INTRODUCTION
The extinct American mastodon, Mammut americanum, is a common member of the late Pleistocene mammalian fauna of North America, and it is especially abundant in the Great Lakes region with hundreds of known occurrences (for a recent overview of sites, see Widga et al., Reference Widga, Lengyel, Saunders, Hodgins, Walker and Wannamaker2017). Many of these specimens derive from the late-glacial Bølling-Allerød chronozone (14.7–12.9 cal ka BP [before 1950]; Rasmussen et al., Reference Rasmussen, Bigler, Blockley, Blunier, Buchardt, Clausen and Cvijanovic2014). The time window for the extinction of mastodons is estimated by Widga et al. (Reference Widga, Lengyel, Saunders, Hodgins, Walker and Wannamaker2017) to be 12,790–12,520 cal yr BP, in the early Younger Dryas chronozone. In the upper Midwest, USA, the vegetation at the time of mastodon abundance was mixed coniferous-deciduous woodland, reconstructed from pollen assemblages, with no apparent modern analogs (but see Birks, Reference Birks2003). It was dominated by Picea and Larix, associated with various deciduous trees, especially Fraxinus nigra (Williams et al., Reference Williams, Shuman and Webb2001, Reference Williams, Shuman, Webb, Bartlein and Luduc2004; Dyke, Reference Dyke2005; Gonzales et al., Reference Gonzales, Williams and Grimm2009; Gill et al., Reference Gill, Williams, Jackson, Donnelly and Schellinger2012). The abundance of Fraxinus nigra indicates a wet climate with high winter precipitation (Gonzales et al., Reference Gonzales, Williams and Grimm2009). However, elsewhere in North and Central America, mastodons are associated with other kinds of vegetation and habitats (e.g., Halligan et al., Reference Halligan, Waters, Perrotti, Owens, Feinberg, Bourne and Fenerty2016).
The American mastodon would have been a keystone herbivore in late Pleistocene terrestrial ecosystems, so any new information on its paleobiology, and especially its diet, may help elucidate its paleoecology and may shed light on the cause of its extinction near the end of the Pleistocene. Some early comments on mastodon diet were based on reports of material identified as “stomach contents” associated with specimens preserved in lacustrine settings (e.g., Dreimanis, Reference Dreimanis1968), but there was generally little evidence provided for the anatomical source of these samples or information concerning the season or cause of death. There are also reports on mastodon fecal material (e.g., Laub et al., Reference Laub, Dufort and Christensen1994; Newsom and Mihlbachler, Reference Newsom and Mihlbachler2006), often in association with skeletal remains. Fecal material, assuming it is correctly attributed, is a legitimate indicator of some aspect of mastodon diet, but the macrofossil assemblage could be biased toward more indigestible components of what was actually ingested, such as twig remnants. Some of the other components of the meal may possibly be detected by aDNA analysis of the purported fecal material. Other important sources of information on mastodon diet have been studies of stable isotope composition of mineralized tissues (generally suggesting a C3-dominated, or “browsing” diet, of leaves, twigs, and bark; Koch et al., Reference Koch, Hoppe and Webb1998), studies of tooth morphology (e.g., Saunders, Reference Saunders1996) and microwear textures (Green et al., Reference Green, Semprebon and Solounias2005, Reference Green, DeSantis and Smith2017; Smith and DeSantis, Reference Smith and DeSantis2018), reports of phytoliths recovered from dental calculus (Gobetz and Bozarth, Reference Gobetz and Bozarth2001), and plant remains in a “molar socket” (Teale and Miller, Reference Teale and Miller2012). Direct evidence of the composition of ingested material from preserved intestinal contents is extremely rare but has been reported (e.g., Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991). Here we make multiproxy analyses of intestinal contents from the two mastodon sites investigated by Lepper et al. (Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991) and Bearss and Kapp (Reference Bearss and Kapp1987) to provide direct insight into what mastodons actually ingested. Comparative analyses of the lake sediments surrounding the remains indicate the habitats of the mastodons and the availability of food plants. The intestinal contents provide additional clues about the season of death. Thus, we aim to reconstruct the habitats of the two mastodons and their food preferences.
The Burning Tree mastodon, Ohio
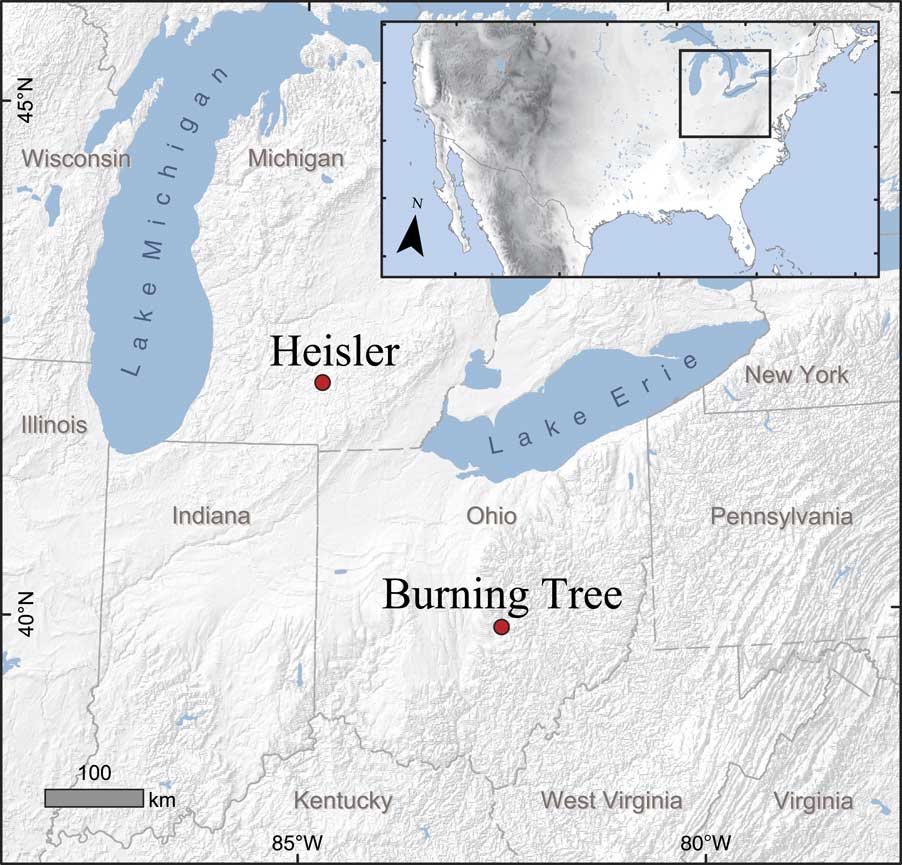
The Burning Tree site is a small infilled pond in a depression within the till of the St. Johns lobe of the Wisconsinan (last) glaciation, which probably melted prior to 15,000 yr ago (Shane, Reference Shane1989). The bones identified as coming from a single almost fully grown male mastodon were discovered in 1989 during mechanical excavation of the <0.5 ha pond on an undulating moraine in Licking County, Ohio (39°58′45″N, 82°27′10″W; 274 meters above sea level [m asl]) (Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991) (Fig. 1). Organic and clastic deposits overlying the till were 3.6 m thick.
