INTRODUCTION
Macroalgae constitute a polyphyletic set of multicellular eukaryote taxa belonging to chlorophytes (‘green algae’), rhodophytes (‘red algae’) and phaeophyceans (‘brown algae’), and are considered to be sensitive to variation in environmental factors, including nutrient enrichment (Bokn et al., Reference Bokn, Duarte, Pedersen, Marba, Moy, Barron, Bjerkeng, Borum, Christie, Engelbert, Fotel, Hoell, Karez, Kersting, Kraufvelin, Lindblad, Olsen, Sanderud, Sommer and Sorensen2003; Becherucci et al., Reference Becherucci, Santiago, Benavides and Vallarino2016), sedimentation, and availability of light (Daly & Mathieson, Reference Daly and Mathieson1977; Devinny & Volse, Reference Devinny and Volse1978; Chapman & Fletcher, Reference Chapman and Fletcher2002; Shepherd et al., Reference Shepherd, Watson, Womersley and Carey2009). The European Union's Water Framework Directive (WFD, 2000/60/EC) specifies the use of macroalgae as biological quality elements (BQEs) for monitoring the ecological status of coastal waters in EU Member States. Additionally, the EU's Marine Strategy Framework Directive (MSFD, 2008/56/EC) refers to macroalgal species composition, biomass and annual or seasonal variability as biological features for the monitoring of marine waters. Consequently, a number of different approaches to macroalgae as ecological indicators have been developed for use in the Mediterranean Sea, including the Ecological Evaluation Index (EEI) (Orfanidis et al., Reference Orfanidis, Panayotidis and Stamatis2001), and the cartography of littoral rocky-shore communities (CARLIT) index (Ballesteros et al., Reference Ballesteros, Torras, Pinedo, Garcia, Mangialajo and de Torres2007; Blanfuné et al., Reference Blanfuné, Thibaut, Boudouresque, Mačić, Markovic, Palomba, Verlaque and Boissery2017). Most of the indices which have been developed focus on assemblages established on natural substrata, and there has been little investigation into the applicability of macroalgal fouling communities to environmental monitoring.
Macroalgal assemblages on artificial substrata provide several advantages in terms of environmental monitoring when compared to assemblages on natural substrata. Artificial substrata are present in many coastal environments, occurring both in anthropogenically impacted and in near-pristine sites. Many artificial structures – such as surface buoys or concrete jetties – are produced or constructed to a relatively uniform standard, and therefore comparable substrata can be found in a wide variety of environments. In contrast, natural substrata are much more heterogeneous and are likely to vary considerably in both nature and texture even at relatively small spatial scales (Ramos et al., Reference Ramos, de Teran, Puente and Juanes2016). Therefore, the use of communities on artificial substrata in environmental monitoring programmes facilitates the development of a standardized sampling strategy by eliminating the recurrent problem of substratum heterogeneity. Furthermore, the species comprising a macroalgal fouling assemblage in a given site tend to be a subset of the entire macroalgal species suite which can colonize hard substrata in that site (Foster, Reference Foster, Abbott, Foster and Ekland1980; Firth et al., Reference Firth, Thompson, White, Schofield, Skov, Hoggart, Jackson, Knights and Hawkins2013). Community structure is therefore simplified in comparison to assemblages established on natural substrata, which may facilitate comparison of assemblages across a wide variety of environments. From an economic perspective, artificial structures such as surface buoys are relatively cheap and easily deployed. Furthermore, they are inconspicuous and do not have significant negative impacts on the surrounding environment. Therefore, these structures are ideally suited for use in dedicated environmental monitoring programmes.
Studies investigating the sensitivity of macroalgae to changes in water quality have mostly focused on communities established on natural substrata and knowledge regarding those on artificial substrata is therefore somewhat lacking. Nonetheless, macroalgal fouling assemblages have been shown to respond to changes in environmental conditions, particularly turbidity and nutrient enrichment (Falace & Bressan, Reference Falace and Bressan2002). Moderate eutrophication may speed up the establishment of fouling communities in originally oligotrophic areas (Mayer-Pinto & Junqueira, Reference Mayer-Pinto and Junqueira2003), while at higher levels, eutrophication negatively affects species biomass and diversity and favours the growth of opportunistic, ephemeral taxa (Duarte, Reference Duarte1995; Cloern, Reference Cloern2001; Mayer-Pinto & Junqueira, Reference Mayer-Pinto and Junqueira2003; Worm & Lotze, Reference Worm and Lotze2006; Kraufvelin et al., Reference Kraufvelin, Ruuskanen, Nappu and Kiirikki2007). Organisms in the Chlorophyta, Rhodophyta and Ochrophyta have been shown to respond differently to increasing concentrations of dissolved nitrates and phosphates: the abundance and species richness of rhodophytes and ochrophytes decreases, while certain chlorophyte species show an increase in abundance (Mayer-Pinto & Junqueira, Reference Mayer-Pinto and Junqueira2003; Sliskovic et al., Reference Sliskovic, Jelic-Mrcelic, Antolic and Anicic2011). This has been linked to the high reproductive capacity and rapid growth rate of most chlorophyte species (Mayer-Pinto & Junqueira, Reference Mayer-Pinto and Junqueira2003), as well as the nitrophilic characteristics displayed by Ulva spp. and certain other chlorophyte taxa (Arévalo et al., Reference Arévalo, Pinedo and Ballesteros2007). Turbidity plays a role in determining initial community composition by influencing the period of motility and the settling ability of macroalgal propagules (Christie & Shaw, Reference Christie and Shaw1968; Jones & Babb, Reference Jones and Babb1968; Fletcher & Callow, Reference Fletcher and Callow1992; Holmes et al., Reference Holmes, Harriott and Banks1997).
To date, very few studies have evaluated the potential utilization of macroalgal fouling communities in environmental monitoring. The aim of this study was therefore to test the hypothesis that different degrees of anthropogenic impact, defined in terms of turbidity and concentration of dissolved nitrates and phosphates, will influence the taxon richness, composition and abundances of the macroalgal component of marine fouling assemblages on buoys in the Maltese Islands. This was assessed with a view to the utilization of these macroalgal assemblages as bioindicators in environmental monitoring programmes. The present investigation is one of the first to consider the response of macroalgal fouling communities on polyvinylchloride (PVC) buoys to changes in environmental conditions and their potential application to environmental monitoring.
