Introduction
Primordial germ cells (PGCs) are the embryonic precursors of gametes. In the mouse, the germ-cell lineage is induced from the proximal epiblast of the egg cylinder at embryonic day 6.5 (E6.5) in response to signalling by bone morphogenetic protein 4 (Bmp4) and Bmp8b (Zhao et al., Reference Zhao, Deng, Labosky, Liaw and Hogan1996; Lawson et al., Reference Lawson, Dunn, Roelen, Zeinstra, Davis, Wright, Korving and Hogan1999). PGCs can first be detected at E7.5 as a cluster of alkaline phosphatase-positive cells at the base of the allantois (Ginsburg et al., Reference Ginsburg, Snow and McLaren1990). From E8.5, founder PGCs migrate through the embryo and arrive at the genital ridge around E10.5, where they become known as gonocytes (McLaren, Reference McLaren2003). After a short period of mitosis, germ cells either cease mitosis and enter meiosis in the female, or progressively arrest in G0/G1 over several days in the male (Childs et al., Reference Childs, Saunders and Anderson2008; Zhou et al., Reference Zhou, Meng and Li2010). In mice, the vast majority of oocytes has entered meiosis during embryonic life, and at birth some oocytes are in the transitory stages of prophase (pachytene and early diplotene), while others have entered late diplotene and dictyate in which they apparently remain until meiosis resumes shortly before ovulation (Pedersen & Peters, Reference Pedersen and Peters1968; Pangas & Rajkovic, Reference Pangas and Rajkovic2006).
Germ-cell development in mammals is regulated by transcription factors that ensure appropriate development of PGCs and eventually, oocytes in females and sperm in males. Recently, the transcription factor MAEL has been reported to play an essential role in mouse spermatogenesis (Soper et al., Reference Soper, van der Heijden, Hardiman, Goodheart, Martin, de Boer and Bortvin2008). MAEL is a component of the mouse meiotic nuage and of the PIWI/piRNA pathway implicated in transposon silencing in fruit flies and mice (Costa et al., Reference Costa, Speed, Gautier, Semple, Maratou, Turner and Cooke2006; Aravin et al., Reference Aravin, van der Heijden, Castaneda, Vagin, Hannon and Bortvin2009. The Drosophila homologue of mouse Mael, maelstrom, is required for posterior positioning of the microtubule-organizing centre in oocytes (Clegg et al., Reference Clegg, Findley, Mahowald and Ruohola-Baker2001). In mice, Mael expression is restricted to testis and ovary, and is developmentally regulated (Bonilla & Xu, Reference Bonilla and Xu2008). However, the role of Mael in mouse oogenesis has not been reported.
In the present study we were interested in the role of Mael in mouse oogenesis. The model for this study was based on the culture of fetal ovaries. By using this model, hundreds of fully grown oocytes at the germinal vesicle (GV) stage are obtained from each fetal ovary after 7 days of culture (De Felici, Reference De Felici and Mazo1991; Bonilla & del Mazo, Reference Bonilla and del Mazo2010). In addition, we were interested in analysing the role of Mael during differentiation of embryonic stem cells (ESC) into germ cells in vitro (Hübner et al., Reference Hübner, Fuhrmann, Christenson, Kehler, Reinbold, De La Fuente, Wood, Strauss, Boiani and Schöler2003; Toyooka et al., Reference Toyooka, Tsunekawa, Akasu and Noce2003; Clark et al., Reference Clark, Bodnar, Fox, Rodriquez, Abeyta, Firpo and Pera2004; Geijsen et al., Reference Geijsen, Horoschak, Kim, Gribnau, Eggan and Daley2004). The expression of Mael was blocked in fetal ovary explants in culture and in ESC during germ line differentiation process using RNAi technology.
Material and methods
Experimental animals
All experimental procedures that involve the use of mice were performed under the regulations established by our Institutional Bioethics Committee on animal care, in accordance with those approved by the National Institutes of Health (Bethesda, MD, USA).
