Introduction
The global epidemic of obesity and its comorbidities, including cardiovascular disease, insulin resistance and type 2 diabetes mellitus (T2DM) is a growing threat to populations across the world, with the number of diabetic adults doubling over the last 30 years.Reference Danaei, Finucane and Lu1 This alarming increase in metabolic disease has been partly attributed to increased consumption of added sugars.Reference Johnson, Appel and Brands2 Large-scale epidemiological studies have associated consumption of one or more sugar sweetened beverage (SSB) per day with an increased risk of developing high blood pressure and metabolic syndrome.Reference Fung, Malik and Rexrode3, Reference Dhingra, Sullivan and Jacques4 The association between sugar intake, weight gain and the risk of non-communicable diseases has resulted in the American Heart Association advising that daily added sugar intake be limited to 100 and 150 calories for women and men respectively,Reference Johnson, Appel and Brands2 equivalent to ~5–10% of daily energy intake. More recently, the World Health Organization recommended that guidelines for added sugar intake be reduced to 25 g per day, less than a single can of SSB.Reference Te Morenga, Mallard and Mann5 In western countries, however, average daily consumption of added sugars substantially exceeds these recommendations; estimated at 15% of total energy intake in the United States, United Kingdom and Australia.Reference Johnson, Appel and Brands2, Reference Willett and Ludwig6, Reference McLennan and Podger7
Sucrose (SUC) (cane/table sugar) is the most commonly used sweetener worldwide, however the use of high fructose corn syrup-55 (HFCS-55), primarily used in the United States, is becoming more widespread. Following ingestion, SUC (disaccharide of glucose and fructose) is hydrolyzed into its monosaccharides before absorption, whereas the free glucose and fructose in HFCS-55 (55% fructose, 42% glucose) can be absorbed directly into the circulation.Reference Tappy and Lê8 The absorption of free fructose is limited by the physiological capacity of the GLUT transporters, which can result in malabsorption if consumed in large amounts.Reference Douard and Ferraris9 The metabolic effects of SUC and HFCS-55 are thought to more closely mimic those of fructose consumption alone; hence the adverse metabolic outcomes associated with excess sugar consumption have been attributed to the fructose component. Approximately 50–70% of fructose is taken up by the liver on first passReference Bizeau and Pagliassotti10 where, unlike glucose, its metabolism is insulin independent and thus virtually unregulated. Furthermore, hepatic fructose metabolism induces a transient insulin resistant state, impacting on glucose metabolism when glucose and fructose are metabolized simultaneously.Reference Wei, Wang, Topczewski and Pagliassotti11 Although the hepatic cellular metabolism of glucose and fructose is similar, the metabolic outcomes associated with their oxidation differ, resulting in a cellular metabolic tussle between energy storage and lipid synthesis.Reference Lustig12, Reference Regnault, Gentili, Sarr, Toop and Sloboda13 Despite SUC and HFCS-55 containing a similar concentration of fructose, it has been suggested that the ‘free’ fructose in HFCS-55 poses a greater risk to the progression of T2DM than bound fructose, as is found in SUC.Reference Bray, Nielsen and Popkin14
It has long been established that the nutritional environment experienced during critical windows of development in fetal and early postnatal life, can result in permanent programming of physiological systems.Reference Alfaradhi and Ozanne15, Reference McMillen and Robinson16 Consumption of SSBs before pregnancy has been associated with increased risk of developing gestational diabetes,Reference Chen, Hu, Yeung, Willett and Zhang17 which is known to be a risk factor for obesity and T2DM in the offspring.Reference Nelson, Matthews and Poston18 Soft drinks and fruit juices are a substantial contributor to total energy consumption during pregnancy,Reference Siega-Riz, Bodnar and Savitz19 and SSB consumption has been linked to an increased risk of preeclampsia,Reference Borgen, Aamodt and Harsem20 preterm deliveryReference Englund-Ögge, Brantsæter and Haugen21 and increased birth weight.Reference Phelan, Hart and Phipps22 Despite these risk factors, there is limited information on the effect of maternal intake of sweeteners such as SUC and HFCS-55, at concentrations equivalent to those in typical western diets, on maternal and offspring health.
