen blood drop every 10 days. On those dates, lizard mass was determined. Lizards were fed mealworms or crickets to satiation and were showered with cool water hourly from 13:00 until 19:00 when temperatures exceeded 29°C. Each of the smears (made every 10 days), was treated with Giemsa stain and total parasitaemia determined as parasites per 1000 erythrocytes.
Virulence measures recorded were chosen based on previous studies on the effect of P. mexicanum on fence lizards (see reviews in Schall, Reference Schall1996, Reference Schall, Lewis, Campbell and Sukhdeo2002). S. occidentalis fence lizards are small animals (recipients at 80 days=8·7–16·4 g), so virulence measures requiring a blood sample could be taken only once (at day 80) to avoid stressing the animals and causing anaemia not related to infection. P. mexicanum infections grow slowly compared to the Plasmodium infecting mammals, and maximal parasitaemia occurs at about day 80 in experimental infections, and there is no evidence of synchronized emergence of merozoites (Eisen and Schall, Reference Eisen and Schall2000; Osgood and Schall, Reference Osgood and Schall2003; Osgood et al. Reference Osgood, Eisen, Wargo and Schall2003; Vardo-Zalik and Schall, Reference Vardo-Zalik and Schall2008). Therefore, we assumed that any costs of infection would be patent on day 80. Virulence measures were as follows. (1) Change in body mass: lizards were weighed every 10 days when blood smears were taken. (2) Proportion of immature erythrocytes: the blood smear made on day 80 post-injection was examined to count 100 erythrocytes from all areas of the smear under 1000× magnification. Immature cells are distinguished by a larger, rounder nucleus and their darker, bluer colour. This measure was taken 3 times for each lizard, and the mean of all 3 values was used. (3) Blood haemoglobin: on day 80 post-injection, the blood haemoglobin level was determined by the cyanmethaemoglobin method in which the concentration of haemoglobin is linearly related to absorbance at 540 nm detected using a spectrophotometer (Schall, Reference Schall1990a). (4) Blood glucose: glucose was measured using a FreeStyle glucose monitor which utilizes a glucose dehydrogenase pathway to estimate glucose concentrations in whole blood (Voituron et al. Reference Voituron, Storey, Grenot and Storey2002). (5) Blood clotting: in humans, Plasmodium infection is associated with changes in blood clotting (Ghosh and Shetty, Reference Ghosh and Shetty2008), and in preliminary studies we also noted differences in clotting rate for infected and non-infected lizards. Therefore, we scored each lizard on day 80 post-injection as ‘rapidly clotting blood’ if the blood immediately (within 30 sec) formed a blood clot in a heparinized capillary tube (used to take blood for the haemoglobin measures). All procedures followed a protocol approved by the University of Vermont Institutional Animal Care and Use Committee.
Data were analysed using the statistical programs JMP 6.0 and Statview 5.01. To detect a treatment effect (clonal number), we used a nested ANOVA, with individual lizards nested within replicates, and replicates nested within treatment groups. This allowed partitioning of variance due to lizard variation in genotype and physiology, source of infected blood for each treatment (different donors), and effect of clonal diversity itself (Gotelli and Ellison, Reference Gotelli and Ellison2004). Other analyses used χ2, or t-tests with a significance level set at P<0·05.
RESULTS
All 78 recipient lizards established infections within 1 month, except 1 lizard that did not show parasites in the blood until day 70 post-injection. All experimental infections had reached their maximal parasitaemia by day 80. Experiment-wide mortality was 12/78 (15%) infected lizards and 2/21 (10%) non-infected controls, with no significant difference for infected versus uninfected (χ2 tests, P>0·05). This result is highly unlikely to be a Type II error due to small sample size because sample size of infected/non-infected would need to be 400 for each for a significant result (for 15% vs 10% mortality). Mortality reduced the total sample size for other analyses to 66 experimentally infected lizards (17 1-clone, 19 2-clone, 5 3-clone and 25 >3-clone) and 19 controls.