In vitro culture of fetal oocytes
Fetal ovaries were dissected from 17.5 days post-coitum mouse CD-1 fetuses and each ovary was cut into three fragments with fine needles (G 5/8) under a stereomicroscope. Ovary explants were cultured in 1 ml Waymouth medium (Gibco) supplemented with 5% horse serum, 2.5% fetal calf serum (both heat inactivated) and 100 μg/ml penicillin–streptomycin in 4-well dishes (Nunc) as described previously (De Felici, Reference De Felici and Mazo1991; Bonilla & del Mazo, Reference Bonilla and del Mazo2010). Half of the medium was changed every day starting from day 3 of culture, to allow the explants to attach to the bottom of the wells. Each well contained one ovary. The cultures were maintained at 37°C in a humidified incubator under 5% CO2 in air for 7 days, when the number of oocytes at the GV stage that had a diameter of 40–70 μm grown out of each fetal ovary cultured was analysed.
Mouse embryonic stem cell maintenance and differentiation
Mouse ESC maintenance was carried out according to the protocol described on the International Gene Trap Consortium (IGTC) website (http://www.genetrap.org/info/protocols/baygenomics/feederES.html). Feeder-independent ESC line ES-E14TG2a (E14) was used. ESC were cultured in ESC culture medium (ES cell medium: Glasgow Modified Minimal Essential Medium (GMEM) medium (Sigma)) supplemented with 2 mM glutamine (Gibco-BRL), 1 mM sodium pyruvate (Gibco-BRL), 1× non-essential amino acids, 10% (v/v) fetal bovine serum (characterized, HyClone), 0.1 mM beta-mercaptoethanol, and 1000 units per ml of leukocyte inhibitory factor (LIF, Chemicon) at 37°C in a humidified 5% CO2 incubator. ES cells were passaged every 2 days, and the medium was changed on alternate days.
Germ-cell differentiation was based in the generation of embryoid bodies (EBs) from mouse ES cells to induce germ-line differentiation (Keller, Reference Keller1995; Ling & Neben, Reference Ling and Neben1997). Briefly, 400 cells in 30 μl differentiation medium drops (ESC culture medium without LIF). BMP4 at a final concentration of 50 ng/ml was used at this stage to induce the differentiation of ESC into germ cells (Makoolati et al., Reference Makoolati, Movahedin and Forouzandeh-Moghadam2011); cells were placed onto the inside of the lid of 96-well plates using a 12-multichannel pipette. Each well was half filled with sterile water to maintain humidity. The lid was flipped in a smooth, steady manner to invert and it was placed onto the 96-well plate. The plates were incubated for 5 days at 37°C with 5% CO2. After 2 to 3 days in culture the EBs had formed. On day 5, the EBs were transferred into 10-cm plates (20 EBs/plate) that contained 10 ml of differentiation medium supplemented with retinoic acid at a final concentration of 2 μM in order to promote germ-cell differentiation (Chen et al., Reference Chen, Jia, Wang, Zhou, Leng, Duan and Kang2012). The plates were incubated for 7 more days at 37°C with 5% CO2. Analysis of genes expressed in undifferentiated ESC, Oct4 (Synonym: Pou5f1), Nanog and Stella, the germ-cell markers acrosin (Acr), boule-like (Boll), deleted in azoospermia-like (Dazl), growth differentiation factor 9 (Gdf9), mouse vasa homologue (Mvh), protamine 2 (Prm2), zona pellucida glycoprotein 3 (Zp3), and the Sertoli cell marker Mis was carried out on day 12 of differentiation.
Transfections
A Stealth short interfering RNA (siRNA; Invitrogen) was designed to Mael (NM_175296.4) using the BLOCK-iT RNAi Designer at www.invitrogen.com/rnaidesigner. The sense (5′→3′) strand of the synthetic oligonucleotide duplex was GGCCUUCAGAGAAGCAGAAACUUGU. A siRNA designed to Dazl (NM_010021; sense strand CACAACUUCUGAGGCUCCAAAUUCA) was used as a positive control, as its essential role in the differentiation of ESC into pre- and post-meiotic germ cells has been reported (Haston et al., Reference Haston, Tung and Reijo Pera2009; Kee et al., Reference Kee, Angeles, Flores, Nguyen and Reijo Pera2009; Kerr & Cheng, Reference Kerr and Cheng2010). Medium GC Negative Control RNAi duplex (with no homology in vertebrate transcriptome) was also used in our experiments.