Recently, there have been attempts to determine the specific effects of excess maternal fructose consumption on maternal and offspring health. These studies, largely carried out in rodents, have implicated consumption of fructose in isolation in the development of metabolic dysfunction in the mother, including increased lipogenesis and hepatic insulin resistance, and adverse effects on placental and fetal development.Reference Regnault, Gentili, Sarr, Toop and Sloboda13, Reference Goran, Dumke and Bouret23–Reference Rodríguez, Panadero and Roglans28 The metabolic effects of fructose in combination with glucose are different to fructose alone, however few studies have investigated the effects of SUC or HFCS-55 during pregnancy on maternal health and the health of the developing offspring.Reference Samuelsson, Matthews, Jansen, Taylor and Poston29–Reference D’Alessandro, Oliva, Fortino and Chicco33 Those that have differ significantly in study design, and concentration and delivery of the sweetener in question, in addition to maternal and fetal/newborn outcomes reported. To our knowledge, no studies have directly compared maternal consumption of SUC and HFCS-55 at physiologically relevant doses, therefore, the aim of this study was to determine the effect of these sweeteners, during pregnancy and lactation on metabolic outcomes in the dam and newborn offspring. We hypothesized that maternal consumption of either SUC or HFCS-55 before and during pregnancy and lactation, would be associated with reduced glucose tolerance, altered lipid metabolism and increased fat deposition in the mother, in addition to increased birth weight of the newborn offspring. We further hypothesized that these effects would be sugar-specific, and would be worse in dams consuming HFCS-55 compared with dams consuming SUC.
Method
Animals and experimental design
All procedures were approved by the SA Pathology Animal Ethics Committee for the University of South Australia. A total of 61 eight-week-old female virgin Albino Wistar rats and 15 eight-to-twelve-week-old male rats were used in this study. Rats were obtained from Laboratory Animal Services and housed at the Reid Animal Facility at the University of South Australia in individually ventilated cages under a 12 h light-dark cycle at a room temperature of 22°C. All rats were given ad libitum access to standard laboratory rat chow (Specialty Feeds, Glen Forrest, WA, Australia; 14 kJ/g) and water throughout the acclimatization and experimental periods. Upon arrival, rats were acclimatized for 1 week then randomly assigned to either control (CONT; n=25), SUC (n=19) or HFCS (n=17) treatment groups.
Dams in SUC and HFCS groups were given ad libitum access to a 10% w/v sugar-sweetened beverage, sweetened with either SUC (CSR, Australia) or high fructose corn syrup-55 (Natures Flavors, USA), respectively, throughout the 10-week study period. A 10% w/v sugar beverage was chosen, as this is approximately equivalent to the calories per ml in commercially available SSBs (1.8 kJ/ml). SUC and HFCS-55 beverages were made fresh in the animal facility using autoclaved tap water, and were replaced on average every 48 h or when required. Food, water and SUC and HFCS-55 beverage consumption were monitored weekly for all dams. Dams commenced the diet 4 weeks before mating, and remained on their respective diets throughout the pregnancy (3 weeks) and lactation periods (3 weeks; 10 weeks of sugar consumption in total as summarized in Fig. 1).

Fig. 1 Schematic representation of study design, summarizing control (CONT; black bar), sucrose (SUC; white bar) and high fructose corn syrup (HFCS)-55 (HFCS; grey bar) intervention period, intraperitoneal glucose tolerance test (IPGTT), postmortem tissue collection, and mating and offspring birth.
Mating and postmortem
After a minimum of 4 weeks on their respective diets all dams were mated. Pro-estrus in the dams was determined using a vaginal impedance reader (Model MK-11; Muromachi Kikai Co., Osaka, Japan), with an impedance reading >3 kΩ as positive for pro-estrus. Between one to two females were caged with one male for mating for up to 48 h. Mating was confirmed by the presence of sperm in a vaginal smear, and was designated as gestation day 0. Offspring were born naturally at 22.4±0.07 days gestation. Within 24 h of birth, all pups per litter were counted and weighed. Two male and two female pups per litter (where possible) were killed by decapitation (CONT, n=16 litters, male n=28, female n=26; SUC, n=15 litters, male n=24, female n=22; HFCS, n=11 litters, male n=16, female=18), and blood samples were collected, pooled for sex. Key internal organs including heart, liver, adrenals, kidney and brain were dissected and weighed. There were no dissectible fat deposits in the newborn pups in any treatment group. The remaining offspring per litter were used as part of an ongoing study.
Three weeks post-partum, after the remaining pups were weaned, dams were culled with an overdose of CO2. Blood was collected via cardiac puncture and tissues were dissected, weighed and collected. All blood samples were centrifuged at 4°C for 10 min at 3000 rpm and plasma was stored at −80°C.
