INTRODUCTION
Parablennius ruber (Valenciennes, 1836), the red or Portuguese blenny, is one of the nine species of blennioid fish occurring in the Azores (Oliveira et al., Reference Oliveira, Almada, Almeida, Santos and Gonçalves1992). It spawns in winter in rock crevices where the male guards the eggs until they hatch (Azevedo & Homem, Reference Azevedo and Homen2002).
Originally described from France, P. ruber was often synonymized with Parablennius gattorugine (Linnaeus, 1768), until its identity was established independently by Almeida (Reference Almeida1982) and Bath (Reference Bath1982). Later, P. ruber has been reported as abundant from the Azores and Madeira waters by several authors (see list in Arruda, Reference Arruda1997), but it is apparently rare elsewhere, and this has undoubtedly led to its classical common name (Portuguese blenny). In 1983, Devesa & Solórzano (Reference Devesa and Solórzano1983) reported it from Galicia (north-western Spain) as Blennius ruber. Recently, Almada et al. (Reference Almada, Domingues, Monteiro, Almada and Santos2007) have confirmed the presence of this species in western Europe while Goodwin & Picton (Reference Goodwin and Picton2007) summarized all the records for the red blenny in the British Isles. The latter authors mention that it has probably always been resident in this area, but could have been overlooked in past faunal surveys. Almada et al. (Reference Almada, Domingues, Monteiro, Almada and Santos2007), however, suggest that the species rarity at those latitudes may be linked to a temperature-related inability to establish viable populations. These authors further hypothesize that long distance larval transport from the Azores may, in a considerable measure, be the source of the P. ruber populations on the Atlantic shores of western Europe, rather than a resident breeding population.
One way to test the hypothesis of Almada et al. (Reference Almada, Domingues, Monteiro, Almada and Santos2007) is to look for oceanic P. ruber larvae. Unfortunately, there is no complete description of the eggs and larvae of this species, apart from the brief textual description of the eggs and embryos given by Santos (Reference Santos1987). This paper contributes to fill this gap by describing and illustrating for the first time the development of the eggs and early larval stages of this species.
MATERIALS AND METHODS
Definitions of measurements follow Hubbs & Lagler (Reference Hubbs and Lagler1964). Measurements were performed with an ocular micrometer to the nearest 0.01 mm. The illustrations of Figure 1 and Figure 2 were prepared with the aid of a camera lucida. Specimens examined were deposited in the Museu Nacional de Historia Natural–Museu Bocage (Lisbon), with the collection numbers MB55-0001, MB55-0002, MB55-0003, MB55-0004, MB55-0005, MB55-0006, MB55-0007 and MB55-0008.

Fig. 1. Eggs (1.02 mm diameter) at different development stages: (A) medium stage egg; (B) transitional stage; (C) late stage egg.
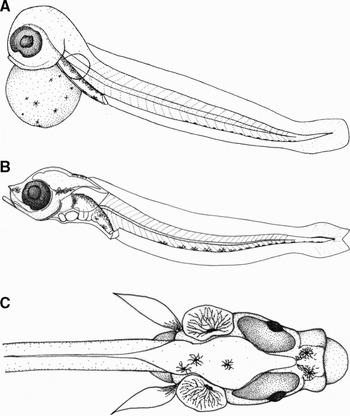
Fig. 2. Larvae at different development stages: (A) yolk-sac larva (4.65 mm TL); (B) preflexion larva, lateral view (4.51 mm TL); (C) preflexion larva, dorsal view of the anterior section.
Reproductive males of Parablennius ruber were located by SCUBA diving in two protected bays (Capelas and Santa Iria) on the north coast of São Miguel Island, Azores, Portugal. The field work was carried out from March to May 2007. Nests were found on crevices of vertical or steeply sloping rock faces, at depths ranging from 0 to 8 m.
Parental males of P. ruber were momentarily and carefully separated from the nests, and a sample of the guarded eggs removed with a plastic pipette. No eggs were removed from nests whose parental male was not present. Once in the laboratory the eggs were placed on a Petri dish in seawater at room temperature (20° to 22°C). A drop of methylene blue was added to prevent infections (Faria et al., Reference Faria, Borges, Gil, Almada and Gonçalves2002). The eggs were observed and photographed daily. Larvae were reared in the laboratory from these wild eggs. Newly hatched larvae were transferred to a 20 l constantly aerated aquarium and kept until the complete absorption of the yolk sac. Eggs and larvae were fixed in 4% buffered formalin (1–2 weeks) and conserved in 70% ethyl alcohol. The egg capsules were removed and the embryos distended and dyed with methylene blue in order to allow further observations of the structures.