Fetal ovary explants from E17.5 fetuses in culture were transfected for 24 h on day 3 of culture, when the ovary explants were attached to the bottom of the 4-well dishes. Complexes that consisted of 40 pmol BLOCK-iT Fluorescent Oligo, Negative Control siRNA, Mael-siRNA or Dazl-siRNA and 2 μg/ml lipofectamine 2000 (all from Invitrogen) were used for transfections, as recommended by the manufacturer. To assess transfection efficiency, the ovary explants were transfected with 40 pmol BLOCK-iT Fluorescent Oligo. Uptake of the Fluorescent Oligo was visualised by fluorescence microscopy at 16–18 h on treated cells.
ESC (30–40% confluent) were transfected with complexes that consisted of 40 pmol Negative Control RNAi duplex or 40 pmol stealth siRNA and 2 μg/ml lipofectamine 2000. After 16–18 h, ESC were trypsinized, pelleted by centrifugation, and resuspended by trituration into a single-cell suspension in ESC differentiation medium. ESC were then cultured as indicated above to induce their differentiation into germ cells via embryoid body formation. To assess transfection efficiency, ESC were transfected with the BLOCK-iT Fluorescent Oligo. Uptake of the Fluorescent Oligo was visualised by fluorescence microscopy at 6 h after transfection.
Reverse transcription polymerase chain reaction (RT-PCR)
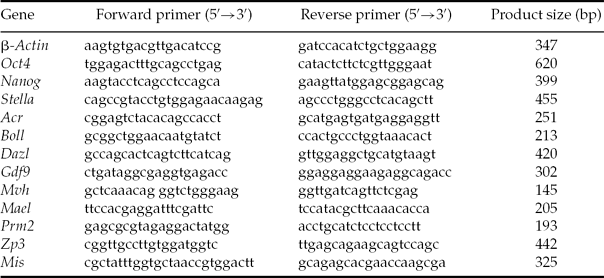
Total RNA was extracted from 13.5–18.5 dpc fetal ovaries, neonatal ovaries, fetal ovary explants in culture and ESC using RNeasy Kit (Qiagen) according to the manufacturer's recommendation. The first-strand synthesis was performed using 5 μg RNA, random primers, and reverse transcriptase Superscript II (Gibco-BRL) at 42°C for 1 h. PCR was performed for 30 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min. Gene-specific primers were designed using the Primer3 program (MIT, available at http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The primers used in this study to amplify mouse transcripts are listed in Table 1.
Table 1 Sequences of forward and reverse primers used for reverse transcription polymerase chain reaction (RT-PCR) reactions

Quantitative PCR
All reactions were performed using the ABI Prism 7700 Sequence Detection System (Applied Biosystems). Each PCR reaction was carried out in triplicate in a 25-μl reaction volume using 12.5 μl of 2× SYBR Green master mix, 9.5 μl of water, 2 μl of 3.75–5 μM primer mix and 1 μl of cDNA. Reaction conditions were: 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. Mouse liver RNA was reverse transcribed and used to construct standard curves. Values for each gene were normalised to the expression levels of Gapdh. Real-time PCR product specificity was confirmed by melt curve analysis and gel electrophoresis.
Statistics
Three independent experiments were performed to demonstrate reproducibility. Student's t-test was used to evaluate the difference between groups, and differences at P < 0.05 were considered to be significant. SAS statistical software (SAS Institute) was used to perform statistical analysis.
Results
Mael expression in fetal and neonatal ovaries
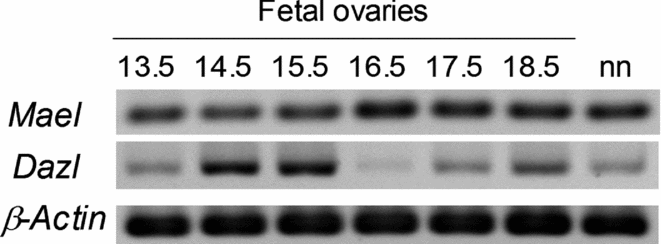
The developmental expression of Mael in embryos was analysed during the development of fetal and neonatal ovaries. Mael was highly expressed in 13.5–18.5 dpc and neonatal ovaries. Dazl was also expressed in 13.5–18.5 dpc and neonatal ovaries, but its expression was changing during fetal development (Figure 1).

Figure 1 Developmental expression pattern of Mael and Dazl. Gene expression was analysed by reverse transcription polymerase chain reaction (RT-PCR) using cDNAs prepared from 13.5–18.5 dpc and neonatal (nn) ovaries.