Intraperitoneal glucose tolerance test (IPGTT)
IPGTT were performed on a subset of dams randomly selected from each treatment group at three time points during the experimental period (CONT, n=17–20; SUC, n=12–16; HFCS, n=13–15 dams for each of the three-time points): immediately before commencing the diet (0 week IPGTT), following 4 weeks on the respective diets and before mating (end of the pre-pregnancy period; 4 week IPGTT) and at 3 weeks post-partum (end of lactation; 10-week IPGTT).
Briefly, animals were fasted for at least 15 h before the IPGTT. Plasma glucose samples were determined using a hand-held glucometer (FreeStyle Glucometer; Abbott Diabetes Care) from a drop of blood from the tail vein (~60 µl). A baseline blood glucose concentration was determined 10–15 min before commencing the IPGTT (baseline sample). A 50% glucose solution (2 g/kg; Phebra, NSW) was then administered via intraperitoneal injection. Tail vein blood samples were collected immediately before (0 min), and at 5, 10, 15, 30, 60 and 120 min after glucose administration for the determination of plasma glucose concentration. Area under the curve (AUC) was calculated using the trapezoidal rule.
Determination of plasma hormone and metabolite concentrations
All hormone and metabolite analyses described below were performed on a representative subset of dams across each of the three treatment groups (CONT, n=11; SUC, n=10; HFCS, n=11 dams). Plasma insulin, glucose and non-esterified fatty acid (NEFA) concentrations only were measured from a subset of litters in male and female neonate samples (CONT, male n=7, female n=8; SUC, male n=6, female n=5; HFCS, male n=7, female=7).
Plasma glucose, alanine amino transferase (ALT), uric acid, total cholesterol, high-density lipoprotein (HDL) cholesterol (Thermo Electron, Pittsburgh, PA, USA), and NEFA (WAKO Pure Chemical Industries Ltd., Osaka, Japan) were determined using a Konelab 20X (Thermoscientific, Vantaa, Finland). Plasma insulin and leptin concentrations were measured by immunoassay with rat specific insulin (ALPCO Diagnostics, Salem, NH, USA) and leptin (Crystal Chem, Downers Grove, IL, USA) kits according to the manufacturers specifications. Inter- and intra-assay coefficients of variation were <1% for all assays. Low-density lipoprotein (LDL) cholesterol was calculated as the difference between total and HDL cholesterol concentrations.
Statistical analysis
All data are presented as mean±s.e.m. All data were assessed for normality using the Shapiro–Wilk test. The effect of CONT, SUC or HFCS consumption on maternal IPGTT AUC, body weight at postmortem, organ weights, plasma metabolite and hormone concentrations were determined using a one-way ANOVA with a Bonferroni post-hoc. The effect of CONT, SUC or HFCS consumption on maternal weight throughout the study period, maternal glucose concentrations during the IPGTT, and average daily food and fluid consumption throughout the study period were determined by repeated-measures ANOVA. The effect of maternal CONT, SUC or HFCS consumption on pup body weight and tissue weights were determined using a nested two-way ANOVA, while litter size, pup sex ratio, and plasma glucose, insulin and NEFA concentrations were determined by a two-way ANOVA, both with maternal dietary treatment and sex as factors with a Bonferroni post-hoc. All statistical analyses were performed using Stata12 (StataCorp LP, USA). The relationship between fat mass and plasma leptin concentrations was determined across the three treatment groups using a bivariate correlation (SPSS Inc., Chicago, IL, USA). A probability of <5% (P<0.05) was considered statistically significant in all analyses.
Results
Effect of SUC and HFCS consumption on dam chow and fluid intake
Average daily energy intake was significantly higher in SUC and HFCS dams throughout the study period, however average daily energy intake of SUC dams converged with CONT dams during the last 2 weeks of lactation (Fig. 2a). The increased energy intake was a result of preferential intake of the SSB over the chow, since average daily chow consumption was reduced in both the SUC and HFCS dams compared with CONT (Fig. 2b). The decrease in average chow consumption resulted in a moderate decrease in protein intake during the pregnancy and lactation periods, such that SUC dams consumed between 25 and 30% less protein than CONT, while HFCS dams consumed between 14 and 21% less protein than CONT dams during pregnancy and lactation. Average daily fluid intake (SSB and/or water) was significantly increased in SUC and HFCS dams compared with CONT, however there was no significant difference in daily fluid consumption between SUC and HFCS groups (Fig. 2c).