RESULTS
Description of the spawn
The eggs were deposited on a single layer about 20–40 cm2 in area. Eggs in different stages of development were found in the same nest. The eggs were spherical (with a slight flattened attachment to the substrate), the mean diameter of 1.02 mm (SD = 0.03, range: 0.97–1.05, N = 16) varying very little with the development stage. Recently laid eggs were orange, and turned to glittering greyish as the development went on and the eyes became more apparent.
Description of the eggs
The middle egg development stage (sensu Kendall et al., Reference Kendall, Ahlstrom, Moser, Moser, Richards, Cohen, Fahay, Kendall and Richardson1984) is shown in Figure 1A. At this phase the yolk was completely spherical. The embryo began to be recognizable and presented a dilatation in the region where the head would eventually appear. The oil globules were recognizable as small spheres (0.02 mm) in the yolk, which contained 7–13 punctuated melanophores that began to be stellated. The eyes were not formed yet, but a small dilatation was shaped in the area where they would eventually be formed. The yolk diameter averaged 0.73 mm (SD = 0.04, range 0.68–0.82, N = 8), with 3–8 brown, round spots, bigger than the melanophores. No fin folds were present. The tail bud tip was rounded and attached to the yolk.
In the next 24–30 hours the eggs suffered great transformations (Figure 1B). The yolk began to be bilobulated (kidney shaped) (major axis 0.73 mm and minor axis 0.51 mm). The eyes were a jelly-like, flimsy mass, unpigmented except for the grey upper part. The iris began to be recognizable as a slight circular silhouette. The pectoral fins buds were weakly distinguished. The melanophores were typically stellated. The embryo did not move in the egg.
Figure 1C represents a late stage of development (late stage egg), shortly before hatching. The eyes were big (major axis 0.26 mm) and iridescent blue. The iris was dark and stood out of the eye plane. The heart was easily recognizable and evident as two lobules. The yolk was strongly bilobulated (major axis 0.82 mm and minor axis 0.49 mm). The melanophores in the yolk were few (0–7) and branched. The dorsal, caudal and anal fins formed a continuous fold. The pectoral fins were small without rays. The digestive tube and peritoneal cavity were dorsally pigmented with branched melanophores. Small orange chromatophores were sometimes visible on the head, between the eyes. The embryo rotated and trembled continuously inside the egg, with whip-like movements of the tail. The eyes moved independently. The blood circulation was clearly observable. The blood was transparent inside the eyes and reddish along the tail. There was one marked vessel that crossed the yolk, transporting dark red blood.
Description of the larvae
Recently hatched larvae (yolk-sac larva) (Figure 2A) measured 4.65 mm total length (TL) (SD = 0.25, range 4.19–5.10, N = 21). They settled to the bottom of the aquarium, from where they would occasionally swim around for up to a few minutes. As far as the yolk was too heavy, longer periods of swimming were not observed. The mouth was not completely open and the jaws and lips were not formed yet. The anus was open. The eyes were fully pigmented. The yolk was perfectly spherical, with branched melanophores. The peritoneal cavity was dorsally pigmented, and the digestive tube had 2–3 ventral melanophores in the terminal section, close to the anus. The tail was rounded at the tip, with a sometimes inconspicuous row of melanophores along the ventral section. The pectoral fins were small (6.2% TL) and rounded, without rays. Though still in development, they allowed the larvae to swim for short periods. The caudal, dorsal and anal fins formed a continuous fold. Mean dimensions and counts (including myomeres) of this stage are given in Table 1.
Table 1. Mean dimensions (mm) and counts diagnostic of the preserved yolk-sac and preflexion larvae of the red blenny, Parablennius ruber.

A three day larva (preflexion larva) is presented in Figure 2B. The head was pointed. The mouth was big, open and prominent (Figure 2C). The anus was open. The jaws were strong with thick formed lips. The operculae were open and gills were visible. The peritoneal cavity was dorsally pigmented by branched melanophores. The terminal region of the digestive tube, near the anus, was also ventrally coloured by melanophores. The eyes were bigger in this stage (see Table 1) and began to have an orange shade all along their perimeter. The head had three melanophores at the top, and the snout had two. There was one melanophore on each otic capsule. The sagittae and lapilli otoliths were observable as small glittering scales in the otic capsules. Orange chromatophores were scattered between the eyes, on the snout and above the brain. The body presented a ventral row of branched melanophores. There were dorsal and ventral melanophores on the tail tip. The pectoral fins were relatively long (9.4% TL), pointed and pigmented by a single melanophore at the base. No fin rays were observed. The caudal section of the fin fold was no longer rounded, but forked. Notochord flexion had not occurred yet. Table 1 provides the mean dimensions and counts of the three day larva.
