Introduction
Stray cats (Felis catus) harbour a variety of parasitic nematodes of both veterinary and zoonotic significance (Okulewicz et al., Reference Okulewicz, Perec-Matysiak, Buńkowska and Hildebrand2012; Wright et al., Reference Wright, Stafford and Coles2016). Among these nematodes are Toxascaris leonina, Toxocara cati and Toxocara canis, in the family Ascarididae (Urquhart et al., Reference Urquhart, Armour, Duncan, Dunn and Jennings1996; Gibbons et al., Reference Gibbons, Jacobs and Sani2001; Pawar et al., Reference Pawar, Lakshimkantan, Hasan, Poornachandar and Shivaji2012), which are important intestinal helminths (Parsons, Reference Parsons1987). Toxocara cati and T. canis are more pathogenic for kittens, puppies and children, with the potential for visceral larva migrans (VLM) and ocular larva migrans (OLM) in the latter. Potentially, T. leonina may also emerge as a zoonotic agent causing VLM in humans (Prokopic & Figallova, Reference Prokopic and Figallova1982; Kim & Huh, Reference Kim and Huh2005; Tarsitano et al., Reference Tarsitano, Greco, Decaro, Nicassio, Lucente, Buonavogliam and Tempesta2010; Okulewicz et al., Reference Okulewicz, Perec-Matysiak, Buńkowska and Hildebrand2012).
Unlike Toxocara, where infective larvae migrate through the lungs, the entire life cycle of T. leonina takes place in the gut of the definitive host (dog, cat or other carnivore) and the eggs are shed with the faeces of the host. Outside the host, infective second-stage larvae (L2) develop inside the eggs within approximately 1 week under optimal climatic conditions (Soulsby, Reference Soulsby1982). Eggs may remain infective for several months in cold and humid climates, but die rapidly in dry and hot seasons. Both felids and canids become infected by ingesting infective eggs in contaminated food or water (Labarthe et al., Reference Labarthe, Serrao, Ferreira, Almeida and Guerrero2004; Dalimi et al., Reference Dalimi, Sattari and Motamedi2006; Dubna et al., Reference Dubna, Langrova, Napravnik, Jankovska, Vadlejch, Pekar and Fechtner2007; Reperant et al., Reference Reperant, Hegglin, Fischer, Kohler, Weber and Deplazes2007; Itoh et al., Reference Itoh, Kanai, Tominaga, Kawamata, Kaneshima, Chikazawa, Hori, Hoshi and Higuchi2011). The second-stage larvae hatch from the eggs in the intestine of definitive hosts and penetrate the gut wall, where they undergo two cycles of moulting. The larvae then return to the gut lumen, moulting to adults, and become sexually mature.
When paratenic rodent hosts ingest T. leonina eggs, the hatched larvae migrate through the tissues and may persist in the tissues for long periods of time (Wright, Reference Wright1935; Epe, Reference Epe2009; Traversa, Reference Traversa2012). When another paratenic host ingests the infected rodent tissues, the infective larvae (L2) migrate to different organs, where they undergo encystment, but development to adult worms will not take place. When a definitive host then ingests such infected rodents, the tissue cysts disseminate the infective larvae (L2), which move directly to the gut where they complete development to adult worms (Sprent, Reference Sprent1959).
Toxascaris leonina eggs are colourless and oval, with a maximum size of 85 × 75 μm, and possess a smooth shell about 2 μm thick with no striations or albuminous coat (Dunn, Reference Dunn1978; Gonzales et al., Reference Gonzalez, Carbonell, Urios and Rozhnov2007). The eggs are resistant to both climatic and chemical exposure, which allows them to remain viable in the environment for long periods. Ambient temperature, humidity and soil features are the predominant factors affecting the duration until the eggs moult into the larval stages (Sommerfelt et al., Reference Sommerfelt, Cardillo, Lopez, Ribicich, Gallo and Franco2006). Toxascaris leonina eggs are able to adapt to various climatic conditions, such as temperature, and are able to embryonate in the dark (Okoshi & Usui, Reference Okoshi and Usui1968; Feney-Rodriguez et al., Reference Feney-Rodriguez, Cuellar Del Hoyo and Guillenllera1988; Anderson, Reference Anderson2000).