Fig. 2 The effect of control (CONT), sucrose (SUC) and high fructose corn syrup (HFCS) consumption before (4 weeks) and during pregnancy (3 weeks) and lactation (3 weeks) on maternal (a) average daily energy intake, (b) average daily chow intake, and (c) average daily fluid intake comprised of SSB and/or water throughout the study period. CONT is represented by circles, SUC by squares and HFCS by triangles. *Denotes difference between SUC and HFCS group relative to CONT at each time point (P<0.001); #SUC, HFCS and CONT groups different to each other at each time point (P<0.005); ^HFCS group different to SUC and CONT groups at each time point (P<0.001).
Effect of SUC and HFCS consumption on dam weight and body composition
There was no difference in maternal weight across the three treatment groups before commencing the study (CONT, 221.8±3.5; SUC, 220.9±4.3; HFCS, 216.3±5.4 g), throughout the study (data not shown), or at postmortem (CONT, 308.6±4.9; SUC, 317.0±4.8; HFCS, 309.8±7.6 g). Furthermore, there was no effect of treatment on percentage weight gain (CONT, 28.0±1.0; SUC, 30.7±0.9; HFCS, 30.0±1.1% total weight gain) during the study period.
There was no effect of SUC or HFCS consumption on maternal relative liver, adrenal, kidney or pancreas weights when compared with CONT (Table 1). Maternal relative heart weight was decreased in the SUC dams compared with HFCS dams only (Table 1), however there was no difference in absolute heart weight (data not shown). Maternal SUC consumption, but not HFCS consumption, was associated with a significant increase in the relative visceral, subcutaneous (Table 1) and total fat mass (Fig. 3), when compared with the CONT group.

Fig. 3 Effect of sucrose (SUC) and high fructose corn syrup (HFCS) on maternal relative total adipose tissue weight. Data are presented as mean±s.e.m., control (CONT) black bar, SUC white bar and HFCS grey bar. Different letters denote mean values that were significantly different (P<0.05).
Table 1 Effect of maternal sucrose (SUC) or high fructose corn syrup (HFCS) consumption on relative liver, heart, adrenal, kidney (left and right), pancreas, and visceral and subcutaneous adipose tissue weight expressed a percentage of body weight

CONT, control.
Data are presented as mean±s.e.m.
a,bDifferent superscript letter denote mean values that were significantly different from each other (P<0.05).
Effect of SUC and HFCS consumption on maternal glucose tolerance
There was no difference in maternal fasting blood glucose concentration between any of the treatment groups, either before commencing the dietary treatments (0 week), immediately before mating (4 weeks after the start of the dietary treatments) or 3 weeks post-partum (10 weeks after the start of the dietary treatments; Table 2). There was no effect of treatment on IPGTT plasma glucose concentrations or AUC at the beginning of the study (0 weeks) or at the end of lactation (10 weeks) (Table 2). However, there was an effect of treatment on IPGTT plasma glucose concentrations immediately before pregnancy (week 4; 10 min peak glucose concentration, Table 2), and a trend towards increased AUC in the SUC dams compared with both HFCS and CONT dams (P=0.057).
Table 2 Effect of maternal sucrose (SUC) or high fructose corn syrup (HFCS) consumption on intraperitoneal glucose tolerance test (IPGTT) blood glucose concentrations and area under the curve (AUC) at the start of the study, before pregnancy and before postmortem
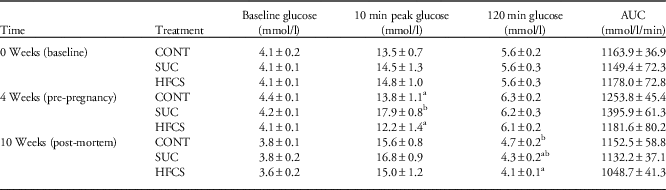
CONT, control.
Data are presented as mean±s.e.m.
a,bDifferent superscript letter denote mean values that were significantly different across the three treatment groups at the same sampling period (P<0.05).
Effect of SUC and HFCS consumption on maternal plasma hormone and metabolite concentrations
At postmortem, there was no difference in plasma glucose concentrations between the three treatment groups (Table 3), however plasma insulin concentrations were significantly higher in HFCS dams compared with SUC dams (Table 3) with no statistical difference between CONT and HFCS dams (P=0.06 as determined by Bonferroni post-hoc analysis). There was no difference in maternal plasma ALT or uric acid between the treatment groups (Table 3).