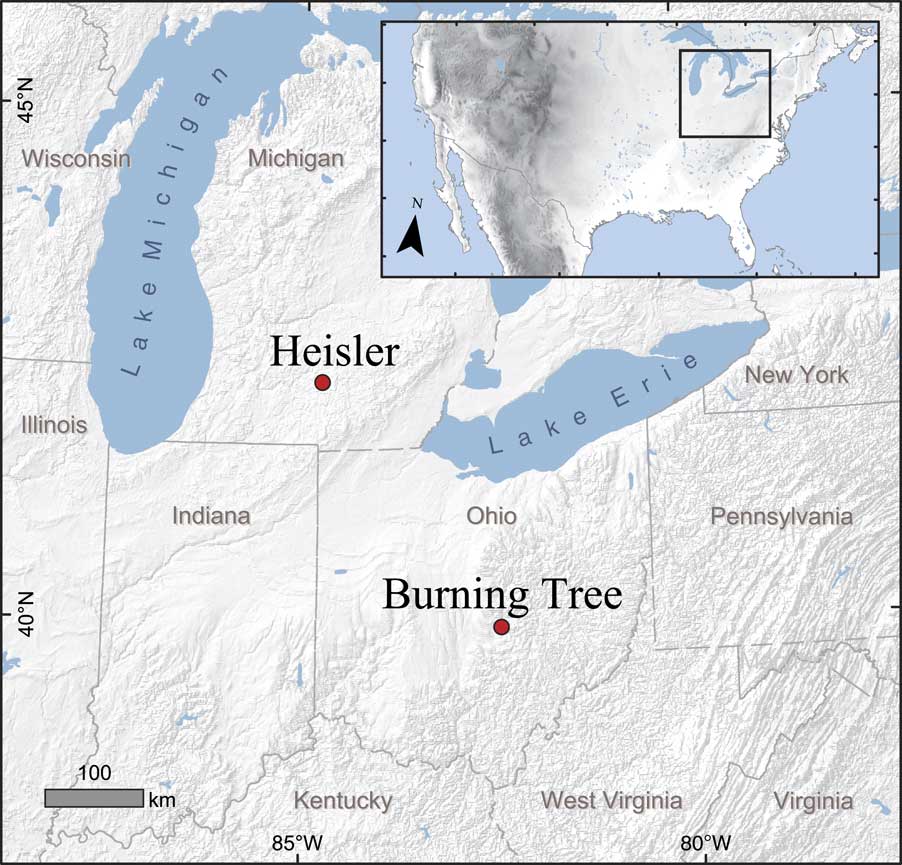
Figure 1. (color online) Map showing the locations of the Burning Tree and Heisler mastodon sites in Ohio and Michigan, respectively.
The macrofossil assemblage from the lake sediment associated with the bones contained abundant seeds of aquatic plants (Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991). Pollen spectra were dominated by Picea, Abies, and Pinus, characteristic of late-glacial forests along the southern margin of the Wisconsinan ice sheet in midcontinental North America (e.g., Dyke, Reference Dyke2005). These trees are characteristic of the boreal and boreal-nemoral biomes in North America today. The Burning Tree site is currently in the temperate forest biome, and the landscape is largely agricultural.
The material interpreted as intestinal contents was encountered as a coherent, cylindrical mass of plant material about 60 cm long and 12 cm in diameter and was recovered during excavation of a sequence of ribs and thoracic vertebrae. It was distinguished by a distinct color (reddish brown) and pungent odor different from that of the surrounding lake sediment (Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991). There was a distinct parting between the cylinder and the adjacent lake sediment, indicating that there had been no effective commingling of these materials. The entire cylinder of reddish-brown material was collected, as well as a comparative sample of the surrounding lake sediment.
In contrast to the lake sediments, the cylinder contained grassy material, mosses, and nonconiferous twigs and far fewer remains of aquatic plants. It was interpreted as part of the intestine containing the remains of the mastodon’s recently ingested food. The occurrence of Carex spp. and fen-herb seeds in this sample led to the inference that the mastodon died in late summer. This was supported by analyses of laminar features in tusk dentin (Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991).
Bacteriological analyses showed that the lake sediment contained taxa known to occur in sediment and/or freshwater environments, whereas the cylindrical mass included species common in mammalian intestinal tracts (Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991). More refined microbiological analysis by Rhodes et al. (Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998) was more quantitative and uncovered greater bacterial diversity. Enterobacteriaceae characteristic of intestines were found by culture and confirmed by DNA and ribosomal sequencing. They predominated in the cylindrical mass of plant material but were rare in the surrounding sediment. This provided strong support for the hypothesis of the intestinal origin for the cylindrical sample. More information on the Burning Tree mastodon is provided in the Supplementary Materials.
The Heisler mastodon, Michigan
Bearss and Kapp (Reference Bearss and Kapp1987) describe the discovery of the Heisler mastodon. It was found in 1984 in Clarence Township of Calhoun County, Michigan (42°23′21″N, 84°44′16″W; 287 masl) (Fig. 1), by Lester and James Heisler while laying tile to improve drainage of a small depression approximately 40 m in diameter in a cultivated field. The natural vegetation of the area would be temperate deciduous forest, but the land is currently cultivated. The depression was an internally drained late Pleistocene lacustrine basin within the till of the Saginaw lobe of the Wisconsinan ice sheet. Beneath the zone of degraded, cultivated peat, the sedimentary sequence extended downward through unweathered peat, peaty marl, marly gyttja grading into gray clay, and finally sands and gravels overlying till. The studied material was recovered from a depth of about 1.5 to 2 m in peaty marl.
Most of the bones at this site are derived from one young male mastodon. They occurred in multiple clusters across the pond basin, most of which consisted of disarticulated bones, although when bones of a given cluster were identified, they were found to represent several discrete portions of the carcass, each made up of several bones (Fisher, Reference Fisher1987). The material interpreted as intestinal contents of the Heisler mastodon was found in 1985, not in association with skeletal units suggestive of an abdominal derivation (as in the case of the Burning Tree mastodon), but with one of the few clusters of bones that retained primary articular relationships among bones comprising a set of anatomically disjunct skeletal units. The putative intestinal material consisted of a zone of finely ground plant debris several centimeters thick in most places, surrounding the lateral and lower aspect of a hemispherical mass of sand and gravel about 30 cm in plan diameter (see figs. 1B and 2 in Rhodes et al., Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998). This hemisphere is inferred to have originally been a spheroidal mass of sand and gravel, surrounded on all sides by a zone of finely ground plant debris, all retained within tissue of the large intestine. The lower portion of this spheroidal mass would have settled into the fine-grained, anoxic sediment of the pond bottom and was preserved as an intact hemispherical feature, but protracted exposure of the upper portion to oxygenated pond water allowed intestinal tissue to decompose and some of the plant debris and juxtaposed sediment to be dispersed, leaving a roughly horizontal surface of truncation. Two more examples of this type of feature occurred elsewhere at the Heisler site. In each case, the sand and/or gravel was anomalous within the pond setting and had no physical connection to any lower stratigraphic unit such as would be expected for a sedimentary dike.
The hypothesis we are working with is that the masses of sand, gravel, and associated plant debris were “clastic anchors” (Fisher, Reference Fisher1989, Reference Fisher1995, Reference Fisher1996; Rhodes et al., Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998; see the Supplementary Materials) made by humans introducing clastic material into sections of the mastodon’s large intestine, after emptying at least some of its normal contents (Rhodes et al., Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998), as part of a strategy of underwater meat storage (Fisher, Reference Fisher1995). Remaining intestinal contents formed the layer of plant debris around the clastic material. The pollen content of the plant debris associated with a clastic anchor was analyzed by R.O. Kapp and G. Snyder (cited in Fisher, Reference Fisher1996) and compared with the pollen assemblage in the surrounding sediment. Surprisingly, although the dominant pollen type in the peaty marl was Picea, no Picea pollen was found in the anchor-associated zone of plant debris (Fisher, Reference Fisher1989, Reference Fisher1996). An independent analysis of the record of dentin apposition in a tusk of the Heisler mastodon suggested it had died in the autumn (Fisher, Reference Fisher1987), a season when Picea pollen would not have been common. Analyses by R.I. Ford (personal communication 1992 to D.C. Fisher) showed that the material contained remains of female Picea cones, interpreted as late-autumn forage. See the Supplementary Materials for further information.
Microbiological analysis by Rhodes et al. (Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998) on samples from the Heisler site supported the hypothesis that the peripheral zone of plant debris (surrounding clastic material) was intestinal in origin, although fewer taxa could be cultured than from the Burning Tree material. Accordingly, the contrast between the intestinal material and the surrounding sediment was not as strong as that observed for comparable materials at the Burning Tree site.
METHODS
Our approach was to investigate the diet of our mastodon specimens by multiproxy microfossil and macrofossil analyses of the intestinal contents. Similar analyses of the embedding sediments were used to determine the environments in which they lived. Besides pollen, we also analyzed non-pollen palynomorphs (NPPs; van Geel, Reference van Geel2001; van Geel and Aptroot, Reference van Geel and Aptroot2006), plant macrofossils, and Mollusca, in order to obtain more complete environmental reconstructions and to be able to improve the comparison between intestinal contents and lake sediments. A similar approach was used by Lepper et al. (Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991) on the material from the Burning Tree site, but our analyses are more detailed and use more proxies. We also document our data photographically (Figs. 2–6).

