Introduction
Many hymenopterans exhibit parental care, with females building nests and providing food for the offspring (Trumbo, Reference Trumbo1996; O'Neill, Reference O'Neill2001; Michener, Reference Michener2007). Although females of solitary species do not help each other in these tasks, they often prefer to nest close to conspecifics, establishing large aggregations of nests (Inouye, Reference Inouye2000; Michener, Reference Michener2007; Černá et al., Reference Černá, Zemenová, Macháčková, Kolínová and Straka2013). Nesting in an aggregation can be costly because a returning female must spend some time looking for her own nest among many similar nest entrances, and mistakenly entering another female's nest can lead to a potentially harmful fight with the nest's owner (Guédot et al., Reference Guédot, Bosch, James and Kemp2006), females can also suffer usurpation of their nests (Kim, Reference Kim1997; Bosch & Vicens, Reference Bosch and Vicens2006). Large aggregation can also attract parasites (Filella et al., Reference Filella, Bosch, Llusià, Seco and Peñuelas2011). Several non-exclusive causes of aggregation formation have been proposed, such as philopatry (Steffan-Dewenter & Schiele, Reference Steffan-Dewenter and Schiele2004), a heterogeneous distribution of resources (Potts & Willmer, Reference Potts and Willmer1997) or preferences for a neighborhood of conspecifics for a more effective joint defense against parasites (Hager & Kurczewski, Reference Hager and Kurczewski1985).
Parasites attacking nests of solitary Hymenoptera often prey upon more than one host species. If the presence of more host individuals in an aggregation reduces the risk of parasitism, females should prefer nesting in aggregations of not only the same species but also of other species with which they share parasites. If the mere presence of many host individuals has a confusing effect on parasites attempting to attack the nest, as Hager & Kurczewski (Reference Hager and Kurczewski1985) suggested, then even species that do not share common parasites should gain from nesting in heterospecific aggregations.
The question of whether solitary females are attracted to or deterred from heterospecific nesting aggregations also has an anthropogenic aspect. In addition to the concerns whether managed honeybee colonies can ensure stable pollination and food production (Aizen & Harder, Reference Aizen and Harder2009; Smith et al., Reference Smith, Loh, Rostal, Zambrana-Torrelio, Mendiola and Daszak2013; Potts et al., Reference Potts, Roberts, Dean, Marris, Brown, Jones, Neumann and Settele2015), interest has been directed also towards other pollinators, and the breeding of solitary bees nesting gregariously, such as Osmia spp., for pollination purposes has become increasingly popular (Vicens & Bosch, Reference Vicens and Bosch2000; Bosch et al., Reference Bosch, Kemp and Trostle2006; Artz et al., Reference Artz, Allan, Wardell and Pitts-Singer2013, Reference Artz, Allan, Wardell and Pitts-Singer2014; Pitts-Singer, Reference Pitts-Singer2013). Wild solitary insects have more and more opportunities to encounter large managed aggregations of other solitary species and to potentially nest in them as the nesting material provided is typically in excess relative to the number of bees in the breeding aggregations. Understanding the relationship between the existence of large bee aggregations and the decisions of other insects about whether to nest in these locations is important for estimating the effect of such breeding aggregations on wild populations of insects.
We addressed the question of how the existence of an aggregation of one species of solitary bee affects the nesting choices of other solitary Aculeata females and whether there is a relationship between the existence of aggregations and the risk of parasitism. We created aggregations of red mason bees (Osmia bicornis L.), a species that is widespread in Europe in the wild and that is commercially reared for pollination purposes, and compared the numbers of nests built by wild Aculeata in trap-nests with and without red mason bee aggregations. We also compared nest parasitism rates to test whether nesting preferences might relate to minimizing parasitism risks.