MATERIALS AND METHODS
Sample collection and processing
Seven sites around the Maltese Islands (Figure 1) were selected for sampling based on the availability of water column physico-chemical data and the presence of polyvinylchloride (PVC) buoys. The expected degree of anthropogenic impact was assessed based on abiotic data collected during a previous monitoring survey conducted between June 2000 and March 2004 (Axiak, Reference Axiak2004). At each site, 10 spherical buoys of circumference 70–90 cm were sampled during the period July–September 2015; these were all moored to concrete blocks on the seabed, had been present in the site for at least 2 years, were fully colonized by fouling organisms and were evenly distributed throughout the sampling site, with a minimum 2 m of separation between each buoy and 5 m distance from the shore. All fouling organisms were removed from the entire surface of each buoy and transferred to separate mesh bags having a 1 mm mesh size. The samples collected were preserved with 5% formalin in seawater and the macroalgal component of the fouling assemblage was subsequently sorted and identified to at least genus level. All identified taxa were dried at 70°C for ~14 h and weighed to obtain values of dry biomass.
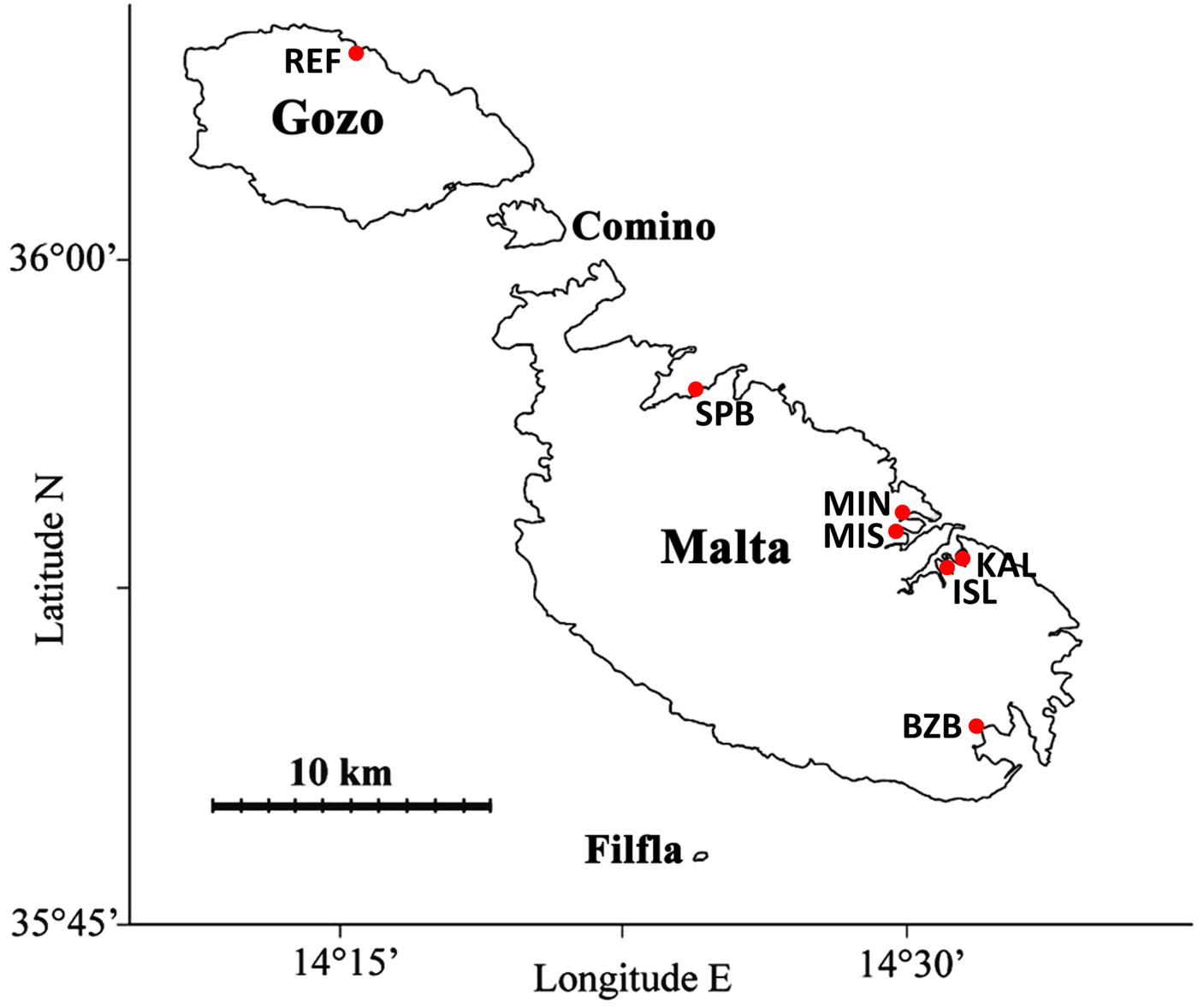
Fig. 1. Map of the Maltese Islands showing the sites sampled (REF, Marsalforn; SPB, St Paul's Bay; MIN, Manoel Island N; MIS, Manoel Island S; KAL, Kalkara; ISL, L-Isla; BZB, Birżebbuġa).
Data analysis
All statistical analyses were carried out using PAST v. 3 (Hammer et al., Reference Hammer, Harper and Ryan2001), IBM SPSS Statistics 23 (IBM Corp., 2015), and PRIMER 6 (Clarke & Gorley, Reference Clarke and Gorley2006). Measurements of seawater nitrate concentration (μg at N L−1), phosphate concentration (μg at P L−1) and beam attenuation coefficient (BAC) (m−1) for each site were obtained from the monitoring survey carried out by Axiak (Reference Axiak2004). This dataset was normalized through a natural logarithmic transformation and standardized by conversion to z-scores. These were then subjected to a hierarchical cluster analysis using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA) and Euclidean distance as the similarity measure. Principal Components Analysis (PCA) was applied to visualize the relative similarity of the study sites according to the abiotic data considered. Mean macroalgal taxon richness and mean total macroalgal biomass (standardized to account for variation in surface area of the buoys) were calculated for each site and these variables were checked for normality using the Shapiro–Wilk test. As both variables had a non-normal distribution, a Kruskal–Wallis test was used in conjunction with post-hoc pairwise Mann–Whitney tests to identify differences between sites. K-dominance curves were plotted for each sampled site to compare taxon-abundance distributions. Diversity profiles were plotted for each site using Hill numbers for values of α from 0.0 to 4.0.
Multivariate analyses were carried out on genus presence-absence and standardized biomass datasets to investigate differences between sites based on both taxon composition and taxon abundances. One-way analysis of similarity (ANOSIM) was used in conjunction with post-hoc pairwise one-way ANOSIM to identify differences between sites in terms of taxon composition and taxon abundances. The Jaccard similarity measure was used in the case of presence-absence data, while Bray–Curtis similarity was used in the case of biomass data. Subsequently, the similarity percentages breakdown (SIMPER) procedure with Bray–Curtis similarity was used to identify those taxa contributing most to any observed pairwise differences between sites. All samples were subsequently pooled and an overall multi-group SIMPER was performed to identify those taxa contributing most to the observed pattern of differences between sites in terms of both species composition and species abundances.