Transfection efficiency in mouse fetal ovary cells and in ESC
Transfection conditions were optimised using cellular internalisation of a fluorescently labelled short RNA duplex (BLOCK-iT Fluorescent Oligo) as an indicator of transfection efficiency. Transfection efficiency was determined by counting the total number of cells per field versus the number of corresponding cells with visible fluorescence uptake and averaging across different fields (300 cells). The transfection efficiency for mouse fetal ovary cells and ESC was routinely observed to be ≥80%, as reported previously (Moreno et al., Reference Moreno, Salazar, Betancourt, Casas, Ducolomb, González and Bonilla2012).
Inhibition of Mael expression in mouse fetal ovaries in culture and ESC
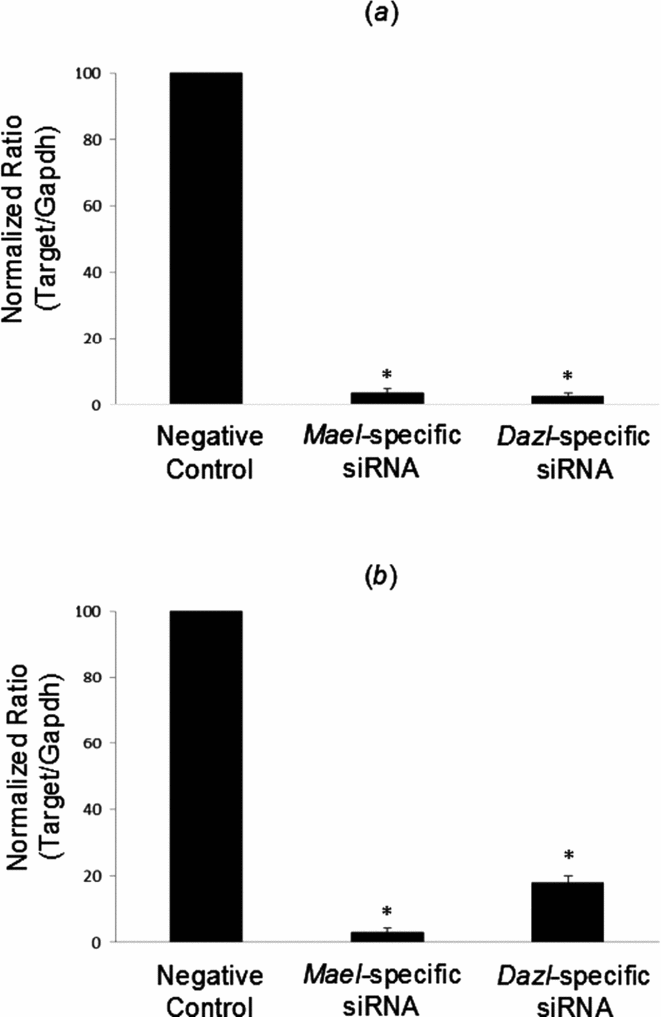
siRNA targeting Mael and the positive control Dazl inhibited their expression by >80% relative to the control after 24 h in mouse fetal ovary cells in culture and ESC, as determined by quantitative (q)RT-PCR analysis (Fig. 2a and 2b, respectively). Hence we decided to use siRNA to knock down expression of Mael in our in vitro germ-cell culture systems to determine the role of Mael in germ-cell development and differentiation, in particular, in oogenesis.

Figure 2 RNAi knockdown of Mael in mouse fetal ovaries and embryonic stem cells (ESC). (a) Mael-specific siRNA and corresponding controls (Negative Control RNAi duplex and Dazl-siRNA, used as positive control) were transfected into mouse fetal ovary explants. At 24 h post-transfection, expression of target and internal control genes was analysed by quantitative reverse transcription polymerase chain reaction (qRT-PCR). (b) Mael-siRNA and corresponding controls were transfected into E14 ESC. At 24 h post-transfection, expression of target and internal control genes was analysed by qRT-PCR. The value for the cells treated with Negative Control RNAi duplex was set to 100%. Expression of Mael and Dazl is normalised to Gapdh and is presented as the fold change in expression relative to negative control. Data are expressed as the mean ± standard error of the mean (SEM). *Statistically significant changes in expression relative to the negative control (P < 0.05).