Materials and methods
The field component of this experiment was conducted in 2014 and 2015. In the spring of each year, 30 trap-nests were prepared for bees. Each trap-nest consisted of a polyvinyl chloride (PVC) tube with nesting material (a bundle of ca. 150 reed straws). Each reed straw was 25 cm long and had a node in the middle. Each trap-nest had wire mesh covering both ends to prevent bee nests from being destroyed by rodents or birds. We added 50 male and 50 female red mason bee cocoons to 15 trap-nests to establish bee aggregations. The cocoons came from a breeding colony that has been maintained at the Institute of Environmental Sciences (Jagiellonian University, Kraków) since 2004. Each cocoon was sexed based on the head morphology of the bee inside after cutting a spyhole in the top of each cocoon (Seidelmann et al., Reference Seidelmann, Ulbrich and Mielenz2010). Cocoons were placed inside smaller PVC tubes placed between the reed straws. The small tubes in the remaining 15 trap-nests were left empty. All trap-nests were placed in meadows partly covered with bushes near the Institute of Environmental Sciences in Kraków on 5–6 May in 2014 and 13–14 April in 2015. The trap-nests were spaced at least 150 m from each other and in the vicinity of blooming trees or bushes to ensure a food base for the emerging red mason bees and other insects establishing nests in the trap-nests. The trap-nests were collected from the field in the first half of July after the end of the flight period of red mason bees and were overwintered outdoors, sheltered from rain.
In February the trap-nests were brought to the laboratory and opened. Reed straws with nests established inside were collected and numbered. The entry to each nest was secured with a piece of transparent rubber pipe closed with cotton wool. Then, the nests were returned to overwintering conditions, and from the beginning of spring (end of March 2015 and beginning of April 2016) they were incubated in the laboratory at room temperature. Emerging insects were collected from the rubber pipes and prepared for further identification. Nests from trap-nests with red mason bee aggregations, from which red mason bees emerged, were immediately cut open and examined for parasites, and cocoons from which red mason bees did not emerge were returned to the breeding colony at the Institute of Environmental Sciences. After the insects ceased emerging, all nests were opened and checked for parasites or dead insects remaining in the nests. The nesting insects and the parasites found in the nests were identified to the lowest possible taxonomic level. If all insects in a nest failed to complete their development or they had emerged before the trap-nest was collected from the field, the nest was classified as belonging to O. bicornis or other species based on nest architecture.
For each trap-nest, we counted the number of red mason bee nests, nests of other species, and nests infected with parasites, and we calculated the proportion of parasitized nests.
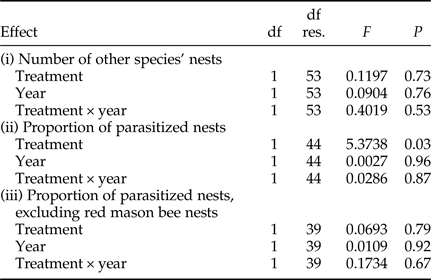
Statistical analyses were performed in R ver. 3.2.3. (R Core Team, 2015) using the package ARTool (Kay & Wobbrock, Reference Kay and Wobbrock2016). Because our data did not meet the assumption of a normal distribution, we performed an aligned rank transform (ART) modification of a two-way analysis of variance (ANOVA). This non-parametric test is equivalent to a classical ANOVA and allowed us to test effect of the treatment (trap-nest with or without a pre-established red mason bee aggregation), year and their interaction in one model (Leys & Schumann, Reference Leys and Schumann2010). The effects of these factors were tested in three separate models: (i) the number of nests established by species other than the red mason bee, (ii) the proportion of parasitized nests among all nests established in a trap-nest, and (iii) the proportion of parasitized nests among nests established in trap-nests by wild insects other than red mason bees. Model (i) tested the preference of solitary insects for nesting in heterospecific aggregations, and models (ii) and (iii) tested for differences in parasitism risks in the presence or absence of a large nesting aggregation.