RESULTS
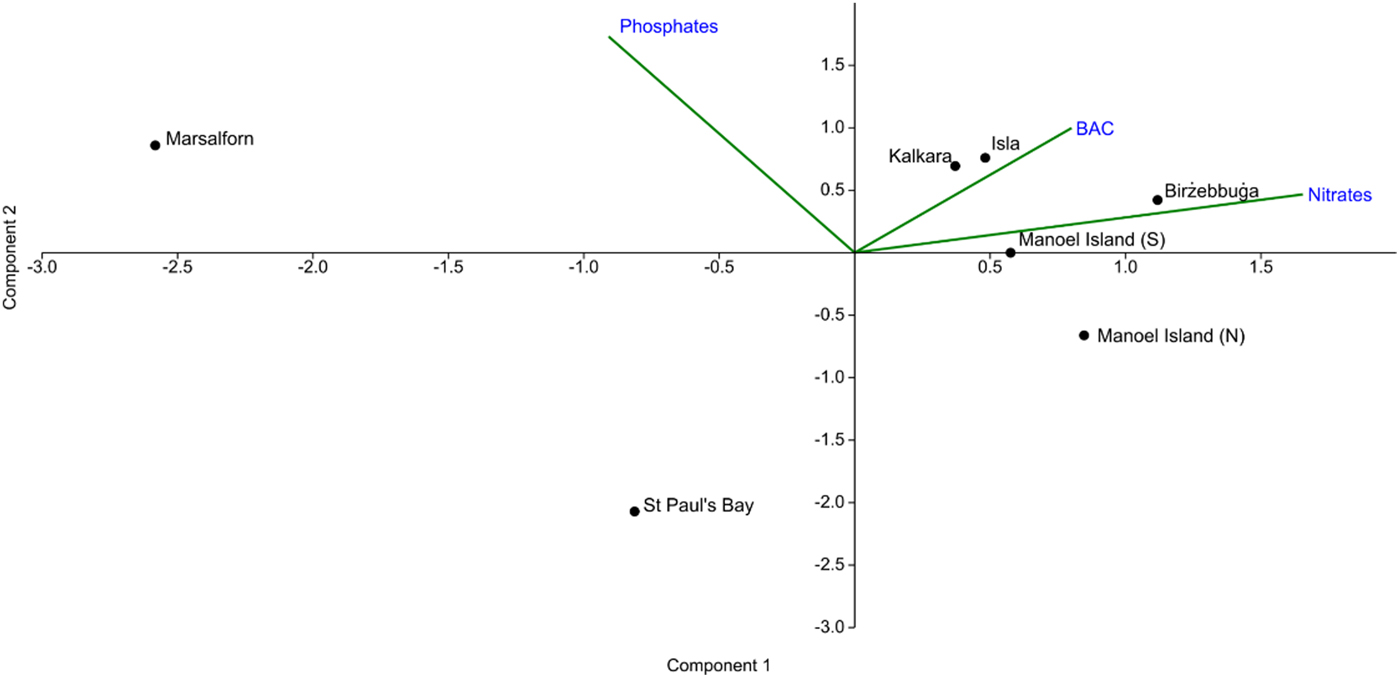
The PCA plot obtained (Figure 2) indicated that Marsalforn, which was a priori taken to be the reference site, is distinct from all other sites based on low mean values of BAC and nitrate concentration and a relatively high mean value of phosphate concentration. St Paul's Bay, which was considered to be an intermediate site, was characterized by a lower mean phosphate concentration but greater positive displacement along the vectors for BAC and nitrate concentration. All other sites were characterized by high BAC and nitrate concentrations, and were considered to be impacted.
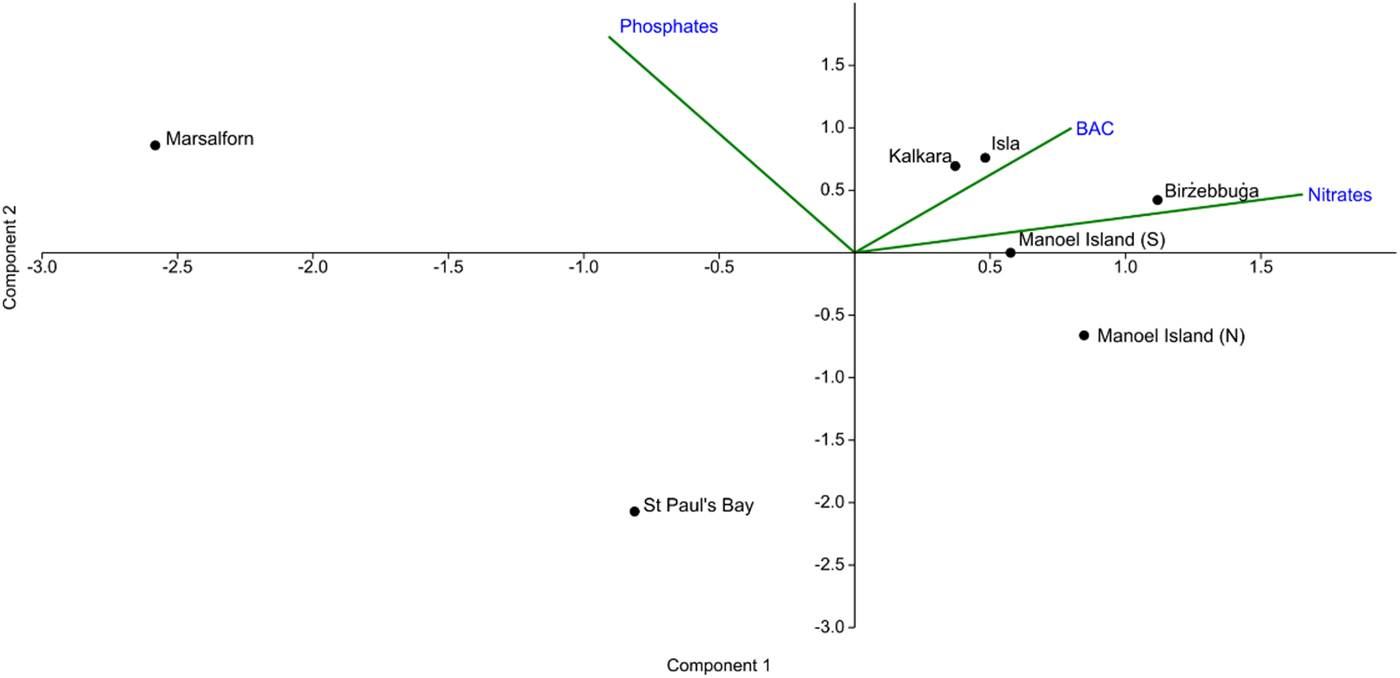
Fig. 2. PCA plot showing ordination of the study sites according to the abiotic factors considered. Component 1 accounts for 59.7% of the total variance of the dataset, while Component 2 accounts for 40.0%.