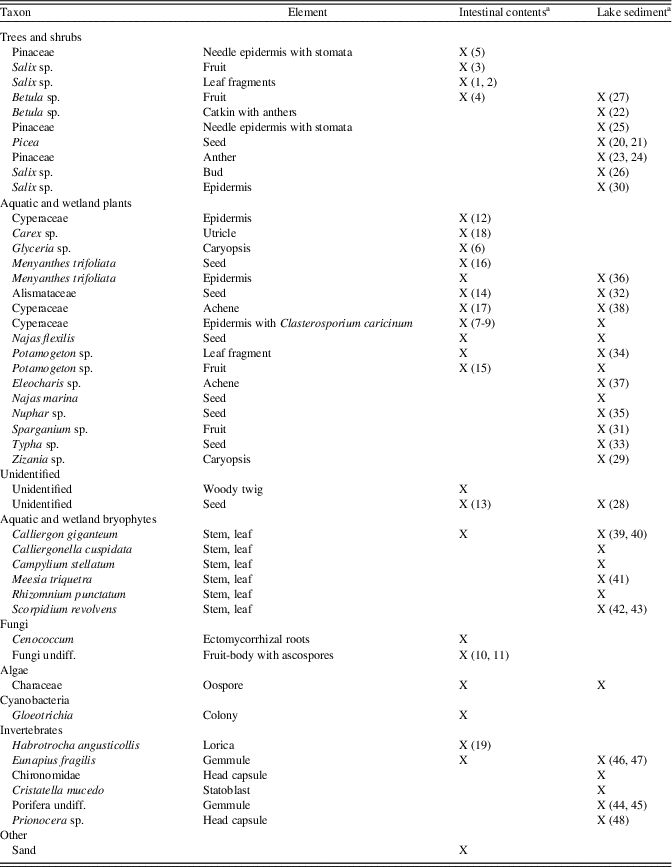
Figure 2. (color online) (1–19) Burning Tree, macrofossils from intestinal contents. (1, 2) Leaf fragments cf. Salix. (3) Salix sp., fruit. (4) Betula sp., fruit (wings not preserved). (5) Pinaceae, needle epidermis with stomata. (6) Glyceria sp., fruits. (7–9) Cyperaceous epidermis showing mycelium and hyphopodia of parasitic fungus Clasterosporium caricinum (HdV-126). (10) Broken fungal fruit-body with two ascospores. (11) Three-septate ascospore from fruit-body (10). (12) Cyperaceous epidermis. (13) Unidentified seed. (14) Alismataceae, seed. (15) Potamogeton sp., fruit. (16) Menyanthes trifoliata, seeds. (17) Various Cyperaceae. (18) Carex sp., utricle. (19) Habrotrocha angusticollis, lorica.

Figure 3. (color online) (20–38) Burning Tree, macrofossils from lake deposit. (20, 21) Picea, winged seeds. (22) Betula sp. (?), catkin with anthers. (23, 24) Pinaceae, anthers. (25) Coniferous needle, epidermis with stomata. (26) Salix sp., bud. (27) Betula sp., fruit (wings not preserved). (28) Unidentified seeds. (29) Zizania sp., fruits. (30) Salix sp., epidermis. (31) Sparganium sp., fruits. (32) Alismataceae, seed. (33) Typha sp., fruit. (34) Potamogeton sp., leaf tip. (35) Nuphar (N. microphyllum or N. variegatum), seeds. (36) Menyanthes trifoliata, epidermis. (37) Eleocharis sp., fruits with perianth. (38) various Cyperaceae.

Figure 4. (color online) (39–49) Burning Tree, macrofossils from lake deposit, continued. (39, 40) Calliergon giganteum. (41) Meesia triquetra. (42, 43) Scorpidium revolvens. (44, 45) Gemmulae of freshwater sponges. (46) Eunapius fragilis, gemmulae. (47) Wall of gemmula of Eunapius fragilis. (48) Prionocera sp., head capsule. (49) Unidentified zoological object.

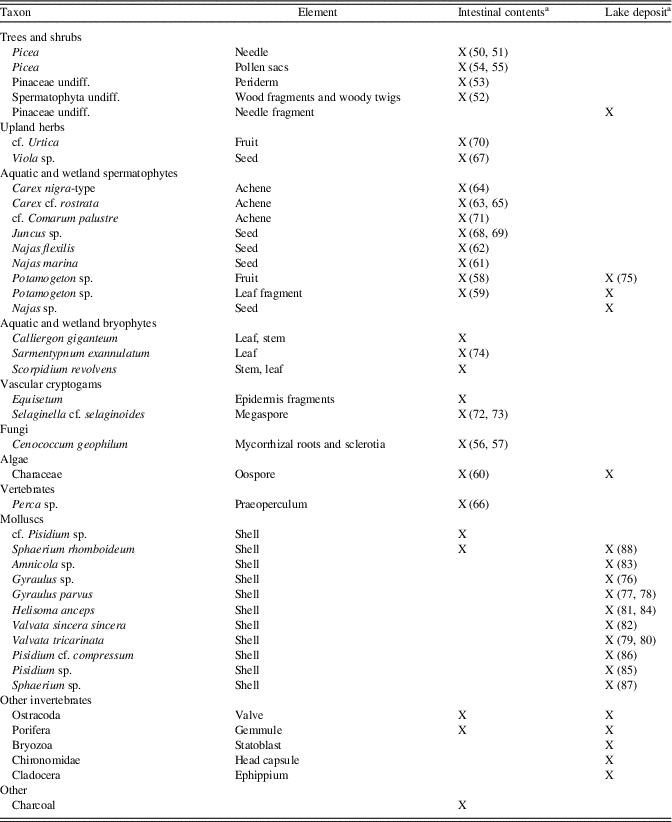
Figure 5. (color online) (50–66) Heisler, macrofossils from intestinal contents. (50, 51) Picea, needle fragments. (52) Twig with leaf scars. (53) Pinaceae, periderm. (54, 55) Picea pollen sacs (54: full of pollen; 55: after squashing, showing separate pollen grains). (56) Mycorrhizal root tip (incomplete) of Cenococcum geophilum. (57) Cenococcum geophilum, sclerotium (broken). (58) Potamogeton sp., fruit. (59) Potamogeton, leaf fragment. (60) Characeae, oospores. The right one showing part of the calcium carbonate coating. (61) Najas marina, seed. (62) Najas flexilis, seed. (63) Carex cf. rostrata, utricle with fruit. (64) Carex nigra type, utricle with fruit. (65) Carex rostrata type, fruit. (66) Perca sp., praeoperculum.

Figure 6. (color online) (67–74) Heisler, macrofossils from intestinal contents, continued. (67) Viola sp., seed. (68, 69) Juncus sp., seeds. (70) cf. Urtica, fruit. (71) cf. Comarum palustre, fruit. (72, 73) Selaginella cf. selaginoides, macrospores. (74) Sarmentypnum exannulatum, leaf. (75–88) Heisler, macrofossils from lake deposit. (75) Potamogeton sp., fruit. (76) Gyraulus sp. (77, 78) Gyraulus parvus. (79, 80) Valvata tricarinata. (81, 84) Heliosoma anceps. (82) Valvata sincera sincera. (83) Amnicola sp. (85) Pisidium sp. (86) Pisidium cf. compressum. (87) Sphaerium sp. (88) Sphaerium rhomboideum.
Samples of the Burning Tree intestinal contents (three aliquots totaling >50 g) and of the surrounding lake sediment (one aliquot >150 g) were selected for the present study from a frozen archive retained in the laboratory of D.C. Fisher since the time of original recovery. There was plenty of material remaining for our analyses. In contrast, the succession of studies attempting to characterize the Heisler intestinal material has significantly depleted the frozen sample archive. We were nonetheless able to select ~100 g from the peripheral zone of plant debris (attempting to exclude clastic material) and ~50 g from the surrounding peaty marl.