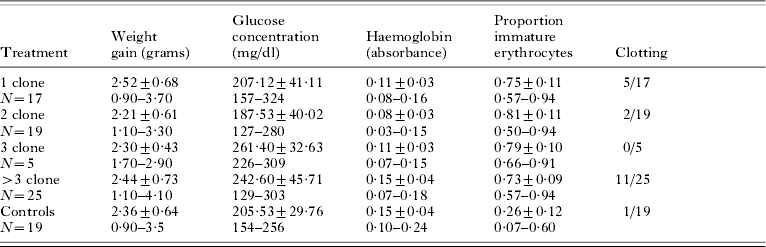
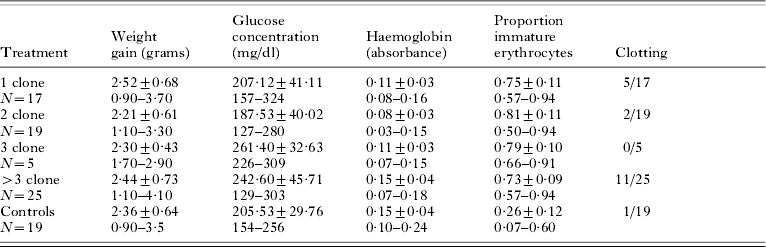
Summary data for all virulence measures are given in Table 2. Almost all lizards gained mass during the experiment. Mass change did not differ for infected versus control lizards (t-test, P=0·88), nor among treatment groups for the infected lizards (F12,72=0·101, P=0·98), with no effect of replicates within treatment groups (F12,72=1·01, P=0·44). The proportion of immature erythrocytes also did not differ for treatment groups (F11,54=1·68, P=0·18), with no replicate effect (F11,54=0·67, P=0·71), but was significantly higher in infected individuals when compared to the non-infected controls (t-test, P=0·0001). Rapid blood clotting was significantly more common in lizards within the >3-clone treatment group versus any other treatment, including the control lizards. To allow a χ2 -test, data for treatment groups 1, 2, and 3 were combined to yield expected values >5 (χ2=13·41, P=0·009). A Post-hoc Cell Contributions test indicated it was only the >3 clone treatment revealed the higher number of rapidly clotting samples. Severe blood clotting prevented determination of blood haemoglobin concentration in 1 control and 18 experimental lizards. Haemoglobin concentration differed among treatment groups, with treatment group 2 having a lower mean haemoglobin concentration than the >3 treatment (F11,36=6·31, P=0·002; Tukey's HSD). A replicate effect within treatments was also observed (F11,36=2·42, P=0·03) within the 2-clone replicate groups. Control lizards had similar levels of haemoglobin in their blood as the 3 and >3 clone treatment group, significantly higher than the 1- and 2-clone treatments (F12,53=10·25, P=0·0001; Tukey's HSD). Glucose concentration also differed among treatment groups, with the infections with fewer clones (treatments 1 and 2) having lower glucose levels than the 3 and >3 treatments (F11,54=6·91, P=0·0005s; Tukey's HSD), with no replicate effect (F11,54=1·57, P=0·16). Blood glucose levels were similar for the control group and infection treatments 1 and 2, but lower than for treatments 3 and >3 (F12,72=6·79, P=0·0001, Tukey's HSD).
Table 2. Summary data (mean±S.D.) and range for 5 virulence measures for western fence lizards (Sceloporus occidentalis) experimentally infected with the malaria parasite, Plasmodium mexicanum
(Treatment groups are infections containing 1, 2, 3 or >3 genetically distinct clones and a control, non-infected group. Measures are determined on day 80 after the experimental infections were initiated. Measures are weight gain over the 80 day experiment, glucose concentration in the blood, a measure of blood haemoglobin concentration (absorbance at 540 nm), proportion of immature erythrocytes and number of animals with blood that clotted very rapidly.)
Effect of total parasitaemia on virulence measures was determined using parasitaemia on day 80 (the final day of the experiment) and the sum of all parasitaemia measures made over the entire experiment. No significant effect relationship was found between parasite load and virulence measures (all P>0·1; Table 3).