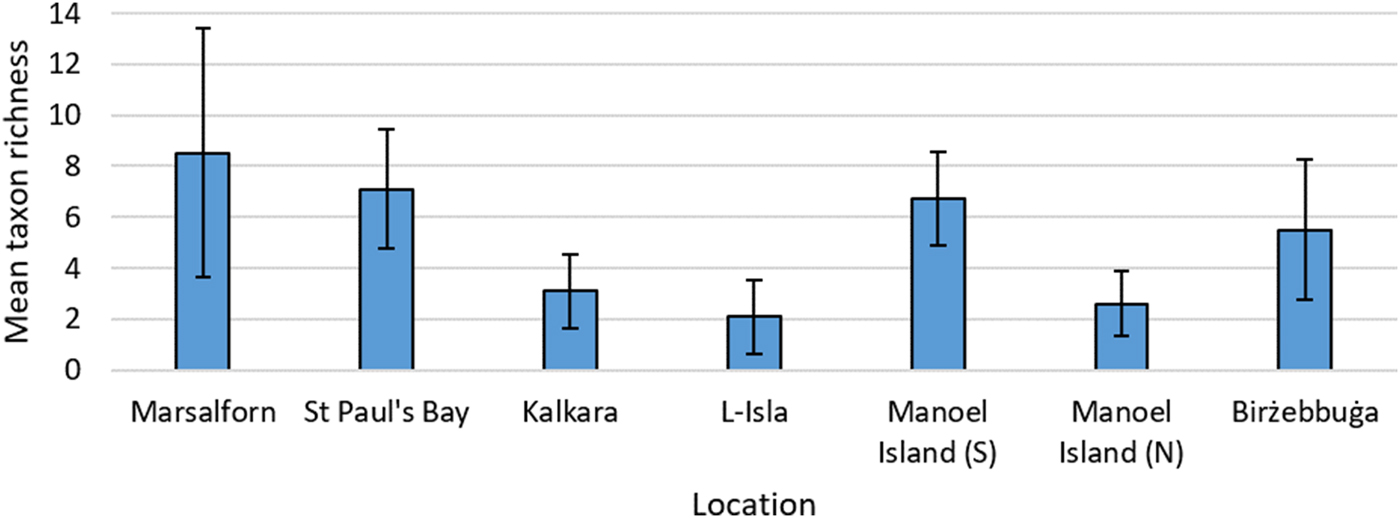
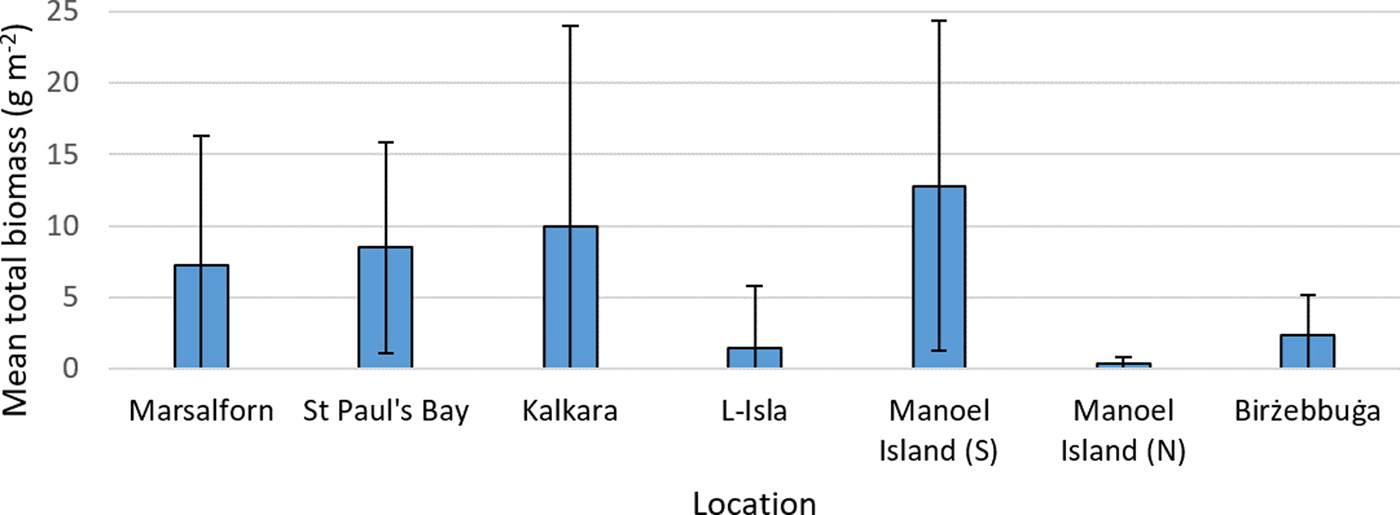
Mean macroalgal taxon richness (Figure 3) was observed to decrease from the reference site (Marsalforn) to the intermediate site (St Paul's Bay), and both supported a higher mean taxon richness than the impacted sites. The sites sampled were shown to differ significantly in terms of this variable (P < 0.05), and post-hoc tests indicate the presence of two main groups: (i) Marsalforn, St Paul's Bay, Manoel Island (S) and Birżebbuġa, and (ii) Kalkara, L-Isla and Manoel Island (N). Marsalforn, St Paul's Bay, Kalkara and Manoel Island (S) were all observed to have relatively high values of mean total biomass (Figure 4). Significant differences were once again observed between sites in terms of this variable (P < 0.05), and post-hoc tests indicated that Marsalforn, St Paul's Bay and Manoel Island (S) did not differ significantly between them and were similar to Kalkara (P < 0.05). L-Isla and Manoel Island (N) also did not show significant differences (P < 0.05). Birżebbuġa differed significantly (P < 0.05) from all sites apart from Kalkara (P = 0.34, U = 37).

Fig. 3. Mean macroalgal taxon richness calculated across all samples for each site. Error bars are ±1 standard deviation.

Fig. 4. Mean total macroalgal biomass for each site. Error bars are ±1 standard deviation.