As a part of control programmes for zoonotic agents, commercial disinfectants are widely used. Numerous disinfectants are available, categorized on the basis on their active ingredients and ability to kill various micro-organisms (Zeweil et al., Reference Zeweil, Rizk, Bekhet and Ahmed2015). Currently, more than 5000 antimicrobial products are used to destroy or suppress the growth of pathogens. Alcoholic compounds are fast acting and highly effective against both bacteria and T. canis eggs (Aycicek et al., Reference Aycicek, Yarsan, Sarimehmetoglu, Tanyuksel, Girginkardesler and Ozyurt2001; Turpin, Reference Turpin2013). Sodium hypochlorite has wide antibacterial activity (Grooms, Reference Grooms2003) and is effective against T. canis eggs (Aycicek et al., Reference Aycicek, Yarsan, Sarimehmetoglu, Tanyuksel, Girginkardesler and Ozyurt2001). Virkon®S has broad-spectrum disinfectant activity against viruses, bacteria and some fungi (Scott & Swetnam, Reference Scott and Swetnam1993; Gasparini et al., Reference Gasparini, Pozzi, Magnelli, Fatighenti, Giotti, Poliseno, Pratelli, Severini, Bonanni and De Feo1995). To date, there is little information regarding its antiparasitic effects, although activity against Gyrodactylus salaris in salmon has been reported (Koski et al., Reference Koski, Anttila and Kuusela2016).
There is a scarcity of information regarding the use of disinfectants against ascarids of pets, particularly T. leonina eggs, in Egypt, although comparisons among a few disinfectants against infective T. cati and T. canis eggs have been reported in Turkey and Spain, respectively (Aycicek et al., Reference Aycicek, Yarsan, Sarimehmetoglu, Tanyuksel, Girginkardesler and Ozyurt2001; Morrondo et al., Reference Morrondo, Díez-Morrondo, Pedreira, Díez-Baños, Sánchez-Andrade, Paz-Silva and Díez-Baños2006). Therefore, the present study was designed to evaluate the efficacy of six commercially available disinfectants against the embryonation and larval development of T. leonina eggs obtained from adult female worms in necropsied cats, to consider whether they might be useful in reducing the zoonotic potential of worms of pets.
Materials and methods
Collection of eggs
Toxascaris leonina eggs were obtained from adult female worms recovered from the small intestines of naturally infected cats. The eggs were either collected by incubating gravid female worms in 0.15 m sodium chloride at 37°C for 24 h to allow egg deposition (Barriga & Omar, Reference Barriga and Omar1992) or by washing gravid female worms with normal saline and then grinding the worms to release the eggs from the uteri. The eggs were then sieved, washed and precipitated several times using 1% formol-saline as described previously (Sabry, Reference Sabry1999).
Exposure of unembryonated eggs to disinfectants
For the in vitro assay, approximately 7000 unembryonated T. leonina eggs were pooled and divided into seven equal groups of approximately 1000 eggs, which were treated in plates as follows. (1) Control: eggs were incubated in 6 ml 1% formol-saline solution to assess normal embryonic development. (2) Sodium hypochlorite: eggs were incubated in 6 ml solution containing 2.5 ml sodium hypochlorite 0.5% + 2 ml egg suspension + 1.5 ml 1% formol-saline. (3) Ethanol: eggs were allowed to settle, centrifuged, the supernatant was removed and then the eggs were incubated in 6 ml 70% ethanol. (4) Virkon®S (DuPont, Wilmington, USA; active ingredients potassium peroxymonosulphate 21.41%, sodium chloride 1.50% and other ingredients 77.09%): eggs were incubated in 6 ml of diluted Virkon (5 g/l distilled water or 30 mg/6 ml distilled water). (5) TH4+ (quaternary ammonium compounds and glutaraldehyde): eggs were incubated in 6 ml of diluted TH4+ (1 ml/200 ml distilled water or 30 μl/6 ml distilled water). (6) Phenol (carbolic acid): eggs were incubated in 6 ml of diluted phenol (100 ml/20 l distilled water or 30 μl/6 ml distilled water). (7) Dettol® (chloroxylenol): eggs were incubated in 6 ml of diluted Dettol (25 ml/l distilled water or 130 μl/6 ml distilled water).