Microfossil preparation followed the standard method of Fægri and Iversen (Reference Fægri and Iversen1989). Macrofossil samples were prepared following Mauquoy and van Geel (Reference Mauquoy and van Geel2007). Identification and ecological interpretation of NPPs followed van Geel (Reference van Geel1978), Pals et al. (Reference Pals, van Geel and Delfos1980), van Geel and Aptroot (Reference van Geel and Aptroot2006), and van Geel et al. (Reference van Geel, Bohncke and Dee1981, Reference van Geel, Coope and van der Hammen1989, Reference van Geel, Buurman, Brinkkemper, Schelvis, Aptroot, van Reenen and Hakbijl2003). Molluscs collected during the macrofossil analysis were identified using Clarke (Reference Clarke1981) and Dillon et al. (Reference Dillon, Ashton, Kohl, Reeves, Smith, Stewart and Watson2013). Bryophytes were identified using Crum and Anderson (Reference Crum and Anderson1981), Lawton (Reference Lawton1971), Nyholm (Reference Nyholm1975), and Vitt and Buck (Reference Vitt and Buck2001). Taxonomic nomenclature follows Flora of North America Editorial Committee (1993+); Angiosperm Phylogeny Group IV (2016); Index Fungorum Partnership (2017); Guiry and Guiry (Reference Guiry and Guiry2017); Flanders Marine Institute (VLIZ) (2017); Royal Botanic Gardens, Kew, and Missouri Botanical Garden (2013); and van Soest et al. (Reference van Soest, Boury-Esnault, Hooper, Rützler, de Voogd, Alvarez de Glasby and Hajdu2017). Photographs were made by J. van Arkel, using a Nikon D2Xs digital camera mounted on a bellows with Zeiss Luminar lenses or with a Micro-Nikkor 60/2.8 lens (macrofossils in glycerine or dry) or mounted on a Zeiss Universal microscope with Nomarski-DIC (microslides). For enhanced depth of field, several photographs were combined using Helicon Focus and Adobe Photoshop software (focus stacking). Plates were composed in Adobe InDesign.
RESULTS
Dating
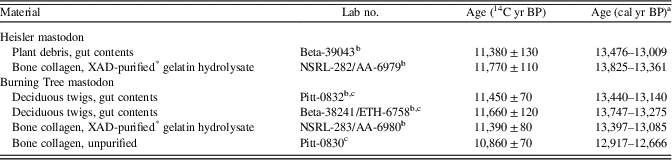
We obtained no new radiocarbon dates from the mastodons. Calibration of previous radiocarbon ages on both mastodons (Table 1) places them within Greenland interstadial 1 (Bølling-Allerød chronozone). For the Heisler mastodon, the radiocarbon date on plant debris in the intestinal contents is ~400 younger than the direct date on XAD-purified bone collagen, and a χ2 test indicates that these dates are not the same at the 95% level of significance (carried out in OxCal 4.3.2; Bronk Ramsey, Reference Bronk Ramsey2009). We do not have a good explanation for the discrepancy, although contamination with younger material might have occurred during excavation or by diffusion of younger humic acids from the overlying sediments. Nevertheless, both dates fall within the Bølling-Allerød chronozone. For the Burning Tree mastodon, the date on unpurified collagen is ~500 yr younger than the date on XAD-purified collagen suggesting likely contamination with younger materials. A χ2 test indicates that the XAD-purified collagen and two dates on twigs in the intestinal contents are similar at the 95% significance level. In fact, all dates except the one on unpurified collagen fall within the Allerød chronozone.
Table 1. Radiocarbon dates from the Heisler and Burning Tree mastodon sites.
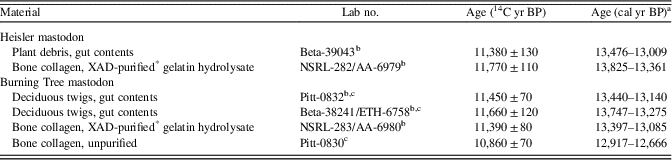
a The 95.4% range, calibrated with OxCal 4.3.2 (Bronk Ramsey, Reference Bronk Ramsey2009) with the IntCal13 calibration curve (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Bronk Ramsey and Buck2013).
b Reported in Rhodes et al. (Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998).
c Reported in Lepper et al. (Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991).
* XAD is a registered trademark of the Dow Chemical Company.
Microfossil and macrofossil results
Burning Tree
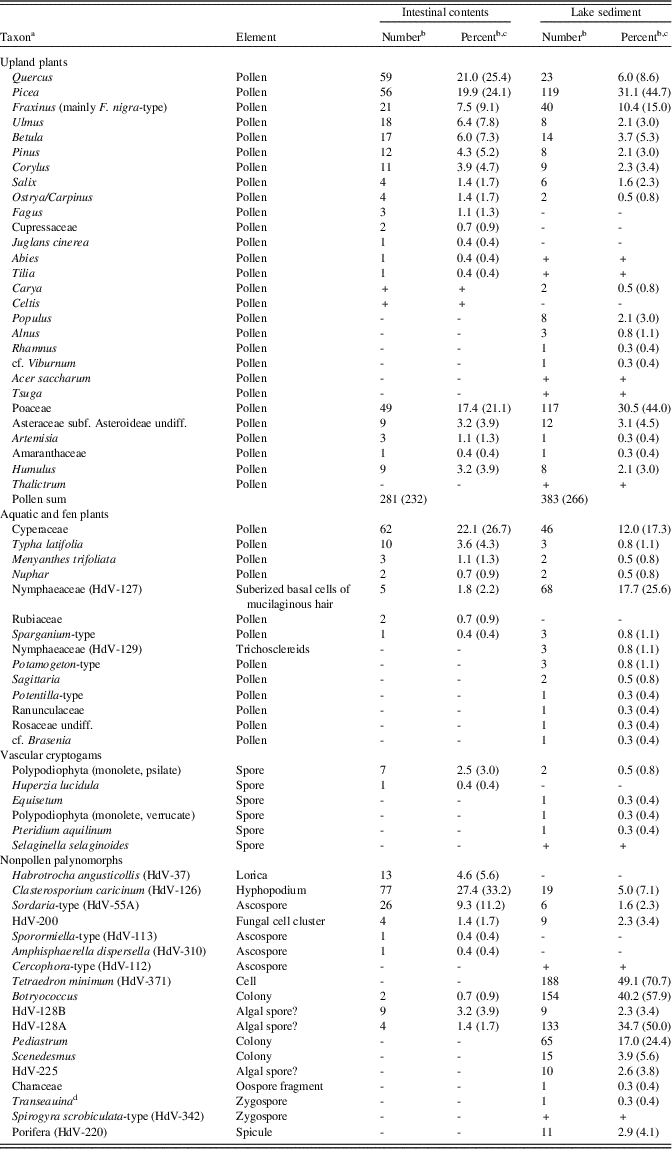
Because the pollen samples from the Burning Tree site have unusually high values of Poaceae relative to other late glacial sites in the upper Midwest, USA, we suspect overrepresentation of local aquatic Poaceae (Zizania, Glyceria). Therefore, microfossil percentages have been calculated with and without Poaceae in the pollen sum (Table 2). Many of the macrofossils are illustrated in Figures 2–4.
Table 2. Burning Tree mastodon, microfossils (pollen, spores, and non-pollen palynomorphs).
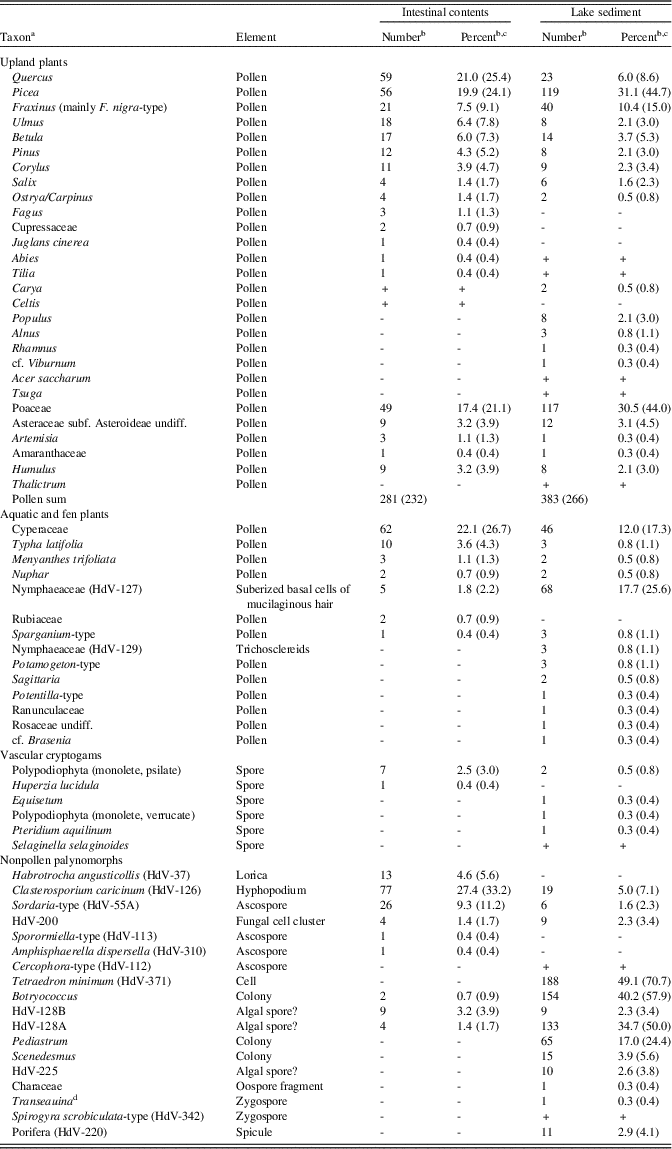
a “HdV” refers to types recognized by the Hugo de Vries-Laboratory, University of Amsterdam (Miola, Reference Miola2012).