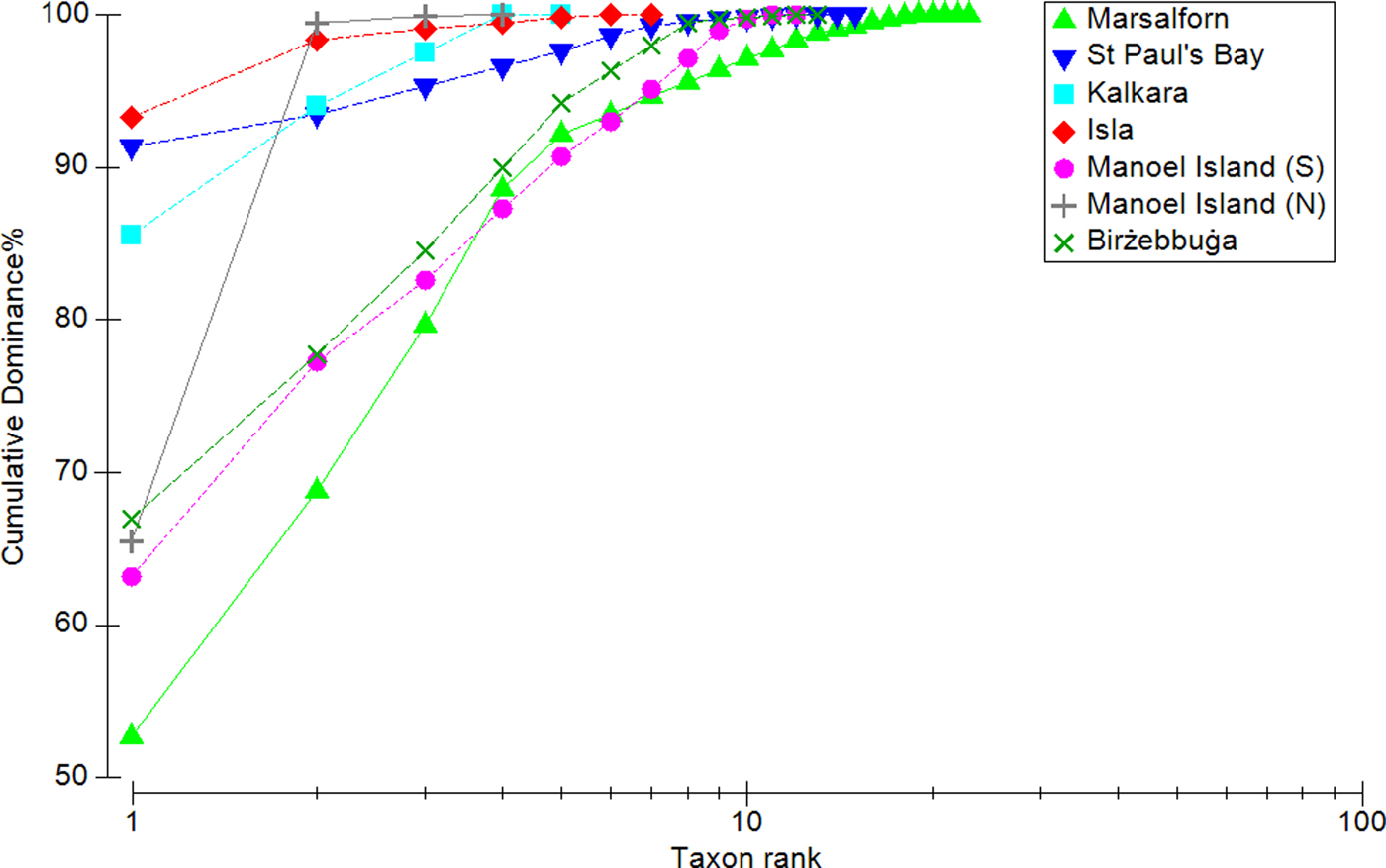
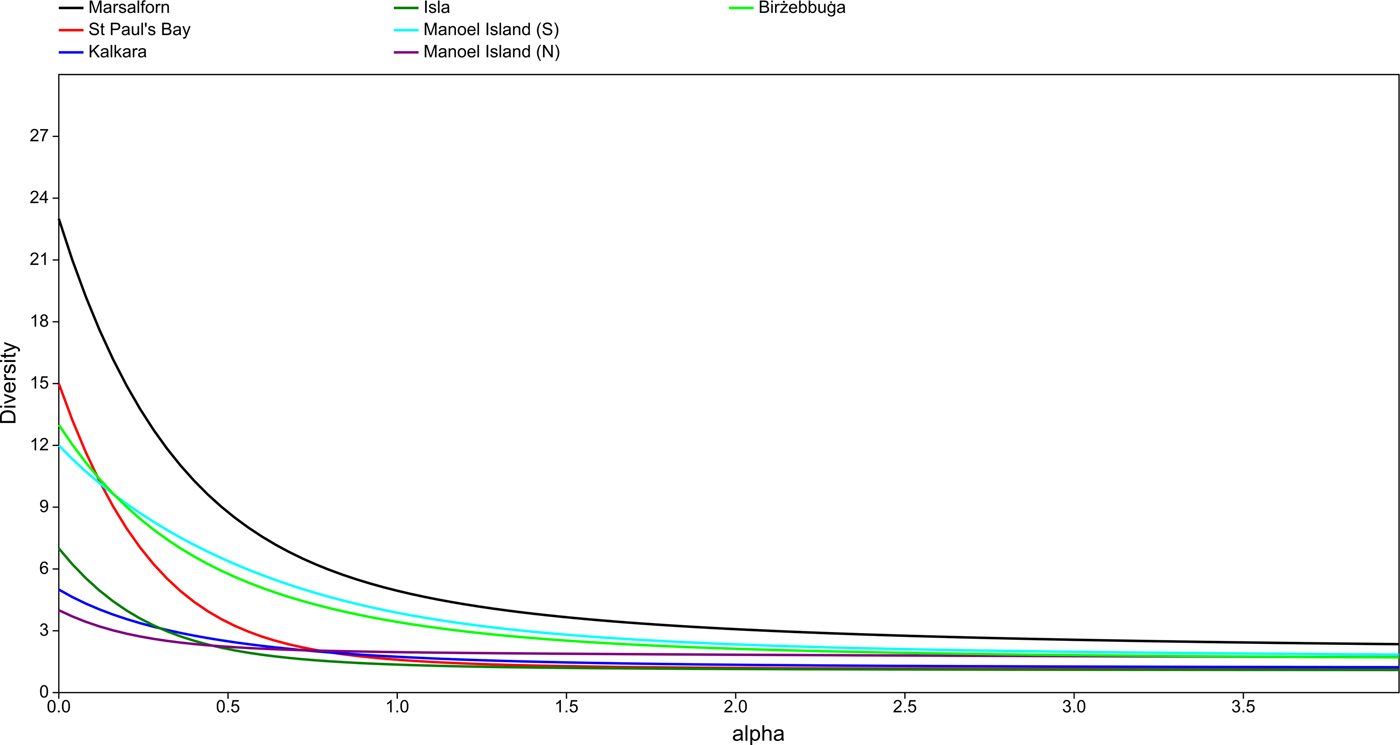
The K-dominance curves (Figure 5) showed all sampled sites to be dominated to some degree by one or a few taxa. Marsalforn produced the steepest curve and therefore has the highest evenness, while Kalkara, L-Isla and St Paul's Bay have the lowest evenness; in the latter sites, a single taxon constituted ~90% of the total mean biomass. In the case of Manoel Island (N), the sample was composed almost entirely of two taxa. The diversity profiles plotted for each site (Figure 6) appear to indicate the presence of three groups: (i) Marsalforn, (ii) Manoel Island (S) and Birżebbuġa, and (iii) St Paul's Bay, L-Isla, Kalkara and Manoel Island (N). The reference site is the most diverse across all values of α; Birżebbuġa and Manoel Island (S) are less diverse and their profiles overlap, indicating that their diversities cannot be separated. All other sites have low diversities and their profiles overlap. It should be noted that the diversity profile for St Paul's Bay does overlap with those for Birżebbuġa and Manoel Island (S) at very low values of α; this indicates that while St Paul's Bay has a relatively high taxon richness, samples from this site are likely to be dominated by one or a few taxa, thus decreasing overall diversity for indices with less emphasis on rare taxa.