Table 3. ANOVA results for parasitaemia and virulence measures

Parasite genotypes were inspected to determine if specific alleles and/or multilocus genotypes were associated with the differences seen between the 1 and 2 compared to the 3 and >3 clone infections. Four of the 5 donor lizards supplied blood for multiple treatment groups, including the single and >3 clone groups, so some of the multilocus genotypes were found in all treatment groups. We then scored the predominant allele at each locus for each infection (the allele that produced the highest peak on the pherogram produced by the genetic analyser). For locus Pmx306, four alleles were scored as predominant among infections and 3 alleles were predominant for locus Pmx732. All of these alleles were predominant in infections across all treatment groups. For locus Pmx747, three alleles were predominant among infections, but 1 was unique to treatment group 1 with a single clone, occurring in 40% of those infections. Treatment groups 1 and 2 did not differ for any virulence measure, so that allele is unlikely to be a driver of differences between the low and high clonal diversity treatments.
DISCUSSION
Prior to this study on Plasmodium mexicanum, two lines of evidence cast light on the role of clonal diversity on the virulence of malaria parasites: the observational field studies on natural P. falciparum infections (with no manipulation of clonal numbers) and experiments on the rodent malaria parasite P. chabaudi in a laboratory model system. Results from the studies on P. falciparum come to substantially different conclusions. High clonal diversity may or may not lead to severe symptoms, and other effects, such as host variation, may overshadow any consequences of clonal diversity (Gupta et al. Reference Gupta, Hill, Kwiatkowski, Greenwood, Greenwood and Day1994; Felger et al. Reference Felger, Smith, Edoh, Kitua, Alonso, Tanner and Beck1999; Smith et al. Reference Smith, Felger, Tanner and Beck1999; Tanner et al. Reference Tanner, Beck, Felger and Smith1999; Ofosu-Okyere et al. Reference Ofosu-Okyere, Mackinnon, Sowa, Koram, Nkumah, Osei, Hill, Wilson and Arnot2001; Leclerc et al. Reference Leclerc, Durand, De Meeus, Robert and Renaud2002; Cui et al. Reference Cui, Mascorro, Fan, Rzomp, Khuntirat, Zhou, Chen, Yan and Sattabongkot2003). The laboratory rodent malaria system allows experimental manipulation of clonal diversity, as well as characterization of specific parasite genotypes. The results demonstrate that the virulence of P. chabaudi differs by both parasite genotype and clonal diversity within infections. In the experiments, mice containing multi-clonal infections of P. chabaudi, usually harboring only 2 clones, often have increased host anaemia, reduced body mass, and other pathologies compared to genetically simple infections (Taylor et al. Reference Taylor, Mackinnon and Read1998; Mackinnon and Read Reference Mackinnon and Read1999, Reference Mackinnon and Read2003, Reference Mackinnon and Read2004; Read and Taylor, Reference Read and Taylor2001; Mackinnon et al. Reference Mackinnon, Gaffney and Read2002; de Roode et al. Reference de Roode, Read, Chan and Mackinnon2003, Reference de Roode, Culleton, Cheesman, Carter and Read2004, Reference de Roode, Helinski, Anwar and Read2005a, Reference de Roode, Pansini, Cheesman, Helinski, Huijben, Wargo, Bell, Chan, Walliker and Readb). The higher virulence of genetically complex infections may be based, at least in part, on the higher cost of mounting an immune attack against multiple parasite clones (Taylor et al. Reference Taylor, Mackinnon and Read1998).