Effect of Mael knockdown on mouse fetal ovaries cultured in vitro
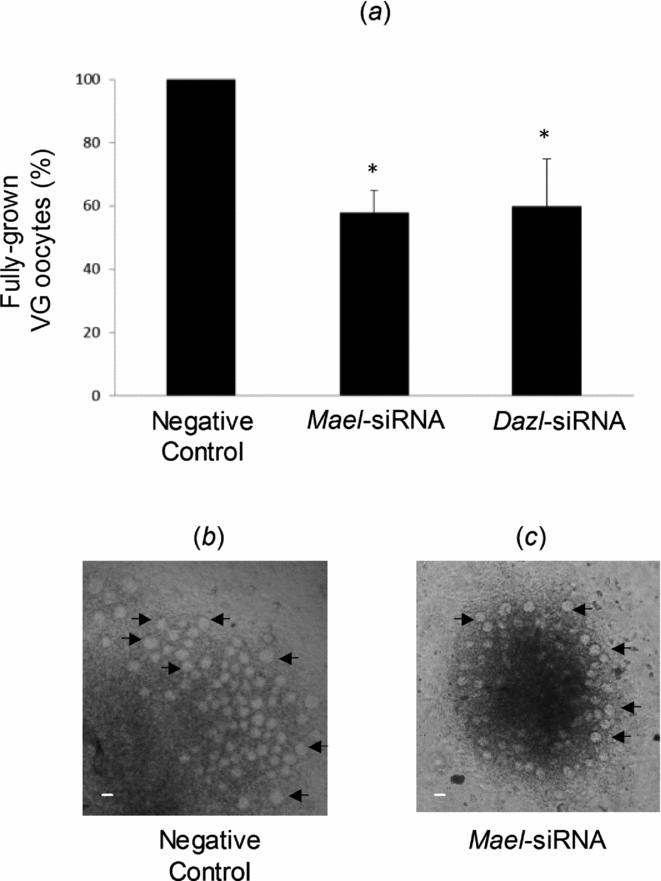
A mean of 198 oocytes at GV stage and showing a diameter of 40–70 μm had grown out of each fetal ovary after 7 days of culture in the negative control. The knockdown of Mael or Dazl expression in fetal ovary explants was accompanied by a significant reduction in oocytes at the GV stage to 58 and 60%, respectively (Fig. 3), thus suggesting an important role of Mael during the early oogenesis in mouse in vitro.

Figure 3 Mael knockdown disrupts the growth of oocytes in fetal ovaries in culture. On day 3 of culture, ovary explants were transfected with Negative Control RNAi duplex, Mael-siRNA or Dazl-siRNA (used as a positive control). (a) The number of oocytes at the germinal vesicle (GV) stage and showing a diameter of 40–70 μm grown out of each fetal ovary was analysed on day 7 of culture. Data are presented as the mean ± standard error of the mean (SEM). *When compared with the paired RNAi duplex negative controls, P < 0.05. (b) Representative fetal ovary explant transfected with Negative Control RNAi duplex. (c) Representative fetal ovary explant transfected with Mael-specific siRNA. Arrows show fully grown GV oocytes. Scale bar: 50 μm.
Effect of Mael knockdown on the differentiation of mouse ESC into germ cells
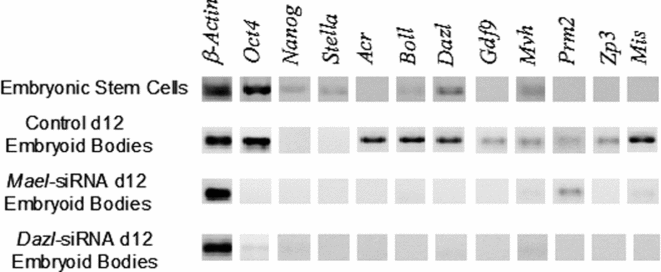
To establish whether Mael knockdown disrupts the differentiation of mouse ESC into germ cells, mouse ESC were transfected with Mael-specific siRNA and their response was analysed after 12 days of differentiation. ESC transfected with the Negative Control RNAi duplex were able to differentiate into germ-cell lineage, as indicated by the expression of germ-cell markers Acr, Boll, Dazl, Gdf9, Mvh, Prm2, Zp3, and the Sertoli cell marker Mis (Fig. 4). Nevertheless, in ESC transfected with Mael-siRNA, expression of most of the germ-cell markers was clearly disrupted, thus indicating an essential role of Mael for the differentiation of ESC into germ cells in vitro. A similar effect was observed when ESC were transfected with Dazl-siRNA (Fig. 4).