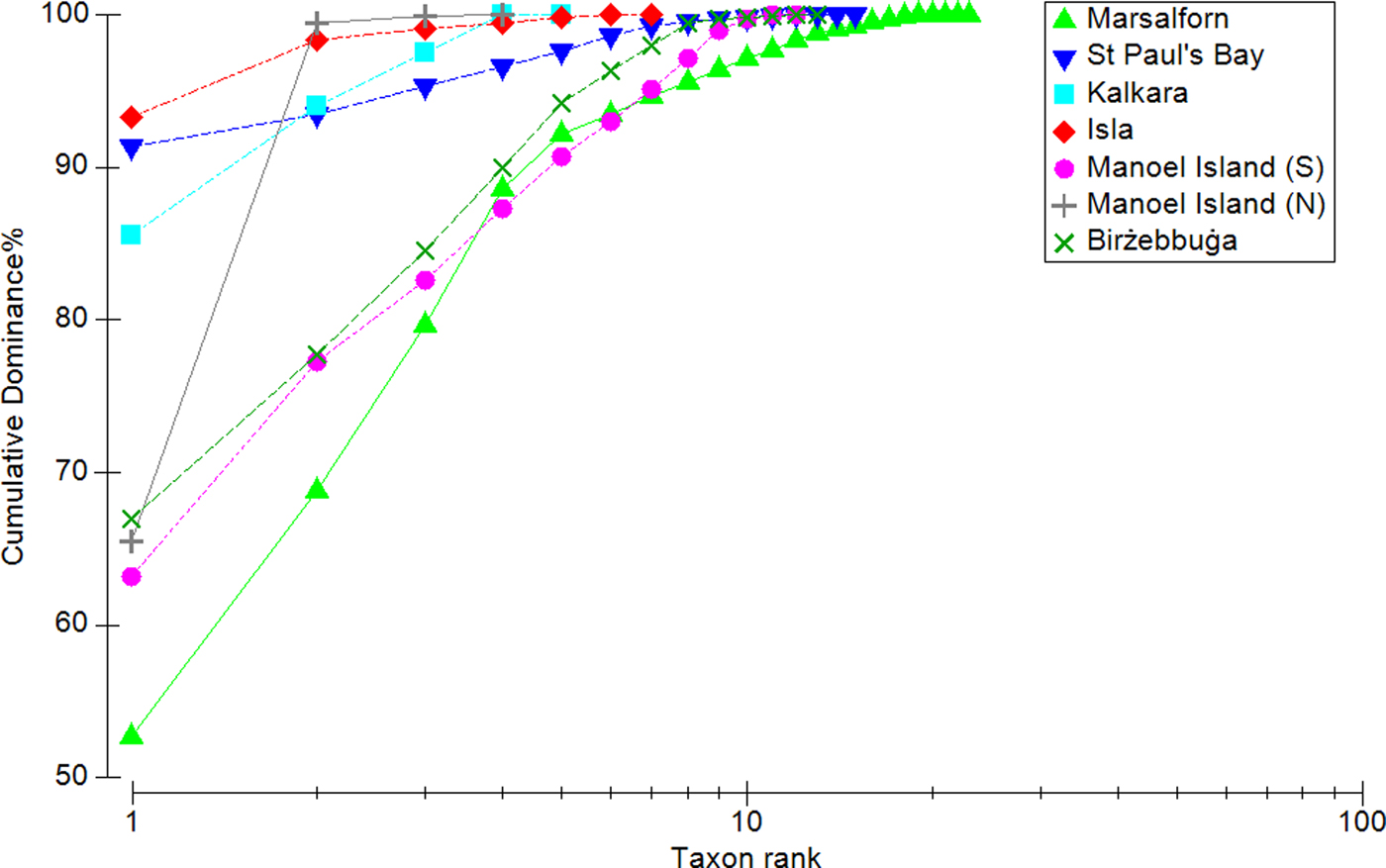
Fig. 5. K-dominance curves for each site, showing species-abundance distributions.

Fig. 6. Diversity profiles for each site, plotted using Hill numbers for values of α from 0.0 to 4.0.