We present here the first manipulative study of the effects of clonal diversity on several virulence measures for the vertebrate host of a natural Plasmodium association. The results present a complex, and unexpected, relationship between the clonal diversity of infections and the effects on the lizard host. Manipulating the number of parasite clones within treatments revealed no effect of infection itself or clonal diversity of infection, on change in body mass when compared to non-infected lizards. The proportion of immature erythrocytes, a measure of the rate of replacement of red blood cells, was higher for infected than non-infected lizards, but with no additional effect of clone number. The surprising finding was that the haemoglobin concentration was highest for the non-infected lizards and those infected with >3 (complex infections), and infections with 3 or >3 clones were associated with the highest glucose levels in the lizard's blood. Thus, for increase in body mass, turn-over of blood erythrocytes, haemoglobin concentration, and glucose levels, infections with the highest clonal diversity either had no additional negative effects or actually appeared to have a positive effect on the lizards. And last, very rapid clotting of the blood was associated with infections with >3 clones. Changes in blood clotting resulting from Plasmodium infection are well known for human hosts, but evaluating these as positive or negative for the health of the host has proven difficult (Ghosh and Shetty, Reference Ghosh and Shetty2008). The total parasite load (meronts and gametocytes) was not associated with any virulence measure, both for a single point in the course of infection (day 80) or combined over the entire infection. The overall conclusion is that infection with 1–2 P. mexicanum clones may have negative consequences for fence lizards, but complex infections induce little harm or may actually benefit the lizard (provided high glucose levels and rapid clotting are beneficial).
We can now compare these results with prior studies on P. mexicanum in natural infections followed in free-ranging fence lizards. Infected lizards in their home territory are just as able to harvest prey as non-infected lizards (Eisen and Schall, Reference Eisen and Schall1997) and have similar growth rates (svl, rather than body mass was measured) (Ressel and Schall Reference Ressel and Schall1989). Our results agree that infection does not reduce growth rate, and also that clonal diversity of infections does not influence growth. All lizards had ready access to plentiful insect prey in their cages, so the results show that the parasite does not interfere with assimilation of these easily captured food items.
Naturally infected fence lizards present a higher proportion of immature erythrocytes in the peripheral circulation compared with non-infected lizards. Such cells contain less haemoglobin, and thus haemoglobin concentration in the blood is lower in infected lizards (Schall et al. Reference Schall, Bennett and Putnam1982; Schall Reference Schall1990a, Reference Schallb, Reference Schall1996; Schall, Reference Schall, Lewis, Campbell and Sukhdeo2002). A similar result for production of immature erythrocytes was seen in the infected versus non-infected lizards in this study, with no difference seen among the treatment groups of infected lizards. Thus, destruction of damaged and/or infected erythrocytes and the subsequent increased production of new blood cells by the host is a product of infection, rather than infection complexity and may be a generalized immune response to Plasmodium infection. A curious result is the similar concentration of haemoglobin in the blood of non-infected lizards and those infected lizards carrying 3 or more clones, despite a higher proportion of immature erythrocytes in all infected treatments. A higher haemoglobin concentration allows more rapid delivery of oxygen to the lizard's tissues, and a greater running stamina, both likely to be ecologically important measures of health (Schall, Reference Schall, Lewis, Campbell and Sukhdeo2002). Previous findings that naturally infected lizards suffer lower haemoglobin concentrations is likely to be a consequence of sampling individuals with low clonality, as infections with high clonality are uncommon in the population of infected lizards at the site (Vardo and Schall, Reference Vardo and Schall2007).
Dunlap and Schall (Reference Dunlap and Schall1995) found that naturally infected fence lizards carry lower concentrations of blood glucose. This conflicts with the experimental study that found no reduction in glucose for lizards with 1 or 2 parasite clones, but an increase in glucose for infections with highest clone numbers (3 and >3) when compared to the non-infected control lizards. This discrepancy could be attributed to differences in methodology, because (i) our lizards were kept in captivity for 80 days, while the previous study kept lizards for a short period of time (<24 h) and (ii) naturally infected lizards caught in the previous study most likely contain only 1–3 clones, as infections with 4 or more clones are relatively scarce at the field site (Vardo and Schall, Reference Vardo and Schall2007). Increased stress from captivity could result in a lower blood glucose level in lizards as observed in our non-infected controls. Wild caught, non-infected lizards have mean blood glucose concentration upon capture of 270mg/dl (Dunlap and Schall, Reference Dunlap and Schall1995), but in our study, this was 25% lower (206 mg/dl) for non-infected control lizards after 80 days of captivity. Wild caught infected lizards (with no information on actual diversity of the infections) had a mean glucose level of 243 mg/dl, close to that seen in the experimental infections carrying 3 or >3 clones, but higher than those with 1 or 2 clones (Dunlap and Schall, Reference Dunlap and Schall1995). This suggests that, despite the stress of captivity reducing blood glucose in these lizards, diverse infections may actively manipulate the host into releasing more glucose into the peripheral blood. Plasmodium parasites feed on host glucose, so any increases in blood glucose concentration could positively affect the parasite's reproductive rate and transmission success (Mehta et al. Reference Mehta, Sonawat and Sharma2005). Additionally, lizards naturally maintain high blood glucose levels (Khanna and Kumar, Reference Khanna and Kumar1975; Chandavar and Naik, Reference Chandavar and Naik2004) suggesting that higher glucose concentrations in infections made up of 3 or more clones may not be harmful, rather, these infections may incur less stress on the lizard host. Thus infections containing 3 or more clones may be beneficial to both parasite and lizard.