b Plus sign (+) indicates type was observed in scans of the slide but not encountered during the quantitative count.
c Percentages were calculated on the sum of terrestrial pollen. Percentages in parentheses exclude Poaceae from the calculation sum.
d Formerly called Debarya Wittrock, 1872; now shown to be an illegitimate homonym of Debarya Schulzer, 1866 (Ascomycota) (Guiry, Reference Guiry2013).
Microfossils from the Burning Tree mastodon intestinal contents are listed in Table 2. Quercus, Picea, Fraxinus, Ulmus, Betula, Pinus, and Corylus are the commonest arboreal-pollen taxa in the intestinal sample, with minor contributions of Abies, Fagus, Juglans, Cupressaceae, Salix, and Tilia. Some wetland and aquatic taxa are present (Typha latifolia, Nuphar, and Botryococcus, as well as probable algal spore types HdV-128A and HdV-128B), but the numbers of these taxa are much lower than in the environmental sample from the lake deposit. Some algae (Pediastrum, Scenedesmus, and Tetraedron minimum) that were extremely common in the lake deposit are absent in the intestinal sample. Spores of the coprophilous fungus Sordaria-type are particularly common. The hyphopodia of the fungus Clasterosporium caricinum were growing on epidermis of Carex species (van Geel and Aptroot, Reference van Geel and Aptroot2006). Habrotrocha angusticollis is a rotifer common in wet mossy habitats.
The macrofossil record from the Burning Tree mastodon intestinal contents (Table 3, Fig. 2) shows that the animal consumed leaves and twigs of Betula, Pinaceae, and Salix. Herbaceous taxa are present such as Glyceria and various Cyperaceae (with the fungal parasite Clasterosporium caricinum), as are semiaquatic and aquatic taxa such as Calliergon giganteum, Menyanthes trifoliata, Alismataceae, Potamogeton, Najas, Characeae, Gloeotrichia, and Eunapius fragilis (sponge gemmulae).
Table 3. Burning Tree mastodon, presence of macrofossils.
The microfossil assemblage of the Burning Tree lake deposit (Table 2) suggests a predominantly forested landscape dominated by Picea associated with Quercus, Fraxinus, Betula, and Corylus, and with lesser amounts of Pinus, Ulmus, Populus, Salix, Alnus, Carya, Ostrya/Carpinus, Rhamnus, and cf. Viburnum. Upland herbaceous taxa (Artemisia, other Asteraceae, and Amaranthaceae) show relatively low values. Cyperaceae and Poaceae dominate the herbaceous taxa. The local aquatic environment is represented by the macrophytes Nuphar, cf. Brasenia, Potamogeton, Sagittaria, Sparganium, and Typha, together with NPPs, including the algae Tetraedron minimum, Botryococcus, Pediastrum, and Scenedesmus, spicules of Porifera, and probably algal spore types HdV-128A and HdV-128B.
The macrofossil record of the Burning Tree lake deposit (Table 3, Figs. 3 and 4) shows that Betula, Pinaceae, and Salix were growing nearby. The bryophytes point to a moist, calcareous habitat. Meesia triquetra is a rich fen indicator, while Rhizomnium punctatum and Calliergon giganteum suggest a swampy, open fen carr. Campylium stellatum and Calliergonella cuspidata are also species of calcareous mires. Zizania aquatica, Menyanthes trifoliata, various Cyperaceae, Sparganium, Typha, and Alismataceae probably occurred in the littoral zone along the lakeshore, while Potamogeton, Najas, Nuphar, and Characeae were growing in the lake. Chironomids, Cristatella mucedo, and Porifera were also part of the lake ecosystem.
Heisler
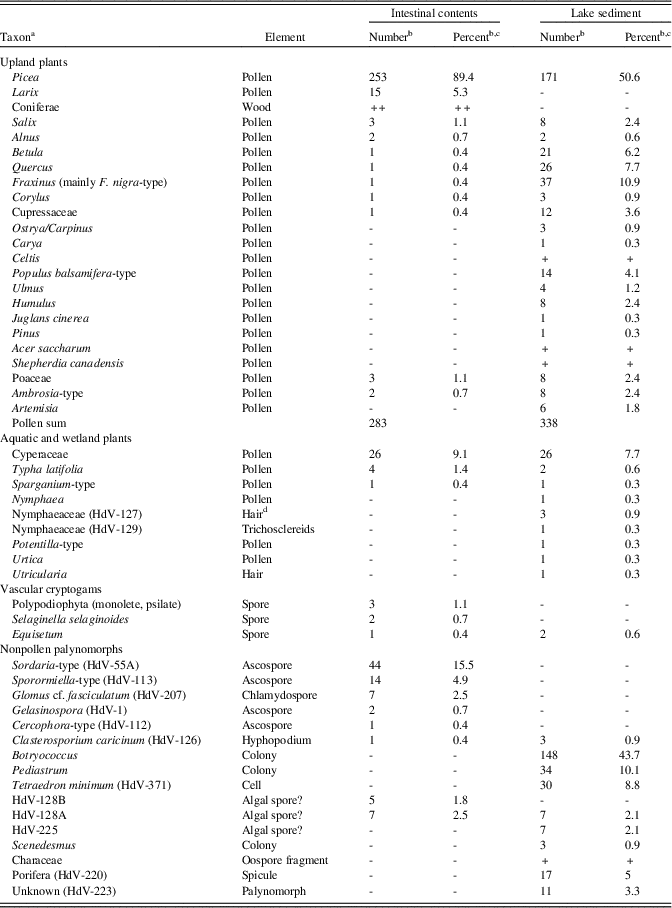
Picea completely dominates the intestinal-content pollen-spectrum with almost 90% (Table 4) in contrast to the (probably multiannual) pollen spectrum of the Heisler lake deposit. The presence of Picea pollen sacs in the macrofossil assemblage suggests overrepresentation of Picea pollen, especially if sacs were squashed and broken during preparation. Larix laricina, which normally occurs in low percentages in lake sediments, is the second-most common tree-pollen type. Other arboreal taxa show low frequencies only. Among the herbaceous taxa are low percentages of Sparganium and Typha. Cyperaceae pollen is common, which fits well with the macrofossil record. Fungal spores of the coprophilous Sordaria-type and Sporormiella-type are common. The latter includes ripe Sporormia spores that have developed slits and spores of the closely related Preussia, both of which are also coprophilous genera (see van Geel and Aptroot, Reference van Geel and Aptroot2006). The presence of chlamydospores of Glomus (formed under the soil surface) shows either that some soil material was ingested or that Glomus was introduced with the clastic material. Aquatic taxa are absent, apart from spores of HdV-128A and 128B, but these can also occur in mires (van Geel et al., Reference van Geel, Coope and van der Hammen1989).
Table 4. Heisler mastodon, microfossils (pollen, spores, and non-pollen palynomorphs).