Results
Out of the 60 trap-nests set up in two seasons, 57 were collected and analyzed, and the remaining three were lost in the field. Of those analyzed, nine did not contain any nests. They were included in analysis: (i) examining the preference for aggregated or solitary nesting but not in analysis, (ii) because it was impossible to calculate the proportion of parasitized nests for an empty trap-nest. In analysis (iii), only nests of wild species were included, and therefore, trap-nests containing only red mason bee nests were excluded for the same reason given for analysis (ii), resulting in 43 trap-nests being analyzed. In trap-nests supplied with cocoons, red mason bees successfully established nesting aggregations (47.4 ± 22.0 red mason bee nests per trap-nest, mean ± SD). However, we found red mason bee nests in only two trap-nests that were not supplied with cocoons; three nests in a trap-nest set in 2014 and four nests in a trap-nest set in 2015 (0.2 ± 0.9 red mason bee nests per trap-nest, mean ± SD). The numbers of wild insects inhabiting our trap-nests were quite small (only two trap-nests, both with a red mason bee aggregation, contained more than 10 nests of other species).
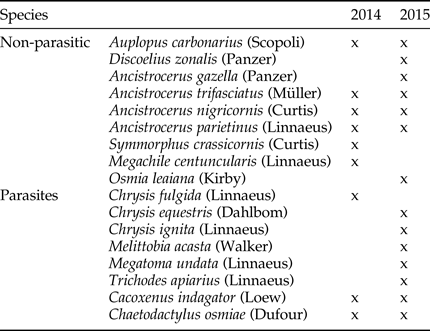
We identified nine species of solitary bees and wasps and at least 10 species of parasites (not all specimens we were able to be identified to the species level; Table 1). One parasite, Monodontomerus sp., was not included in the analysis because it develops inside bee cocoons, and as we did not