DISCUSSION
Nest sites are very similar to those used by other related blennies like Parablennius gattorugine and Coryphoblennius galerita (Linnaeus, 1758) as described by Fives (Reference Fives1986), Lipophrys pholis (Linnaeus, 1758) as described by Faria et al. (Reference Faria, Borges, Gil, Almada and Gonçalves2002) and Lipophrys trigloides (Valenciennes, 1836) as described by Faria et al. (Reference Faria, Gil and Almada2005). The egg size is comparable to that of C. galerita (Fives, Reference Fives1986) but slightly smaller than other blennioid eggs (Fives, Reference Fives1986; Faria et al., Reference Faria, Borges, Gil, Almada and Gonçalves2002, Reference Faria, Gil and Almada2005).
The presence of eggs in different stages of development in the same nests indicates a successive spawning of one or more females, a typical situation in this family (Westernhagen, Reference Westernhagen1983; Kraak, Reference Kraak1996; Faria et al., Reference Faria, Gil and Almada2005).
Our observations on eye formation, embryo movements, moment when blood circulation begins to be recognizable in the eggs and melanophore shape and distribution agree with those of Santos (Reference Santos1987). This author, however, stated that late embryos have no formed pectoral fins, whereas we observed that early in egg development the pectoral fin bud is already perceptible. Newly hatched larvae have small pectoral fins, which allow them to swim for short periods.
The larva of P. ruber has the general characteristics of the Blenniidae family according to Fives (Reference Fives1986): pre-anal region 1/4 to 1/3 of the total length, digestive tube dorsally pigmented, pectoral fins without spines and well developed; being also in agreement with Ré (Reference Ré1999): larvae with intense peritoneal and occipital pigmentation, pectoral fins well developed and sometimes very pigmented.
The larval stage of the red blenny is quite similar to other sympatric blenny species whose larvae have been described.
Recently hatched larvae of P. ruber differs from those of the closely related species, P. gattorugine, as described by Ford (Reference Ford1922) and Lebour (Reference Lebour1927) in that the pectoral fins were pointed, the snout prominent and the tail forked at the 5 mm TL larval stage in P. ruber, whereas P. gattorugine larvae at the 6 mm TL stage have rounded pectoral fins, a little marked snout and a curved tail. The patterns of pigmentation are quite similar in both species at this stage.
Larvae of P. ruber are to some extent similar to those of the widespread P. pilicornis, as described by Faria et al. (Reference Faria, Gil and Almada2006). The main differences of the recently hatched larvae are the form of the pectoral fins (pointed in the red blenny at the 5 mm stage but still perfectly rounded in P. pilicornis at the 6 mm stage), the small or absent yolk-sac (it was present in P. ruber) and the scarce prominence of the snout in the 6 mm TL stage larvae (it was remarkably prominent in the 5 mm TL stage in the red blenny).
Larvae of Blennius ocellaris (Linnaeus, 1758) mainly differ from those of the red blenny in that they hatch with pectoral fins radially pigmented (there is no radial pigmentation in the red blenny), have a ventral row of only 4 to 6 melanophores in the body and tail (there were 14 to 17 in P. ruber) and the snout is almost not prominent at the stage when the yolk is fully absorbed, according to the descriptions and illustrations provided by Ford (Reference Ford1922), Fives (Reference Fives1986) and Halbeisen (Reference Halbeisen1988).
Coryphoblennius galerita newly hatched larvae differ in that they have radially pigmented pectoral fins that extend to the second or third postanal body segment, they hatch with little or no yolk and the top and back of the head is scattered with melanophores (Fives, Reference Fives1986). None of these characters is shared by the early larval stage of the red blenny.
Lipophrys pholis larvae as described by Faria et al. (Reference Faria, Borges, Gil, Almada and Gonçalves2002) are different in the presence of teeth when hatching, the radial pigmentation of the pectoral fins, the absence of yolk, the scattered pigmentation over the brain and the presence of only 7–9 ventral melanophores in the tail.
Lipophrys trygloides recently hatched larvae have little or no yolk, pectoral fins radially pigmented and longer than those of the red blenny, according to Faria et al. (Reference Faria, Gil and Almada2005).
The hypothesis that the Azores may be supplying individuals to the west Atlantic coasts (Almada et al., Reference Almada, Domingues, Monteiro, Almada and Santos2007) predicts that the larvae of this species could be found in the North Atlantic Current, flowing past the Azores to north-eastern Europe. The present description of the larvae of P. ruber will therefore be useful in future oceanographic surveys, but it should be complemented with the description of the late larval and early juvenile stages of this species.
ACKNOWLEDGEMENTS
The authors would like to thank Afonso Prestes and Nuno Pereira for their technical aid, and the anonymous referees for their comments on the manuscript.