Table 3 Effect of maternal sucrose (SUC) or high fructose corn syrup (HFCS) consumption on non-fasting plasma glucose, insulin, leptin, alanine amino transferase (ALT), uric acid, triglycerides, non-esterified fatty acids (NEFA), total cholesterol, high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol concentrations
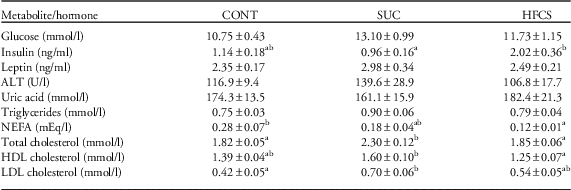
CONT, control.
Data are presented as mean±s.e.m.
a,bDifferent superscript letter denote mean values that were significantly different across the three treatment groups (P<0.05).
There were no significant differences in plasma triglyceride concentrations between treatment groups (P=0.09; Table 3). Dams in the HFCS group had significantly lower plasma NEFA concentrations compared with CONT dams (Table 3). Total cholesterol was higher in the SUC group compared with both CONT and HFCS groups (Table 3). LDL and HDL cholesterol were higher in SUC dams than in CONT or HFCS, respectively (Table 3).
Maternal plasma leptin concentrations were not significantly different between treatment groups despite the increase in fat mass in SUC dams (Table 3). There was, however, a positive correlation between relative total adipose tissue weight and plasma leptin concentrations when all data were combined (y=27.99x+0.94; r 2=0.37, P=0.001).
Effect of maternal SUC or HFCS consumption on the neonate
There was no effect of maternal SUC or HFCS consumption before and during pregnancy on average litter size (CONT, 13.6±0.5; SUC, 12.6±0.8; HFCS, 12.4±0.4 pups per litter) or the ratio of male:female pups per litter (CONT, 1.2±0.2; SUC, 1.2±0.1; HFCS, 1.2±0.1). There was also no effect of maternal dietary treatment on pup weight within 24 h of birth, however males were significantly heavier than females independent of maternal treatment (Table 4). Relative liver weight was significantly lower in pups of HFCS dams independent of sex (Table 4). Relative adrenal weight was higher in pups born to SUC dams compared with both the CONT and HFCS groups (Table 4), while relative heart weight was higher in pups born to SUC dams compared with HFCS only (Table 4). There were no other differences in total or relative organ weights between groups.
Table 4 Effect of maternal SUC and HFCS consumption before and during pregnancy on offspring birth weight (separated by sex), liver, heart, adrenal, kidney (left and right combined) and brain weights expressed as a percentage of body weight (pooled for sex)
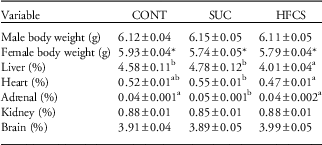
SUC, sucrose; HFCS, high fructose corn syrup; CONT, control.
There was no effect of offspring sex on organ weight, therefore data presented have been pooled for sex. Data are presented as mean±s.e.m.
a,bDifferent superscript letter denote mean values that were significantly different across the three treatment groups (P<0.05).
*Denotes mean body weight significantly different between male and female pups independent of treatment group (P<0.05).
There was no effect of maternal SUC or HFCS consumption before and during pregnancy on plasma glucose or insulin concentration in the pups within 24 h of birth (Figs 4a and 4b). Plasma NEFA concentrations, however, were lower in pups born to HFCS dams compared with CONT (Fig. 4c), and there was no difference between pups born to SUC dams when compared with CONT (P=0.15 as determined by Bonferroni post-hoc analysis).

Fig. 4 The effect of maternal consumption of sucrose (SUC) or high fructose corn syrup (HFCS) before and during pregnancy on neonate (a) plasma glucose, (b) plasma insulin and (c) plasma non-esterified fatty acid (NEFA) concentrations. There was no effect of sex therefore data were pooled for treatment group. Data are presented as mean±s.e.m., control (CONT) black bar, SUC white bar and HFCS grey bar. Different letters denote mean values that were significantly different across the three treatment groups (P<0.05).