An intriguing result from this experiment was the increase in rapid blood clotting among infected lizards with highest clonal number (clotting occurred within 30 sec of being drawn into a heparinized capilliary tube). Blood rapidly clotted for 5% of the control non-infected lizards clotted, and 17% of infections with 1, 2, or 3 clones (with no significant difference). However, 44% of infections carrying >3 clones rapidly clotted within the heparinized capillary tube. The mechanism involved in the increased clotting of infected blood and how this mechanism differs between infections with 1–2 versus 3 or more clones is not presently known. Humans infected with P. falciparum experience reduced numbers of platelets in the blood, but without the expected excessive bleeding response, and perhaps even an increase in clotting during injury (Ghosh and Shetty, Reference Ghosh and Shetty2008). For P. mexicanum infections, thrombocytes (non-mammalian, nucleated platelets) within the lizard's blood stream could increase in numbers or activity for genetically complex infections. At present, it is unclear if the quickness of clotting is driven by host effects or direct action of the parasite. Interpretation of this finding as beneficial or harmful for the lizard is problematic, because rapid blood clotting may benefit the lizard after injury, but if clots form within the circulation, infections made up of 3 or more clones could result in major injury to organs.
A final issue for this and other studies using blood transfer to initiate infection is the possible role of the initial liver stages that result from natural transmission by vector bites and transfer of sporozoites. Although the direct effect of these cells is likely to be very low, it is possible that the immune response activated by sporozoites could be costly. This possibility must remain an open question for studies on Plasmodium virulence, but is unlikely to be resolved for P. mexicanum because of the difficulty in raising sufficient infected vectors (Fialho and Schall, Reference Fialho and Schall1995).
Overall, our findings suggest that low diversity infections are harmful for the lizard host; such infections are the most common at the site (Vardo and Schall, Reference Vardo and Schall2007). However, lizards with complex infections are similar to non-infected animals in blood haemoglobin, and have the highest concentration of blood glucose and the highest rate of blood clotting. Thus, a high genetic diversity of parasites within an infection could be viewed as beneficial to the lizard host, a striking finding. The results for P. mexicanum, a parasite of lizards, P. falciparum of humans, and P. chabaudi in laboratory mice, all demonstrate that parasite clonal diversity is important for the consequences of infection, but the biology is more complex than assumed in standard theoretical models of parasite virulence (Bull, Reference Bull1994; Ewald, Reference Ewald1994, Reference Ewald2004; Nowak and May, Reference Nowak and May1994; May and Nowak, Reference May and Nowak1995; van Baalen and Sabelis Reference Van Baalen and Sabelis1995; Frank, Reference Frank1996; Read and Taylor, Reference Read and Taylor2001; Schall, Reference Schall, Lewis, Campbell and Sukhdeo2002).
We thank the staff of the Hopland Research and Extension Center for offering their usual warm welcome and logistical support, especially C. Vaughn, R. Keefer, and R. Timm. B. Blumberg (2005), S. Reece (2006), M. Robinson (2006) and N. Zalik (2006s) assisted in catching and caring for lizards. The research was funded by grants from the USA National Science Foundation and the Vermont Genetics Network to J. J. S.