Figure 4 Mael knockdown disrupts the differentiation of mouse embryonic stem cells (ESC) into germ cells in vitro. ESC were transfected with Negative Control RNAi duplex, Mael-siRNA or Dazl-siRNA (used as a positive control). ESC were then cultured to induce their differentiation into germ cells via embryoid body formation. Differentiation of mouse ESC into germ cells was carried out on day 12 (d12) of culture by analysis of several germ-cell-specific markers and the Sertoli cell marker, Mis. Experiments were carried out in triplicate. Representative gels are shown.
Discussion
Even when a number of key germ cell-specific genes have been identified, the mechanisms and networks involved in the regulation of germ-cell specification and development remain largely unknown (Thomson et al., Reference Thomson, Liu, Zou, Smith, Meissner and Ramanathan2011). In the present study we were interested in analysing whether Mael, which encodes a transcription factor with an essential role in mouse spermatogenesis, is also necessary for mouse oogenesis and germ-cell differentiation from ESC in vitro.
The developmental analysis of Mael in this study showed that mRNA is detected in ovaries of mouse embryos from 13.5 dpc to 1 day postnatal. A similar expression pattern was found in mouse testes by immunolocalization (Aravin et al., Reference Aravin, van der Heijden, Castaneda, Vagin, Hannon and Bortvin2009). MAEL was expressed in gonocytes at 14.5, 16.5 and 18.5 dpc.
In order to determine if Mael is essential during the early oogenesis in mice in vitro, fetal ovary explants in culture were transfected with Mael-specific siRNA, as this RNAi strategy has been widely used in gene/protein function in several systems, including cultured hamster ovaries (Wang & Roy, Reference Wang and Roy2006) and recently mouse fetal mouse ovaries (Xu et al., Reference Xu, Hua, Zhang, Jiang, Zhang, Ma, Zheng, Sun, Shen, Sha, Cooke and Shi2011; Moreno et al., Reference Moreno, Salazar, Betancourt, Casas, Ducolomb, González and Bonilla2012).
Mael mRNA levels were reduced significantly by 96.2% in comparison with fetal ovaries transfected with Negative Control RNAi duplex. The knockdown of Mael was more efficient than that observed in whole fetal mouse ovaries in culture using siRNA targeting the proliferating cell nuclear antigen (PCNA; mRNA levels reduced by 62%) (Xu et al., Reference Xu, Hua, Zhang, Jiang, Zhang, Ma, Zheng, Sun, Shen, Sha, Cooke and Shi2011) and in mouse fetal ovary explants using siRNA targeting the skin–embryo–brain–oocyte transcription factor Sebox and the germ cell-specific spermatogenesis and oogenesis basic helix–loop–helix transcription factor Sohlh2 (mRNA levels reduced by 80%) (Moreno et al., Reference Moreno, Salazar, Betancourt, Casas, Ducolomb, González and Bonilla2012).