open all the red mason bee cocoons, we could have overlooked some Monodontomerus that had not yet emerged. We found this parasite in nine trap-nests with aggregations of red mason bees, all from the second season, and including these data in the analysis would not change our results.
Table 1. Species of Aculeata and their parasites identified to the species level in the trap-nests.
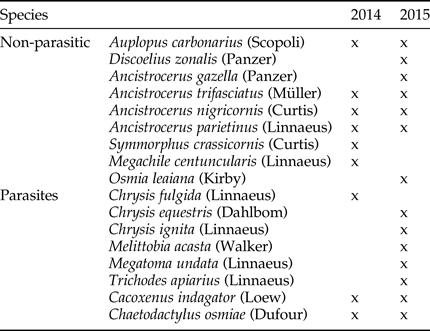
The ‘x’ denotes the year in which a given species was present. Specimens of parasites identified to higher taxonomic levels are not presented in the table, and included representatives of Parasitica (Hymenoptera) and Diptera in 2014 and Cryptinae and Pimplinae (Ichneumonidae, Hymenoptera) in 2015.
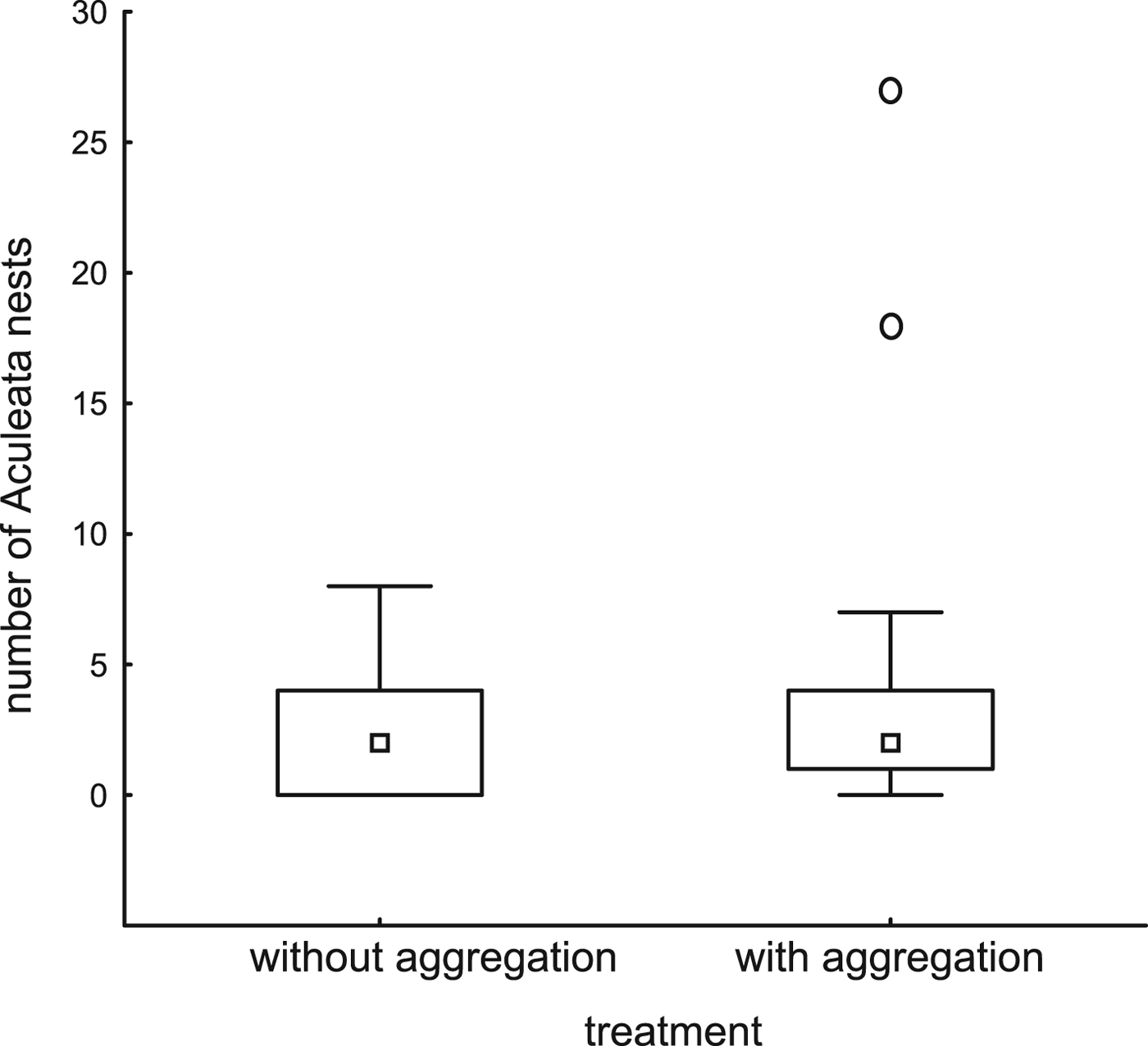