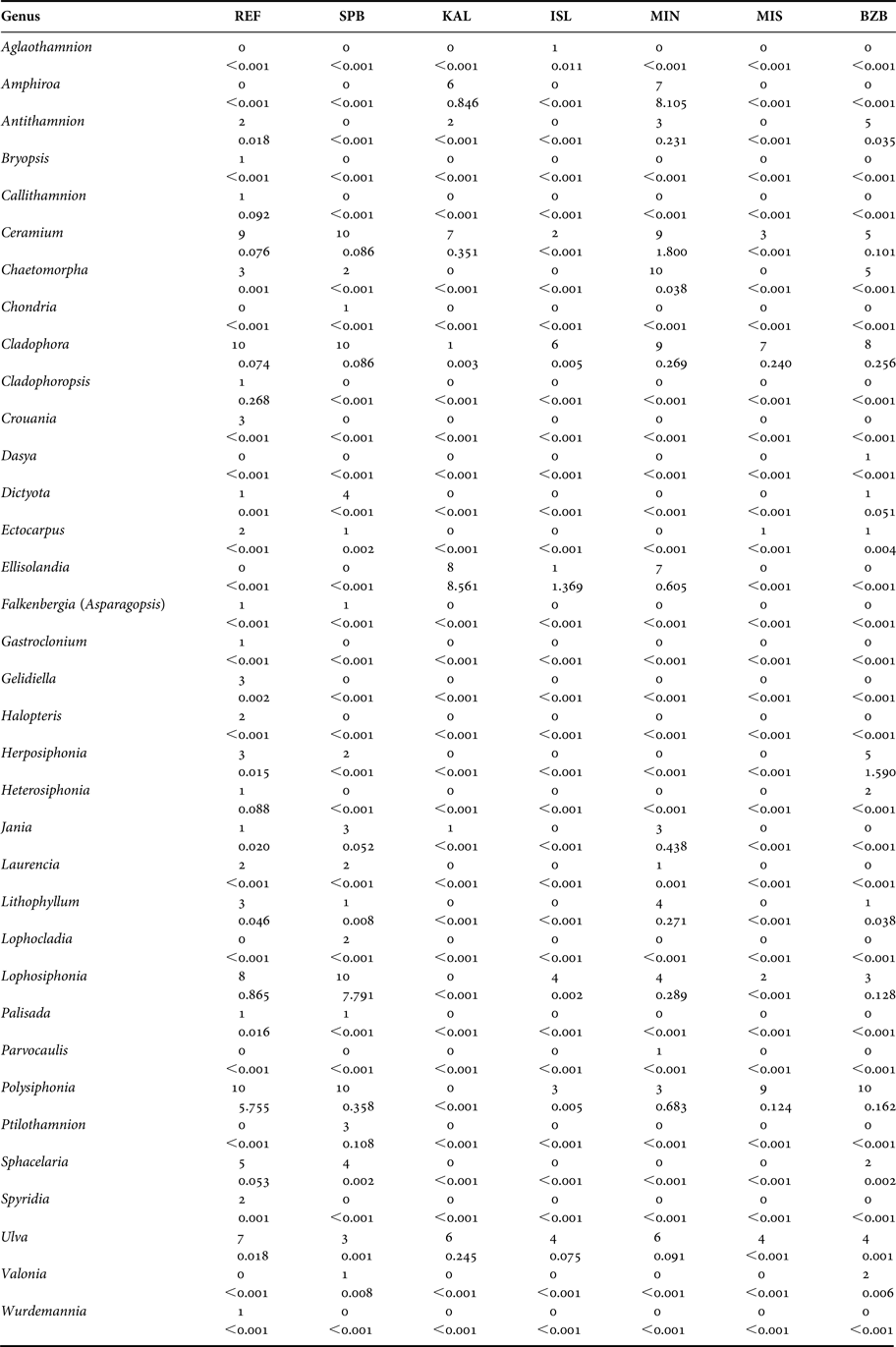
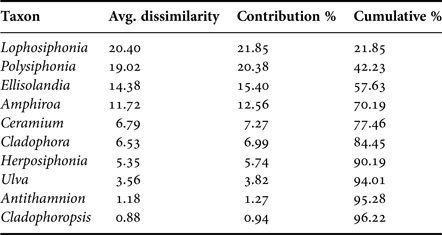
A total of 35 genera were encountered over the course of this investigation (Table 1). One-way analysis of similarity (ANOSIM) on genus presence-absence data using the Jaccard coefficient showed a significant difference between sampled sites (P < 0.05, R = 0.466). Pairwise one-way ANOSIM indicated that Marsalforn and St Paul's Bay did not differ in terms of taxon composition (P = 0.057, R = 0.108). Furthermore, no differences were observed between L-Isla and Manoel Island (N) (P = 0.848, R = 0.119). Otherwise, all sites differed significantly from all other sites. The similarity percentages (SIMPER) procedure using the Bray–Curtis coefficient indicated that the genera contributing most to the overall pattern observed are either filamentous rhodophytes (e.g. Polysiphonia spp.), coralline rhodophytes (e.g. Ellisolandia spp.), or chlorophytes (e.g. Ulva spp.) (Table 2). Pairwise SIMPER procedures using the Bray–Curtis coefficient indicated that filamentous rhodophytes contributed most to differences between the reference and intermediate sites and all other sites, while filamentous rhodophytes and chlorophytes contributed to differences between L-Isla and the remaining sites. Coralline rhodophytes contributed significantly to differences between Kalkara and the remaining sites.
Table 1. Frequency (0–10 buoys, top) and average biomass (g m−2, bottom) of each genus encountered in each site sampled.

Table 2. Pooled SIMPER (Bray–Curtis similarity) results showing the 10 genera contributing most to observed differences in species composition between sites.

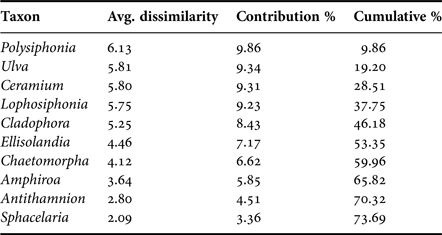
One-way analysis of similarity (ANOSIM) on genus abundance data using the Bray–Curtis coefficient also showed a significant difference between sites (P < 0.05, R = 0.578). Pairwise one-way ANOSIM indicated that all sites differed from all other sites with the exception of Manoel Island (N) and Birżebbuġa (P = 0.19, R = 0.062). The similarity percentages (SIMPER) procedure using the Bray–Curtis coefficient indicated that the genera contributing most to the overall pattern observed are once again filamentous rhodophytes, coralline rhodophytes, or chlorophytes (Table 3). In this case, the coralline rhodophytes contributed more to the observed differences than was the case for taxon composition data. Pairwise SIMPER procedures using the Bray–Curtis coefficient indicated that filamentous rhodophytes contributed most to differences between the reference and intermediate sites and all other sites besides L-Isla, Kalkara and Manoel Island (S), where coralline rhodophytes contributed in an equal amount. Coralline rhodophytes were observed to contribute significantly to differences between Kalkara and all other sites, L-Isla and all other sites, and Manoel Island (S) and all other sites.
Table 3. Pooled SIMPER (Bray–Curtis similarity) results showing the 10 genera contributing most to observed differences in community structure between sites.