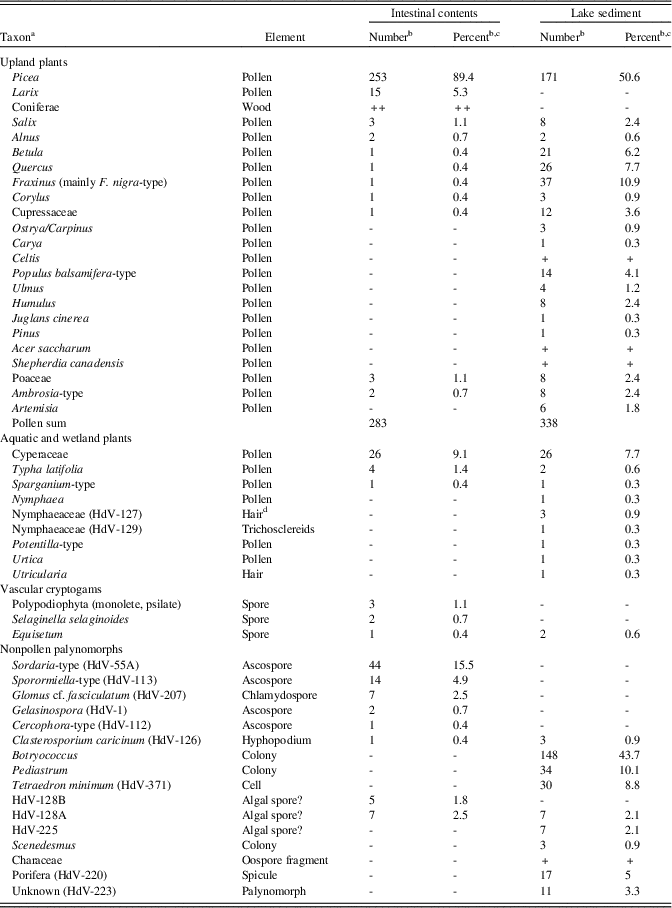
a “HdV” refers to types recognized by the Hugo de Vries-Laboratory, University of Amsterdam (Miola, Reference Miola2012).
b Plus sign (+) indicates type was observed in scans of the slide but not encountered during the quantitative counts.
c Percentages were calculated on the sum of terrestrial pollen.
d Suberized basal cells of mucilaginous leaf hairs.
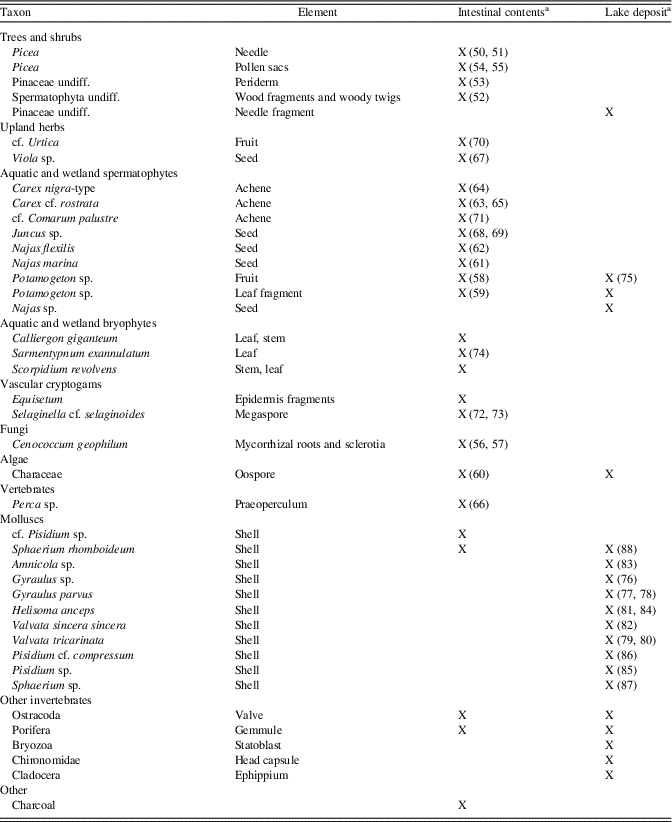
The macrofossil assemblage of the Heisler intestinal sample (Table 5, Figs. 5 and 6) contained Picea needle fragments, Picea pollen sacs (still filled with pollen), periderm of Pinaceae, wood fragments, and some charcoal. Among the herbaceous taxa were Carex species, Viola sp., cf. Juncus, cf. Urtica, cf. Comarum palustre, Equisetum, and Selaginella selaginoides. A variety of aquatic taxa included Potamogeton, Characeae, Najas marina, N. flexilis, Ostracoda, Porifera, and a small fish bone of Perca sp. The three recorded moss species point to a rich fen habitat, probably with seepage of alkaline water. The intestinal material of the Heisler mastodon also contained a few mollusc remains: one adult valve of Sphaerium rhomboideum and three juvenile valves of cf. Pisidium sp. These molluscs may have been introduced during the production of the “clastic anchors,” but some molluscs could have been ingested when the Heisler mastodon was drinking water.
Table 5. Heisler mastodon, presence of macrofossils.
The arboreal pollen record of the Heisler lake deposit (Table 4) is dominated by Betula, Fraxinus, and Picea, with some Quercus, Populus, Cupressaceae, Corylus, and Salix, whereas Alnus, Ostrya/Carpinus, Carya, Juglans, Pinus, and Ulmus show low values. Upland herbaceous taxa (Artemisia, other Asteraceae, and Poaceae) show low percentages only. Aquatic taxa are comparatively rare and include Utricularia, Characeae, Nymphaea, Sparganium, and Typha; the algae Botryococcus, Pediastrum, Scenedesmus, and Tetraedron minimum; and freshwater sponges.
Apart from some Picea needle fragments, the macrofossil record of the Heisler lake deposit is dominated by aquatic taxa, including Potamogeton, Najas, and Characeae, and a variety of invertebrates: Cladocera, Ostracoda, Bryozoa, Porifera, Chironomidae, and numerous Mollusca (Fig. 6, 76–88; Table 5). The mollusc shells were mostly well preserved and some still had a periostracum (thin organic coating), but some showed (bio) erosion. Some shells could not be identified to species level (e.g., juvenile shells). Many juveniles and some adults of Valvata tricarinata, Helisoma anceps, and Gyraulus parvus were recorded, and two juveniles of Amnicola cf. limosa were found. Some of the bivalves were still present as double valved. Double shells and valves of Sphaerium rhomboideum and Sphaerium (Musculium) sp. were recorded (possible candidates: S. lacustre, S. partumeium, and S. securis). One valve of Pisidium cf. compressum and double shells and many valves of Pisidium sp. were recorded. Probably some originally double-valved shells were separated during sampling and sieving of the sediment. The assemblage is typical of fresh calcareous waters with muddy substrates and aquatic vegetation occurring today in the boreal forest region of southern Canada and northern USA.
DISCUSSION
Samples from the lake sediments of the mastodon sites probably represent the complete growing seasons of multiple years, especially as the sediments had probably been stirred up by the process of emplacement of the mastodon parts. In contrast, the intestinal samples are expected to represent short time intervals and therefore overrepresent taxa that were flowering or fruiting near the time of death of the animal. In addition, the mastodons may have been selective in their food choice, which may cause additional overrepresentation and underrepresentation of some taxa.
Environments of the mastodons
The microfossil and macrofossil assemblages from the sediments surrounding the mastodon skeletal units indicate their environment, both regionally, with wind-dispersed forest-pollen types such as Picea, and more locally, with fen and aquatic taxa. The macrofossils, in particular, are probably locally derived, as they are generally not dispersed far from their source (Birks, Reference Birks1973) and represent both aquatic and emergent lakeshore vegetation.
The pollen assemblages indicate that the lakes were surrounded by mixed coniferous-deciduous forests dominated by Picea, together with Larix, characteristic of the late glacial vegetation in the Great Lakes area along with a significant deciduous component, especially Fraxinus, mainly F. nigra. Deciduous taxa were more frequent in the more southerly site (Burning Tree) in Ohio. Open glades were probably rather rare, as upland-herb pollen types have low percentages. It seems likely that mastodons roamed through the forest, browsing the trees, particularly Picea, and foraging and drinking at ponds.
The pollen assemblages from the mastodons’ environments are consistent with others from the Great Lakes region for the Allerød chronozone. They represent a vegetation type that was a mixture of Picea, Larix, and deciduous trees, with especially high values of Fraxinus nigra. These assemblages have no modern pollen analog (Overpeck et al., Reference Overpeck, Webb and Webb1992; Williams et al., Reference Williams, Shuman and Webb2001, Reference Williams, Shuman, Webb, Bartlein and Luduc2004; Jackson and Williams, Reference Jackson and Williams2004). Although these taxa occur together today in the northern Great Lakes region, the modern assemblages are dominated by Pinus and Betula, and Fraxinus nigra is much less abundant (e.g., Williams et al., Reference Williams, Shuman and Webb2001, Reference Williams, Shuman, Webb, Bartlein and Luduc2004; Grimm and Jacobson, Reference Grimm and Jacobson2004; Gonzales and Grimm, Reference Gonzales and Grimm2009; Gonzales et al., Reference Gonzales, Williams and Grimm2009). A modeling study using extended climate-pollen response surfaces suggested that greater precipitation, especially winter precipitation (snow), was a feature during the late glacial period (Gonzales et al., Reference Gonzales, Williams and Grimm2009).