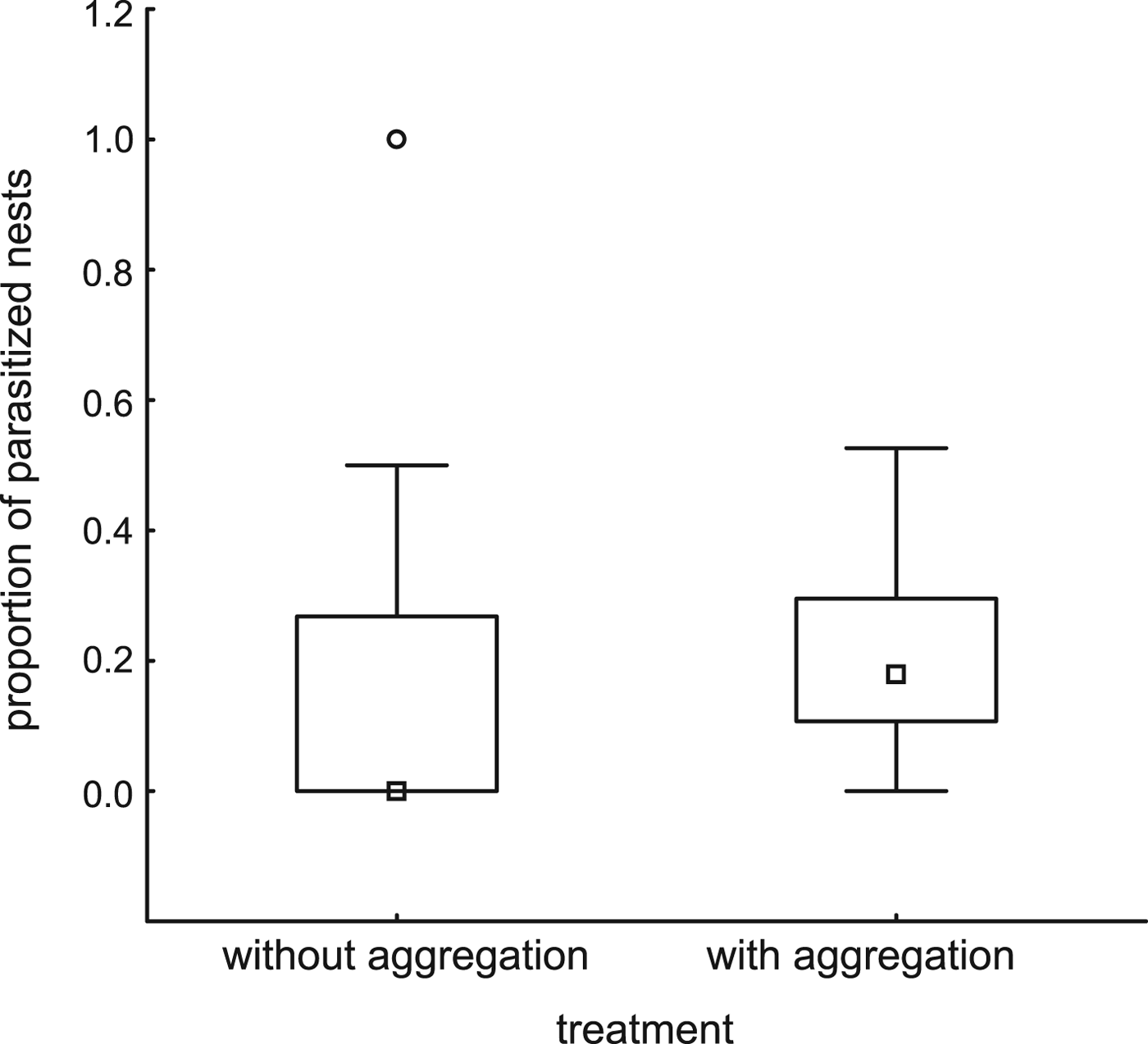
Trap-nests with and without red mason bee aggregations did not differ with respect to the number of other Aculeata nests (P = 0.73, fig. 1, Table 2). Overall parasitism rates were higher in trap-nests with red mason bee aggregations (P = 0.03, fig. 2, Table 2), but the relationship disappeared after excluding red mason bee nests (P = 0.79, Table 2). There were no differences between the 2 years and no interaction between the factors in any of the three models (Table 2).
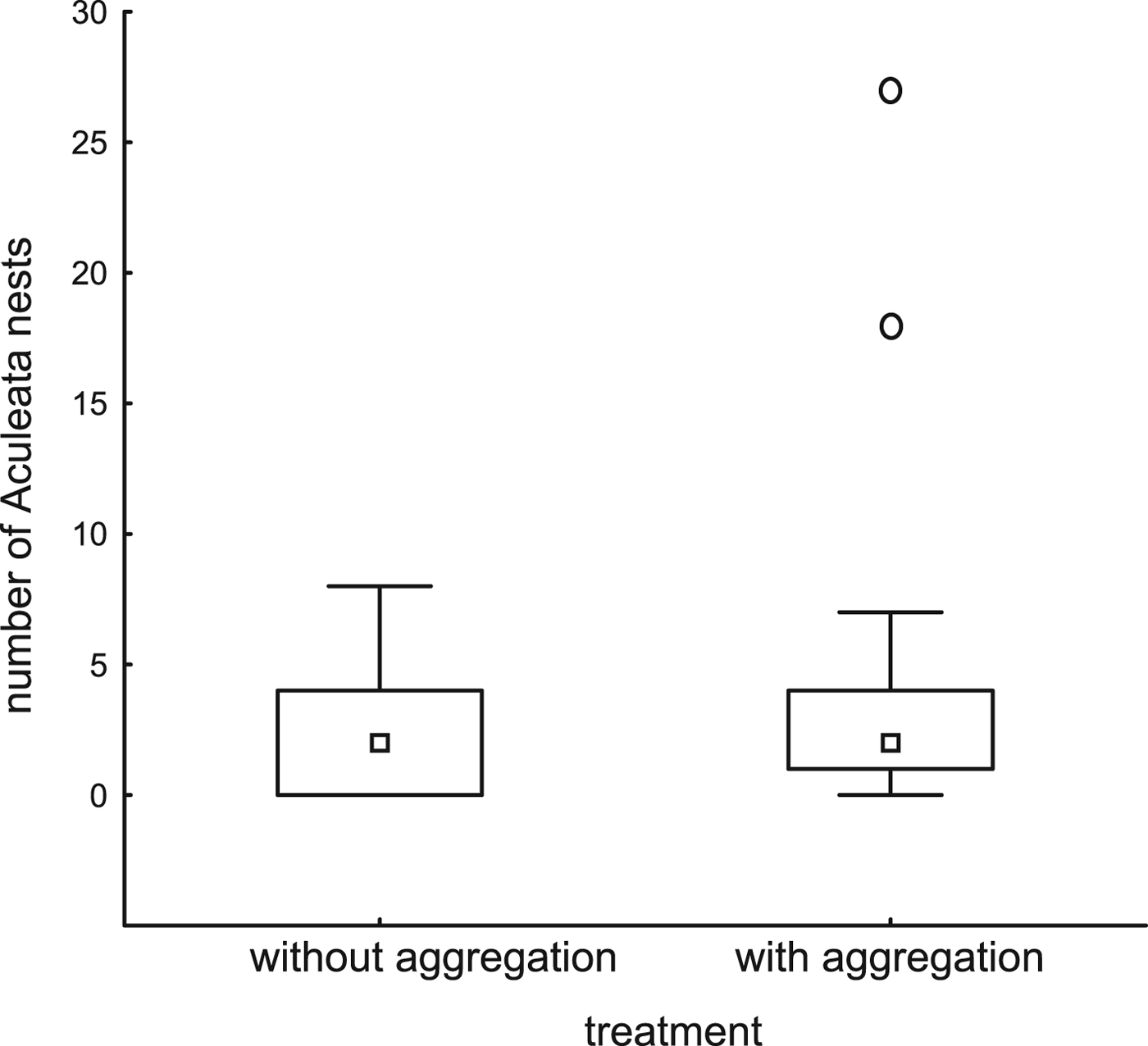
Fig. 1. Number of wild Aculeata nests (other than those of red mason bees) established in trap-nests with or without red mason bee aggregations. The treatments with and without aggregation did not differ significantly (ART ANOVA, F = 0.12, P = 0.73).