Discussion
There is clear evidence that exposure to poor quality western diets, which are typically high in both fat and sugar, during pregnancy, is associated with an increased propensity to obesity and T2DM in both the mother and her offspring.Reference Alfaradhi and Ozanne15, Reference Taylor and Poston34 We report that SUC consumption before and during pregnancy and lactation is associated with altered metabolic and lipid homeostasis in the dam, while HFCS-55 consumption during the same period impacts on neonatal liver growth and circulating NEFA concentrations.
We provided dams with ad libitum access to standard laboratory chow, in addition to a 10%w/v SUC or HFCS-55 beverage, which is comparable to the sugar content of commercially available SSBs. Daily energy intake was increased in both SUC and HFCS dams, however the increase in energy intake from SUC consumption was accompanied by a compensatory decrease in chow consumption relative to controls,Reference Light, Tsanzi, Gigliotti, Morgan and Tou35–Reference Bergheim, Weber and Vos37 an effect not seen in the HFCS group. Fructose consumption has been shown to reduce satiety signals when compared with glucose consumption.Reference Lindqvist, Baelemans and Erlanson-Albertsson38, Reference Page, Chan and Arora39 As HFCS-55 contains 5% more fructose than SUC, we speculate that dams consuming HFCS-55 may have had an altered satiety response in this study. Interestingly, neither sugar resulted in a significant increase in body weight, which was surprising given the increase in daily energy intake of the two groups relative to controls. Daily activity measurements were not taken as part of this study, therefore we cannot comment on the effect of SUC or HFCS-55 on daily energy expenditure, however consumption of equimolar glucose:fructose has been shown to have no effect on activity in adult rats.Reference Sheludiakova, Rooney and Boakes36
We found that maternal SUC, but not HFCS-55 consumption, resulted in increased visceral and subcutaneous fat masses despite no change in body weight. Light et al. Reference Light, Tsanzi, Gigliotti, Morgan and Tou35 showed that HFCS-55 consumption increased visceral fat mass in non-pregnant adult rats to a greater extent than SUC consumption. The differences reported in this study may be due to the physiological adaptations of pregnancy and lactation on adipose tissue metabolism, storage and mobilisation.Reference Butte40 Acute SUC ingestion has been demonstrated to increase hepatic de novo lipogenesis via increased glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, acetyl CoA carboxylase and malic enzyme activities more effectively than equimolar fructose:glucose ingestion.Reference Lee, Szepesi and Hansen41, Reference Michaelis, Nace and Szepesi42 We speculate that the observed increase in fat mass in the SUC dams is due to SUC induced adipocyte hypertrophy,Reference Soria, D’Alessandro and Lombardo43 however adipocyte size and changes in fat mass throughout the study period were not measured, and these data are required to confirm this suggestion. In addition, it is possible that the difference in fat mass resulting from SUC consumption may have been due to differences in fructose absorption following SUC v. HFCS-55 consumption. It is known that fructose is well absorbed when consumed in combination with higher or equal amounts of glucose, such as in SUC.Reference Douard and Ferraris9 In HFCS-55, free fructose concentrations exceed those of glucose, which may result in fructose malabsorption, as absorption in the small intestine is quantitatively limited by the physiological capacity of the GLUT transporters.Reference Douard and Ferraris9 Therefore, SUC dams may have had increased hepatic metabolism of fructose, and thus, increased de novo lipogenesis compared with HFCS dams, resulting in increased fat mass. Increases in adipose tissue mass, specifically increased visceral fat, is associated with local and systemic inflammation, which has been linked to insulin resistance in tissues such as the liver and adipose tissue.Reference Shoelson, Lee and Goldfine44
Fructose-containing sugars have been implicated in promoting an atherogenic lipid profile, and thus contributing to the development of obesity, cardiovascular disease and T2DM.Reference Tappy and Lê8, Reference Wei, Wang, Topczewski and Pagliassotti11 Total plasma cholesterol and LDL cholesterol concentrations were significantly increased in dams consuming SUC compared with controls. SUC consumption has been shown to increase hepatic lipase activity and very low-density lipoprotein output,Reference Cahova, Dankova, Palenickova, Papackova and Kazdova45 potentially contributing to increased LDL cholesterol production. Interestingly, HFCS-55 had no effect on plasma cholesterol concentrations in the dam, which was surprising given the known effect of fructose on PPARα activity and apoB expression.Reference Su, Tsai and Xu46 The observed difference between SUC and HFCS-55 groups, however, is consistent with increased fructose absorption in the dams consuming SUC, as described earlier.