Both lakes were surrounded by marginal sedge-swamps dominated by Carex spp. and containing other telmatic plants (Tables 2–5). The presence of numerous arboreal macrofossils in the Burning Tree lake sediment indicates a short distance between the sampling site and tree species, and therefore that the marginal lake-swamp was probably less extensive than at the Heisler site or that there was an inflowing stream nearby. The pollen and macrofossil records from Burning Tree suggest that the swamp contained abundant Salix, a variety of sedges (Carex and Eleocharis), and the emergent grass Zizania aquatica (wild rice), together with other wet-fen plants. There were also areas of rich fen around the lakes, indicated by the mosses and Selaginella selaginoides. These plants indicate calcareous conditions and may have been deposited near the mastodon carcasses through trampling and disturbance.
Lakes were important water sources for mastodons, and the surrounding sedge marshes were grazed. As indicated by the assemblages of aquatic plants, Najas flexilis, N. marina, Potamogeton spp., Nymphaeaceae, and Chara, the lake waters were calcareous with relatively high conductivity but probably low nutrient status. This is confirmed by the abundance of freshwater mollusca at the Heisler site. The snails and clams typically live in aquatic vegetation and on the muddy bottoms of shallow ponds, as well as in slow-moving rivers with quiet, silt-free, clean, cool, well-oxygenated, and calcium-rich water and abundant macrophytes. Valvata sincera sincera prefers larger calcareous lakes, ranging from the shoreline to 15 m depth. Gyraulus parvus lives in a variety of permanent and temporary water bodies. There are no indications for transport of shells, so we get a spectrum of the in situ mollusc fauna. Land snails of species from marsh or higher banks were absent. Algae such as Botryococcus, Pediastrum, Scenedesmus, and Tetraedron, and invertebrates such as Chironomidae, Cladocera, Bryozoa, and sponges, were also part of the lake ecosystems.
Mastodon meals
Yansa and Adams (Reference Yansa and Adams2012) provided a review of the habitats and diets of mammoths and mastodons in the Great Lakes region of the USA and adjacent Ontario, Canada, as they neared extinction around 13.0 cal ka BP. Our detailed results accord well with their characterization of mastodons as browsers of shrub and tree leaves, including spruce twigs, needles, and bark. They lived in a spruce parkland/sedge wetland environment and later in spruce-dominated forest.
The intestinal samples represent a relatively short time interval (probably only a few days and therefore just part of a season). The assemblages are biased by the specific vegetation of the sites where the animals selected their last meals and their selection of food plants. The microfossil assemblages from the intestinal samples of both mastodons differ from the surrounding lake deposits, especially for nonanemophilous types. Taphonomically, the intestinal contents represent the local vegetation that the mastodons were eating, whereas the lake sediments would have a larger source area and represent the average of several decades of pollen deposition. In most respects, the browsing and grazing behavior of the mastodons was similar. They browsed tree branches and leaves, both conifers and broad leaves, and pulled up herbaceous plants from the lakeshore that included fruits and seeds in the ingested sediment.
At both sites, algae (HdV-128A, Botryococcus, Tetraedron minimum, Pediastrum, and Scenedesmus), remains of Nymphaeaceae leaves (HdV-127), and sponge spicules are common in the lake sediments but rare or even absent in the intestinal samples. The absence of aquatic algae such as Botryococcus, Pediastrum, and Tetraedron in the intestinal samples is remarkable. These algae are common in freshwater and bloom in eutrophic conditions when inorganic phosphate and nitrate are high and the water temperature is warm. The aquatic assemblages indicate that the lakes were oligotrophic to mesotrophic, so blooms were probably rare, especially in early spring when the water was cool, which would explain the absence of algae in the intestines. Larger Pediastrum values in the sediments are probably derived from the Pediastrum population increase in autumn. Pediastrum would have been rare in the water in spring, when the mastodon was taking its last drink.
The intestinal samples from both mastodons contained ascospores of the coprophilous Sordaria-type and Sporormiella-type fungi. These are absent from the Heisler and infrequent in the Burning Tree lake-sediment samples (Tables 2 and 4), which suggests that the mastodons were consuming herbaceous food with wind-dispersed ascospores from fruit-bodies that developed on nearby feces but that these spores were not dispersed by wind or water from land to lake (e.g., Baker et al., Reference Baker, Bhagwat and Willis2013; Gill et al., Reference Gill, McLauchlan, Skibbe, Goring, Zirbel and Williams2013). Studies of mammoth dung (van Geel et al., Reference van Geel, Aptroot, Baittinger, Birks, Bull, Cross and Evershed2008, Reference van Geel, Fisher, Rountrey, van Arkel, Duivenvoorden, Nieman, van Reenen, Tikhonov, Buigues and Gravendeel2011a, Reference van Geel, Guthrie, Altmann, Broekens, Bull, Gill, Jansen, Nieman and Gravendeel2011b) suggest coprophagy (ingestion of the animal’s own dung) by Mammuthus primigenius. The presence of fungal fruit-bodies of coprophilous taxa in mammoth feces was of crucial importance in demonstrating coprophagy. Because we did not find fruit-bodies of dung-inhabiting fungi in the mastodon samples, we can only speculate about possible coprophagy of our mastodons. However, the presence of the ascospores of coprophilous fungi can be expected in any sample of vegetation frequented by large herbivores (van Geel et al., Reference van Geel, Zazula and Schweger2007), because dung and fruit-bodies will have been present, and dispersed ascospores could thus have been consumed unintentionally. The presence of Glomus in the intestine indicates that soil material was ingested, also suggesting that soil was accidentally consumed when vegetation was pulled up.
The Burning Tree mastodon intestine did not contain overwhelming amounts of Picea and Larix pollen, and the percentages of deciduous-tree pollen, Ulmus, Fraxinus, Corylus, and Quercus, and Poaceae and Cyperaceae pollen are relatively great compared with the Heisler mastodon. All these taxa flower in spring, so the differences between pollen samples from the intestine and lake sediment are linked to the season of the mastodons’ death.
The relatively large amount of Cyperaceae pollen in the intestinal sample of the Burning Tree mastodon may indicate that the sedges were flowering when they were eaten. However, the pollen may have been already deposited within the sedge marsh before it was eaten. The large number of Cyperaceae seeds together with other marsh-plant propagules probably originated from the seeding plants or from sediment hauled out with the sedge plants by the mastodon’s trunk. The mastodon also ate a copious quantity of grasses (Glyceria and Zizania). Poaceae pollen is abundant in the lake sediment, and Zizania aquatica fruits (wild rice) were recovered (Table 3; Fig. 3, 29).
The pollen assemblages of trees and upland herbs in the Heisler mastodon spectrum are completely different from the Burning Tree intestinal contents. The Heisler pollen spectrum is dominated by Picea pollen, whereas the sediment sample shows many more arboreal taxa (Table 4). The combination of so much Picea pollen (accompanied by pollen sacs full of pollen) and more than 5% Larix pollen (which was not recorded in the corresponding sediment sample) indicates ingestion during the flowering period and browsing of the Heisler mastodon on young branches with pollinating male cones, especially of Picea. The seasonality of Picea flowering suggests that the Heisler mastodon died in April or May, but see the “Residual inconsistencies” section. Propagules of aquatic and fen plants that form later in the season also occur (Table 5). The mastodon may have ingested these along with sediment while grazing in a sedge marsh around the lakeshore. It could also have ingested a few propagules of aquatic plants, such as Chara oospores and Najas seeds, together with some aquatic invertebrate remains and a fish bone, when drinking the more than 100 L of water required each day.