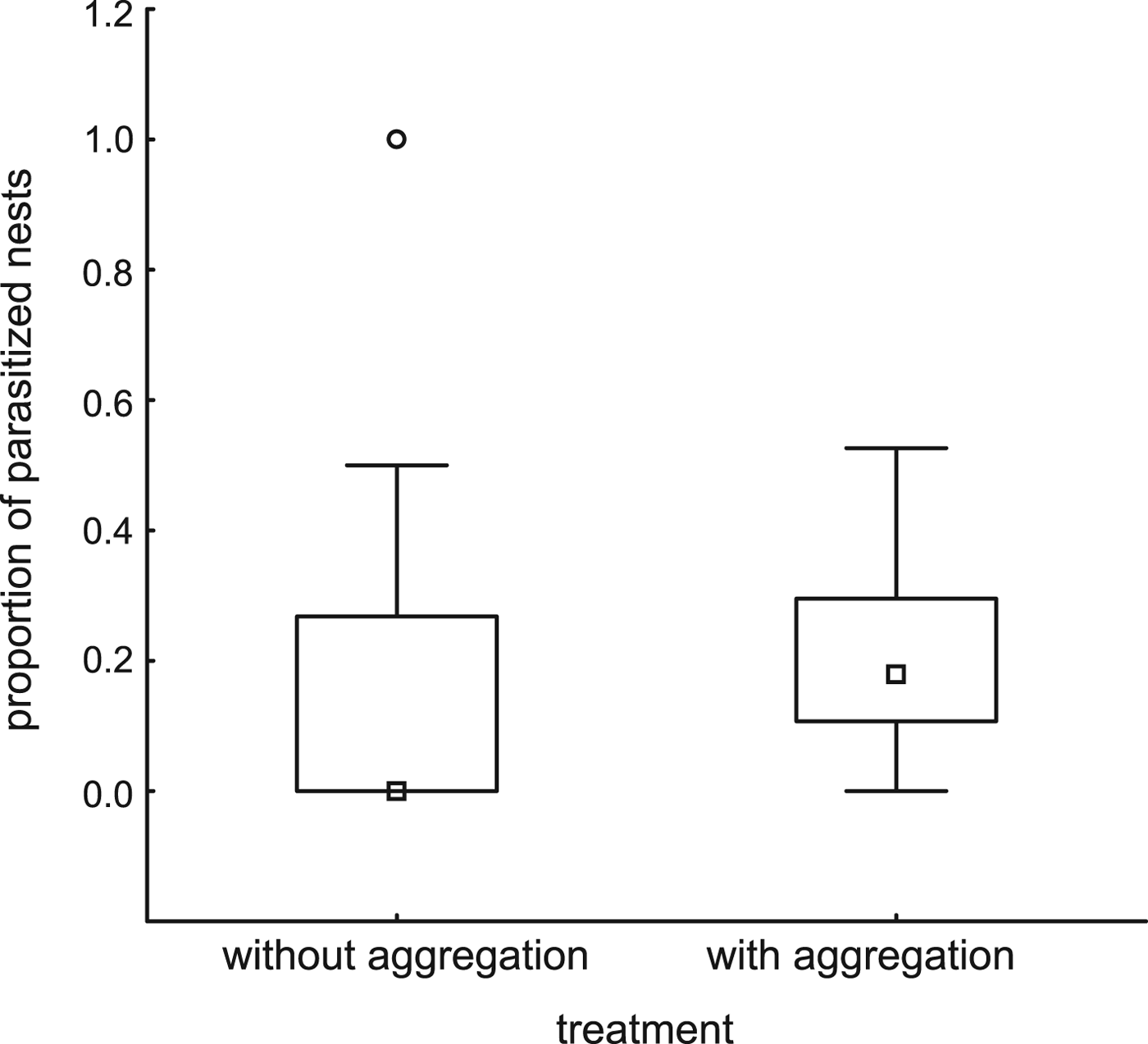
Fig. 2. Proportion of parasitized nests in trap-nests with or without red mason bee aggregation. Parasitism was higher in trap-nests with red mason bee aggregation (ART ANOVA, F = 5.37, P = 0.03).
Table 2. Results of the analysis of variance of aligned rank transformed data. df res., degrees of freedom for the residual.
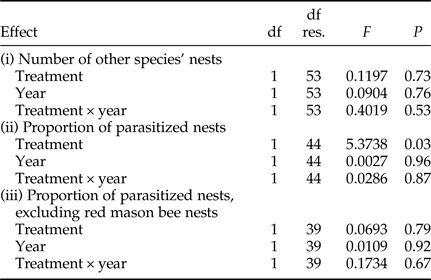
In all models, there were two treatments: trap-nests with or without red mason bee aggregations. Proportion of parasitized nests was calculated from all the Aculeata nests present in a trap-nest (both red mason bee and other species) in model (ii). In model (iii), only nests of species other than red mason bees were included in the analysis.