Consumption of HFCS-55 was associated with a decrease in plasma NEFA compared with controls. Fructose consumption is associated with elevated ectopic fat depositionReference Lustig47 due to an increase in hepatic carbohydrate oxidation and fatty acid synthesis.Reference Mukai, Kumazawa and Sato26, Reference Rodríguez, Panadero and Roglans28, Reference Crescenzo, Bianco and Coppola48 Furthermore, we observed a similar decrease in plasma NEFA concentration in the newborn offspring of dams that consumed HFCS-55.
HFCS-55 consumption resulted in a significant increase in plasma insulin concentration compared with SUC, however it did not reach statistical significance compared with controls. There was no effect of SUC or HFCS-55 consumption on maternal fasting blood glucose or glucose tolerance before pregnancy or at the end of lactation. Fructose consumption induces a transient insulin resistant state in the liver due to the direct effect of fructose on IRS-1 serine phosphorylation,Reference Wei, Wang, Topczewski and Pagliassotti11 resulting in a lack of suppression of gluconeogenic pathways via FOXO-1 activity, increasing plasma glucose concentration.Reference Lustig12 It is possible that the observed increase in plasma insulin in HFCS-55 dams is indicative of fructose-induced hepatic insulin resistance, which would involve a compensatory increase in insulin secretion to maintain euglycaemia, despite increased gluconeogenesis.
Few studies have investigated the effect of exposure to SUC during pregnancy on the fetus or newborn offspring, and those that have produced conflicting results,Reference Samuelsson, Matthews, Jansen, Taylor and Poston29–Reference D’Alessandro, Oliva, Fortino and Chicco33 and to our knowledge none have determined the effects of commercially available HFCS-55 on the newborn offspring. We identified a sugar-specific effect of exposure to SUC and HFCS-55 on neonate adrenal and liver weights respectively. Maternal SUC consumption resulted in increased relative adrenal weight in the offspring at birth, which may indicate an effect of SUC on programming of the hypothalamic–pituitary–adrenal stress axis.Reference McMillen, MacLaughlin and Muhlhausler49 Maternal HFCS-55 consumption, on the other hand, resulted in a significant decrease in relative liver weight in offspring at birth. Maternal fructose consumption has been shown to decrease placental weight, in addition to relative liver weight of offspring at postnatal day 10.Reference Vickers, Clayton, Yap and Sloboda25 Before developing its metabolic profile (at ~E15.5),Reference Guo, Zhang and Huang50 the developing liver is seeded by the migration of haematopoietic cells from the yolk sack, aorta–gonadal–mesonephros region, and possibly the placenta.Reference Kinoshita and Miyajima51, Reference Mikkola and Orkin52 Given the overlap between liver and placental development, it is possible that maternal fructose consumption may affect liver haematopoietic stem cell migration, expansion and maturation via the placenta. These changes may give rise to a sugar-specific decrease in liver weight at birth, which may have long-term metabolic and immune effects in the offspring. The long-term programming effects of maternal SUC and HFCS-55 consumption during pregnancy on the development of obesity and T2DM in the offspring from this cohort are part of an ongoing study.
This is the first study to investigate the effects of SUC and HFCS-55, at concentrations comparable to those found in commercially available beverages, on maternal health during pregnancy, and the first to directly compare the effects of SUC and HFCS-55 on the health of her offspring at birth. Consumption of SUC appears to be associated with metabolic dysfunction in the mother, whereas HFCS-55 consumption appears to be associated with altered hepatic growth and plasma NEFA concentration in the newborn offspring, which may influence postnatal disease risk. The results of this study suggest that consumption of added sugar in the form of SUC or HFCS-55 should be limited during pregnancy to reduce any adverse effects on the mother or the long-term metabolic health of her offspring. In addition, the current study emphasizes the importance of determining the effects of SUC and HFCS-55 independently as the results clearly demonstrate that despite containing the same monosaccharides, the relative concentration and composition of the sugars result in significant differences in the dam and her offspring.
Acknowledgments
The authors wish to thank the staff at the Reid Animal Facility for their assistance during the animal study.
Financial Support
BSM is funded by a Career Development Award APP_1004211 from the National Health and Medical Research Council of Australia. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of Albino Wistar rats and has been approved by the SA Pathology Animal Ethics Committee for the University of South Australia.