DISCUSSION
In general, the results obtained indicated that lower levels of nutrient enrichment and turbidity were associated with higher macroalgal taxon richness and overall abundance. Inverse relationships between these aspects of macroalgal community structure and the abiotic variables considered have also been observed in the case of rocky-shore benthic assemblages (Diez et al., Reference Diez, Secilla, Santolaria and Gorostiaga1999; Arévalo et al., Reference Arévalo, Pinedo and Ballesteros2007), and the phenomenon has been associated with a simplification of macroalgal community structure along a gradient of increasing impact in terms of these abiotic variables (Diez et al., Reference Diez, Secilla, Santolaria and Gorostiaga1999). Lower levels of the abiotic factors considered were also associated with an increase in evenness and diversity, as indicated by the k-dominance curves (Figure 5) and diversity profiles (Figure 6) obtained. The reference site was observed to support the most diverse assemblages overall, followed by Manoel Island (S) and Birżebbuġa, while the remaining sites, including the intermediate site, supported the least diverse assemblages. Since taxon richness at the intermediate site was found to be similar to that of the reference site, this suggests that a large number of the genera encountered in this site were rare or infrequent. This may indicate that the changes in levels of nutrient enrichment and turbidity from the reference site to the intermediate site lead to a reduction in the abundances of genera which are intolerant to such environmental variation, and to dominance of the assemblage by more tolerant genera. This results in a reduction in overall diversity with no change in taxon richness. This is corroborated by the results obtained from multivariate analyses; macroalgal fouling assemblages from St Paul's Bay were similar in taxon composition to the reference site but differed in terms of taxon abundances. Therefore, taxon abundances may respond to changes in environmental conditions before taxon replacement occurs; once again, similar results have been obtained from studies conducted on benthic rocky-shore assemblages (Arévalo et al., Reference Arévalo, Pinedo and Ballesteros2007). This has implications in terms of environmental monitoring as it suggests that macroalgal fouling communities on buoys are sensitive to intermediate levels of nutrient enrichment and turbidity and are able to respond in a manner which is detectable and which is therefore useful for monitoring programmes.
L-Isla and Manoel Island (N) were statistically similar in terms of taxon composition. These sites supported a low taxon richness, and it is therefore possible that the macroalgal fouling assemblages present only comprise genera resistant to increased levels of nutrient enrichment and turbidity. Therefore, the similarity in taxon composition between these sites may be due to the total exclusion of genera intolerant to these environmental conditions in both cases. Previous studies have shown that along a gradient of nutrient enrichment, the most impacted sites are consistently characterized by low taxon richness and the presence of nitrophilic taxa such as Ulva spp. and Cladophora spp. (Arévalo et al., Reference Arévalo, Pinedo and Ballesteros2007; Pinedo et al., Reference Pinedo, Garcia, Satta, de Torres and Ballesteros2007), both of which were recorded from the sites under consideration here. Assemblages from sites which were considered to be impacted all varied both in terms of taxon composition and in terms of abundances. In general, filamentous rhodophytes, filamentous chlorophytes or geniculate coralline forms contributed most to the observed difference between sites. It is possible that the nature of the artificial substratum sampled precludes the establishment of larger, more robust macroalgae and therefore favours the proliferation of filamentous taxa at many of the sites considered. Coralline rhodophytes were found to have a greater contribution to overall differences between sites when taxon abundances were taken into consideration; this may be a result of the relatively large mass per unit volume of coralline macroalgae in comparison to other taxa. Since this study did not investigate a defined environmental gradient across sampling sites, the observed variation between impacted sites is not unexpected. Stochasticity and spatial processes related to dispersal are expected to affect the structuring of macroalgal communities (Okuda et al., Reference Okuda, Noda, Yamamoto, Hori and Nakaoka2010), and therefore differences in taxon composition between sites may be due to these factors.
Previous studies have indicated that macroalgal communities occurring in impacted and non-impacted sites may be distinguished based on the relative dominance of particular taxa or taxon suites (Mayer-Pinto & Junqueira, Reference Mayer-Pinto and Junqueira2003; Arévalo et al., Reference Arévalo, Pinedo and Ballesteros2007). However, the results of the present investigation indicate that this approach may not be useful for environmental monitoring, at least in the local context. Macroalgal fouling communities at the sites sampled were found to be highly variable in terms of taxon composition, and this variation does not appear to be a response to differences in levels of nutrient enrichment or turbidity. Conversely, aspects of community structure such as macroalgal taxon richness, abundance and diversity were influenced by the abiotic factors considered: lower levels of nutrient enrichment and turbidity were associated with higher macroalgal taxon richness, abundances and diversity. Previous studies have also shown that these aspects of community structure tend to be responsive to changes in water quality (Diez et al., Reference Diez, Secilla, Santolaria and Gorostiaga1999; Fabricius et al., Reference Fabricius, De'ath, McCook, Turak and Williams2005; Arévalo et al., Reference Arévalo, Pinedo and Ballesteros2007). Furthermore, the case of St Paul's Bay has shown that taxon richness, abundance and diversity are sensitive to intermediate levels of impact and respond accordingly, which is fundamental to the successful utilization of these assemblages for environmental monitoring purposes. Macroalgal fouling communities on buoys could therefore potentially be used for the purposes of water quality monitoring, at least in the local context, if these aspects of community structure are assessed regularly in target sites over a defined period of time.
In conclusion, the results of this investigation indicated that lower levels of nutrient enrichment and turbidity were associated with higher macroalgal taxon richnesses and overall abundances. Assemblages from all sampled sites were dominated by one or a few genera to some extent. However, in general, lower levels of nutrient enrichment and turbidity were associated with an increase in taxon diversity. Macroalgal fouling assemblages at the intermediate site were similar in taxon composition to the reference site but differed in terms of overall taxon abundances and were less diverse. Therefore, macroalgal abundances may respond to changes in environmental conditions before taxon replacement occurs. Impacted sites all differed both in terms of taxon composition and in terms of abundances, indicating that macroalgal fouling assemblages may be influenced by factors other than those considered in this investigation, including stochasticity. The results of this investigation suggest that macroalgal taxon dominance and taxon composition in fouling communities may not be applicable as indicators of water quality. Conversely, aspects of community structure such as taxon richness, abundance and diversity were influenced by the abiotic factors considered. Therefore, there is potential for the utilization of macroalgal fouling communities for water quality monitoring in the Maltese Islands.
ACKNOWLEDGEMENTS
We are grateful to Professor Enric Ballesteros for help with the identification of certain taxa and to Dr Julian Evans for advice regarding data analysis. We would like to thank both anonymous reviewers for their constructive comments, which contributed greatly to the improvement of this manuscript.
FINANCIAL SUPPORT
Veronica Farrugia Drakard was funded by the ENDEAVOUR Scholarship Scheme (Malta), part-financed by the European Union – European Social Fund (ESF) under Operational Programme II – Cohesion Policy 2014–2020, ‘Investing in human capital to create more opportunities and promote the wellbeing of society’.