Discussion
Contrary to our expectations, the existing aggregation of one species of solitary Aculeata did not affect the attractiveness of trap-nests as potential nesting places for other species. Solitary bees and wasps did not establish more nests in trap-nests with existing aggregations of red mason bees but also did not avoid aggregations. Nesting sites are often limiting for solitary bees and wasps (Tscharntke et al., Reference Tscharntke, Gathmann and Steffan-Dewenter1998; Steffan-Dewenter & Schiele, Reference Steffan-Dewenter and Schiele2008). Although solitary Aculeata are free-flying insects, and some larger species of bees have been shown to cover impressive distances of more than 1 km, under natural conditions they probably fly much smaller distances (Zurbuchen et al., Reference Zurbuchen, Cheesman, Klaiber, Müller, Hein and Dorn2010a , Reference Zurbuchen, Landert, Klaiber, Müller, Hein and Dorn b ). It is possible that the females in our experiment, after encountering one of our trap-nests, decided to stay there regardless of whether it contained an aggregation of O. bicornis because looking for a new nesting site would be too costly and not necessarily successful. If more potential nesting sites were available within a small distance from each other, females might have inspected and decided among several sites. However, the distances between our experimental trap-nests (at least 150 m) might have been enough to prevent females from encountering by chance more than one potential nesting site.
We assumed that one of our experimental nesting conditions (i.e., with or without a red mason bee aggregation) would be markedly more beneficial than the other for nesting females. However, it is possible that either the costs and benefits associated with nesting in a heterospecific aggregation are insubstantial, or that these costs and benefits counterbalance each other. In both cases, there would be no difference in female fitness in relation to the conditions in which they nest, and there would be no reason for them to be choosy in picking nesting sites. We hypothesized that a major benefit of nesting in heterospecific aggregations would be less parasitism. However, we found that the presence of a large aggregation was associated with a higher parasitism rate. After including only the nests of insects other than red mason bees in the analysis, this relationship disappeared. The majority of wild species in our trap-nests were wasps. The species composition of parasites attacking solitary wasps is probably less similar to the parasites attacking red mason bees than is the composition of parasites of different bee species, and the dynamics of host–parasite relationships might also be different in bees and wasps. Indeed, patterns of the density-dependence of nest parasitism on solitary Hymenoptera vary depending on the species studied (Rosenheim, Reference Rosenheim1990). However, as red mason bees were dominant in the trap-nests with pre-established aggregations and were almost absent in trap-nests without aggregations, the higher risk of parasitism we observed may simply result from an overall higher vulnerability to parasitism in red mason bees than in wasps. It would be interesting to conduct an analogous experiment at a locality with a higher abundance of trap-nesting bees to test whether there is a density-dependence of parasitism in bees.
There are several possible, non-exclusive causes of the high parasitism rates observed in the trap-nests with red mason bee aggregations in our experiment. Some nest parasites, such as Melittobia acasta, found in our study, go through several generations per year and easily disperse to nearby nests within an aggregation. Other parasites, such as Chaetodactylus mites, not only move between nearby nests but also hitchhike on the bodies of females when they occasionally enter the wrong nesting holes (Park et al., Reference Park, Kondo, White, West, McConnell and McCutcheon2009). Females of free-flying parasites may be attracted to the volatile compounds released from active nests, and larger aggregations produce stronger cues for them to follow (Filella et al., Reference Filella, Bosch, Llusià, Seco and Peñuelas2011). Another other possible explanation is connected with creating bee aggregations using cocoons from a breeding colony. Although we selected free cocoons and did not supply the trap-nests with old nesting material in which parasites may have persisted, it cannot be excluded that some smaller parasites, such as mites, could have been introduced to our experimental trap-nests with the cocoons. In breeding colonies, parasites can be numerous and diverse and can pose serious problems to the management of such colonies (Krunić et al., Reference Krunić, Stanisavljević, Pinzauti and Felicioli2005), which is an important issue in the context of the impact of bee cocoons introduced to the field, e.g., as pollinators in orchards (Bosch & Kemp, Reference Bosch and Kemp2000).
In summary, our experiment did not show preferences of wild solitary Aculeata in relation to the presence of existing heterospecific nesting aggregations. Trap-nests were probably settled by insects that randomly encountered them in the field. Because the majority of the wild trap-nesting insects in this experiment were solitary wasps, it would be interesting to check whether wild solitary bees have preferences for or against heterospecific nesting aggregations.
Acknowledgements
Paweł Mielczarek helped with constructing the trap-nests and with the field work. The research was supported by Jagiellonian University (DS/BINOZ/INOS/761/15-16) and the Polish National Science Centre (DEC-2013/11/N/NZ8/00930).