Association with human activity
According to Goebel et al. (Reference Goebel, Waters and O’Rourke2008), the best explanation of available genetic, archaeological, and environmental evidence is that humans colonized the Americas around 15,000 yr BP, immediately after the deglaciation of the Pacific coastal corridor. Humans already lived in the Americas before the Clovis culture developed ca. 13 ka BP (Waters and Stafford, Reference Waters and Stafford2007). Humans rapidly migrated eastward along the southern margin of the continental ice sheet, possibly following prey such as mammoth and mastodon (Goebel et al., Reference Goebel, Waters and O’Rourke2008). Based on adjusted 14C dating and a reevaluation of the record of the Clovis culture, Waters and Stafford (Reference Waters and Stafford2007) revised the Clovis time range to 13,125 to 12,925 cal yr BP. This overlaps with the age of our mastodons. Within 200 calendar years, Clovis technology spread throughout North America, including the Midwest, though we need to be clear that neither of the sites we focus on here yielded diagnostic Clovis artifacts. Sites of this age showing evidence of pre-Clovis or Clovis human activity are small and may represent mammoth or mastodon kills, short-term camps, or caches. In southeastern Wisconsin, the Schaefer and Hebior mammoth sites provide evidence of proboscidean hunting or scavenging near the margin of the Laurentide Ice Sheet between 14.8 and 14.2 cal ka BP (Joyce, Reference Joyce2006). Disarticulated remains of a single mammoth at both sites were sealed in pond clay and were associated with unequivocal stone artifacts, probably of pre-Clovis type, and the bones bore consistent signs of butchering—cut and pry marks made by stone tools (Overstreet and Kolb, Reference Overstreet and Kolb2003). Disarticulated skeletons of mammoths and mastodons preserved within lake sediments are widespread in the Midwest (Widga et al., Reference Widga, Lengyel, Saunders, Hodgins, Walker and Wannamaker2017). It seems possible that the practice of caching carcasses in small lakes was a common method of preserving the meat (Fisher, Reference Fisher1989, Reference Fisher1995, Reference Fisher2009). In any case, this is the hypothesis within which we currently understand these two otherwise puzzling occurrences of mastodon intestinal contents.
Residual inconsistencies
We are satisfied that most observations made during this study are compatible with one another and with our growing knowledge of the ecology of late-glacial North America. However, some inconsistencies remain. Why did Kapp (cited in Fisher, Reference Fisher1996) find no pollen of Picea in the intestinal contents of the Heisler mastodon, whereas we found it to be both abundant and accompanied by anthers? Why did Ford report debris of female conifer cones in his samples of Heisler intestinal contents, whereas we recognized no comparable material? The mastodon bones at the Heisler site are all consistent with a single individual, a young male, so it cannot be reasonable to explain the reported differences by conjecturing that there were intestinal contents from more than one individual. However, we can propose three other potential explanations of these inconsistencies:
First, it is possible that these differences simply reflect the heterogeneity of intestinal material in a large mammal that forages sporadically within a complex habitat with a wide variety of potential foods (Green et al., Reference Green, DeSantis and Smith2017). Once past the stomach, there would be little tendency for peristaltic movement to homogenize intestinal contents, so we might well acquire a serially diversified picture of this animal’s diet. Kapp’s material came from one “clastic anchor,” but later analyses, including ours, came from a second anchor. Therefore, although they are part of the same intestine, they sample different parts of it. The mastodon might have eaten only one or two Picea cones from the previous year, which Ford happened to sample. The abundant Picea pollen and anthers we recorded are usually produced in May.
Second, the samples of Heisler intestinal contents used by Kapp, Ford, and Rhodes et al. (Reference Rhodes, Urbance, Youga, Corlew-Newman, Reddy, Klug, Tiedje and Fisher1998) were selected early in the history of study of this site, when enough material remained to focus on the outer portion of the peripheral zone of plant debris (intestinal contents), avoiding material close to the sand and gravel that filled the interior of the “clastic anchor.” By the time our sample was selected, it was difficult to collect enough mass for analysis while maintaining a safe distance from the clastic material, risking contamination of our sample by inclusion of introduced sediment—and pollen associated with it—that would have been acquired from a substrate just beyond the pond margin without having been ingested by the mastodon. Our sample might thus have included both material ingested by the mastodon and material introduced postmortem by humans during production of a “clastic anchor.”
Third, although we have no direct or compelling evidence of coprophagy for mastodons comparable to evidence for woolly mammoths (van Geel et al., Reference van Geel, Aptroot, Baittinger, Birks, Bull, Cross and Evershed2008, Reference van Geel, Fisher, Rountrey, van Arkel, Duivenvoorden, Nieman, van Reenen, Tikhonov, Buigues and Gravendeel2011a, Reference van Geel, Guthrie, Altmann, Broekens, Bull, Gill, Jansen, Nieman and Gravendeel2011b), coprophagy would dramatically increase the potential for observing dietary heterogeneity by combining material ingested by multiple individuals, even during different parts of the year, in a single individual’s intestine.
At present, we cannot rule out any of the three hypotheses described previously, but it is worth noting that they are not mutually exclusive. However, another problem is that our botanically based estimates of season of death for at least the Heisler mastodon (and probably the Burning Tree mastodon) differ from the estimates generated by tusk analysis. Tusk dentin accretes along the surface of a proximal pulp cavity throughout life and typically shows an annual cycle of variation in structure and composition that has been seasonally calibrated by finer-scale increment studies and stable isotope analyses (e.g., Fisher, Reference Fisher1987; Koch et al., Reference Koch, Fisher and Dettman1989). The Heisler and Burning Tree mastodons have not been studied with the full range of techniques (e.g., multiple isotope systems and microscale computed tomography; e.g., Fisher et al., Reference Fisher, Cherney, Newton, Rountrey, Calamari, Stucky, Lucking and Petrie2014) that have been used recently, but both of these specimens were originally reported to show autumn seasons of death (Fisher, Reference Fisher1987; Lepper et al., Reference Lepper, Frolking, Fisher, Goldstein, Sanger, Wymer, Ogden and Hooge1991).
CONCLUSIONS
The combined analysis of macrofossils and microfossils—including NPPs—provides a detailed reconstruction of the late-glacial environment of the Burning Tree and Heisler mastodon sites. Both were surrounded by mixed coniferous-deciduous forest with abundant Picea, mixed with more deciduous trees in Ohio than in Michigan. The pollen spectra and radiocarbon dates fit with the known regional vegetation history of the Great Lakes region. The mastodons lived for at least part of the year near lakes surrounded by marginal sedge fens. Both lakes had calcareous oligo-mesotrophic water, with many Mollusca in the Michigan site. Botanical evidence shows that the mastodons browsed on trees and shrubs, with a preference for Picea, eating leaves and twigs. They used the marginal sedge fen vegetation extensively (mainly sedges and grasses) as additional forage and drank the lake water. They consumed, probably unintentionally, spores of dung fungi and seeds and fruits of fen plants and aquatic plants such as Potamogeton spp. and Najas spp., algae, and invertebrates. Intestinal samples are biased by the food choice of the mastodons and by the short time interval they represent, whereas lake sediment samples indicate the composition of terrestrial and aquatic vegetation at the sites. The botanical evidence implies that the seasons of their last meals were late spring or summer, in contrast to tusk structural evidence that indicates their deaths in the autumn. Ascospores of coprophilous fungi and aquatic algae appeared to be important “differentiating taxa” for intestinal samples and sediments surrounding the mastodons.
The diets of mammoths have been relatively well documented from frozen remains in the Arctic. This study uniquely improves our understanding of the diets and the paleoecology of mastodons in North America compared to previous reports on this topic. Diets of two mastodons have been precisely reconstructed from multiproxy analyses of preserved intestinal contents, which provide direct and incontrovertible evidence of what the mastodons ate. They are compared to similar multiproxy analyses of the surrounding lake sediments that reflect the local terrestrial and aquatic vegetation of the mastodons’ habitats and therefore the food plants available to them. They were herbivorous and seemed to select twigs and leaves, especially of Picea, as well as lakeshore sedges and grasses. The taxa identified in the analyses are presented as photographs, which will allow subsequent redetermination if necessary and will be an aid to future researchers in identifying their fossil specimens.
SUPPLEMENTARY MATERIALS
To view supplementary material for this article, please visit https://doi.org/10.1017/qua.2018.100
ACKNOWLEDGMENTS
The authors thank Annemarie Philip for preparing the microfossil slides. Bob Beerenhout identified a fish bone, Elly Beglinger and Rob van Soest kindly identified gemmulae of Porifera, and René Cappers identified some seeds. John Birks kindly advised about some pollen taxa. We thank Suzette Flantua for the location map. We appreciate helpful comments from the reviewers. The data have been deposited in the Neotoma Paleoecology Database (http://www.neotomadb.org).