The number of fully grown oocytes in GV stage was significantly reduced to 58% in fetal ovaries transfected with Mael-siRNA, in comparison with fetal ovaries transfected with Negative Control RNAi duplex, thus suggesting that Mael plays a relevant role in the early oogenesis in mice in vitro. Similar results were obtained with Dazl-siRNA (development of fully grown oocytes reduced to 60%), the positive control used in this study due to the essential role of Dazl in the differentiation of pre- and post-meiotic germ cells (Haston et al., Reference Haston, Tung and Reijo Pera2009; Kerr & Cheng, Reference Kerr and Cheng2010). The fact that we have not observed a complete ablation of oocyte development in our RNAi experiments, even when Mael RNA was efficiently knockdown may be due to: (1) transfection of 100% of the cells cannot be achieved, especially in organ- and organ-explant cultures. In our study, cells in fetal ovary explants in culture were transfected efficiently (≥80%), but there are always cells that escape transfection and are able to have a normal development; and (2) even in transfected cells, a complete knockout of gene expression by RNAi cannot be achieved because of the characteristic mechanisms of siRNA action (Martinez et al., Reference Martinez, Patkaniowska, Urlaub, Lührmann and Tuschl2002). It is often observed that transcription is efficiently knocked down by 24 h post-transfection, although, by 48 h, levels of inhibition are somewhat attenuated (Hough et al., Reference Hough, Clements, Welch and Wiederholt2006). In our RNAi experiments, oocytes in which Mael transcription knockdown was not achieved efficiently could have had an apparently normal development. However, the normal development of oocytes transfected with Mael-siRNA is still to be confirmed as, in a previous study with mice lacking Sohlh2, it was observed that even when adult female mice are infertile due to lack of ovarian follicles, Sohlh2-deficient ovaries can form primordial follicles. But the primordial oocytes are abnormal at the molecular level because they misexpress several germ cell- and oocyte-specific genes, causing their rapid lost; few are present by 14 days of postnatal life (Choi et al., Reference Choi, Yuan and Rajkovic2008). A similar result was obtained when the role of PCNA in the folliculogenesis in mice was studied (Xu et al., Reference Xu, Hua, Zhang, Jiang, Zhang, Ma, Zheng, Sun, Shen, Sha, Cooke and Shi2011). Although more primordial follicles were observed after PCNA RNAi, the number of primary follicles decreased, accompanied by downregulation of several key regulators of the transition from primordial to primary follicles. Expression analysis of these key regulators in oocytes transfected with Mael-siRNA is being carried out in our laboratory.
As maelstrom is required for microtubule organization in Drosophila oocytes (Clegg et al., Reference Clegg, Findley, Mahowald and Ruohola-Baker2001), it will also be very interesting to analyse whether Mael is important for microtubule organization in mouse oocytes during the early oogenesis and especially during oocyte maturation, when microtubule organization disruption can be easily assessed as it would impact spindle organization, kinetochore assembly, and microtubule growth or responsiveness (Kim et al., Reference Kim, Kim and Lee2008).
Our results also show that Mael mRNA levels in mouse ESC transfected with Mael-specific siRNA were significantly reduced by 97% in comparison with ESC transfected with Negative Control RNAi duplex. Transfection of ESC with the positive control, Dazl-siRNA, resulted in a significant reduction of Dazl mRNA by 82%. Downregulation efficiency of the targeted genes was similar to that observed in other studies in which siRNA was used to block the expression of specific genes in ESC (Hyslop et al., Reference Hyslop, Stojkovic, Armstrong, Walter, Stojkovic, Przyborski, Herbert, Murdoch, Strachan and Lako2005; Hough et al., Reference Hough, Clements, Welch and Wiederholt2006).
Differentiation of male and female germ cells out of the Mael knockdown ESC was clearly disrupted. Expression of the male and female germ-cell markers used was very low or undetectable compared with their expression in ESC transfected with Negative Control RNAi duplex. These results indicate a pivotal role of Mael in germ-cell differentiation from ESC in mouse in vitro and resemble what has been observed in Drosophila, where maelstrom has been reported to be necessary for proper germline stem cell lineage differentiation (Pek et al., Reference Pek, Lim and Kai2009), thus indicating that Mael is a phylogenetically conserved regulator of germ-cell development.
Previous studies have shown that fruit fly maelstrom and mouse Mael genes are necessary for the repression of transposable elements (TE) via a particular class of small RNAs known as piRNAs, for their association with PIWI proteins (O'Donnell et al., Reference O'Donnell, Burns and Boeke2008; Aravin et al., Reference Aravin, van der Heijden, Castaneda, Vagin, Hannon and Bortvin2009; Pek et al., Reference Pek, Lim and Kai2009). Derepression and mobilization of active TEs has been implicated in gene mutation, abrogation or perturbation of gene expression, and DNA damage (Han & Boeke, Reference Han and Boeke2005; Girard & Hannon, Reference Girard and Hannon2008; Soper et al., Reference Soper, van der Heijden, Hardiman, Goodheart, Martin, de Boer and Bortvin2008). Additional studies are necessary to determine if the effects of Mael knockdown during the early oogenesis and the germ-cell differentiation observed in the present study are related to transposon derepression.
In summary, evidence of an essential role of Mael during the early oogenesis and the differentiation of ESC into germ cells in mouse in vitro is presented. Unravelling of the molecular mechanisms underlying these processes is essential for a better understanding of the genetics of infertility.
Acknowledgements
We thank Dr Humberto González, LBE Patricia López and LBE Diego Carrera for assistance with imaging.