All plates were incubated for 7–10 days at 28°C and 80% relative humidity, and shaken daily to allow oxygenation.
Exposure of embryonated eggs to disinfectants
Approximately 7000 unembryonated eggs were allowed to undergo embryonation in 1% formol-saline at 28°C and 80% relative humidity with daily agitation, as above. The embryonated eggs were divided into seven groups of approximately 1000 eggs and then exposed to the treatments described above for 7–10 days.
Evaluation of effects on larval development
Dishes were examined daily to check for alterations in the eggshell and survival of larvae. After 7–10 days, the percentage of both non-developed and larvated eggs was recorded. The reduction in larval development for the treated groups was determined according the equation:
 $$\displaystyle{\matrix{{\left( {{\rm Larvated} \,{\rm eggs} \, {\rm of} \,{\rm the}\, {\rm control}\, {\rm group}} \right) - }\cr {\!\!\!\!\left( {{\rm Larvated} \,{\rm eggs} \,{\rm of} \,{\rm the}\, {\rm treated} \,{\rm group}} \right)}} \over {{\rm Larvated} \,{\rm eggs} \,{\rm of} \,{\rm the} \,{\rm control} \,{\rm group}}} \times 100$$
$$\displaystyle{\matrix{{\left( {{\rm Larvated} \,{\rm eggs} \, {\rm of} \,{\rm the}\, {\rm control}\, {\rm group}} \right) - }\cr {\!\!\!\!\left( {{\rm Larvated} \,{\rm eggs} \,{\rm of} \,{\rm the}\, {\rm treated} \,{\rm group}} \right)}} \over {{\rm Larvated} \,{\rm eggs} \,{\rm of} \,{\rm the} \,{\rm control} \,{\rm group}}} \times 100$$Statistical analysis
Data were analysed statistically using Statistical Package for Social Science (SPSS for Windows (IBM), version 22; SPSS Inc., Chicago, Illinois, USA) to determine if variables differed among treatments. Data were analysed using analysis of variance (ANOVA) tests and subsequent Duncan's multiple range test to determine the differences of means. Results were expressed as means ± SD. Probability values of less than 0.05 (P ≤ 0.05) were considered to be significant.
Results
The effects of disinfectants on unembryonated eggs
Both sodium hypochlorite and phenol had non-significant effects on unembryonated toxascarid eggs compared to the control untreated eggs (table 1), resulting in 2.8 and 21.0% reductions in larval development, respectively.
Table 1. The effect of various disinfectants on the embryonation of Toxascaris leonina eggs.
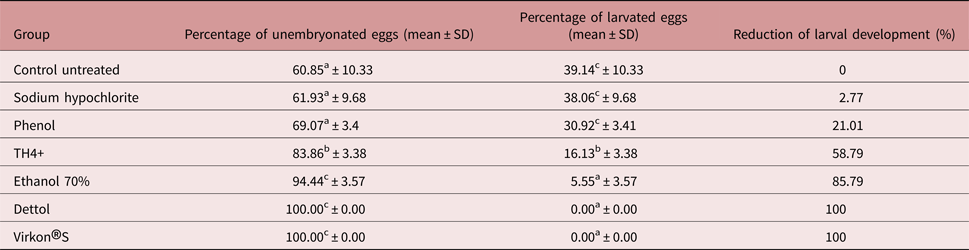
Superscripts of the same letters in the same column mean non-significant findings.
Superscripts of different letters in the same column mean significant (P ≤ 0.05) findings.
TH4+ and ethanol had significant (P ≤ 0.05) effects on unembryonated toxascarid eggs compared to the control untreated eggs (table 1), resulting in 58.8 and 85.8% reductions in larval development, respectively (table 1).
Both Dettol® and Virkon®S treatment resulted in 100% reduction in larval development (P ≤ 0.05) (table 1). Virkon®S induced early embryonic lysis in treated eggs after 24 h (fig. 1).
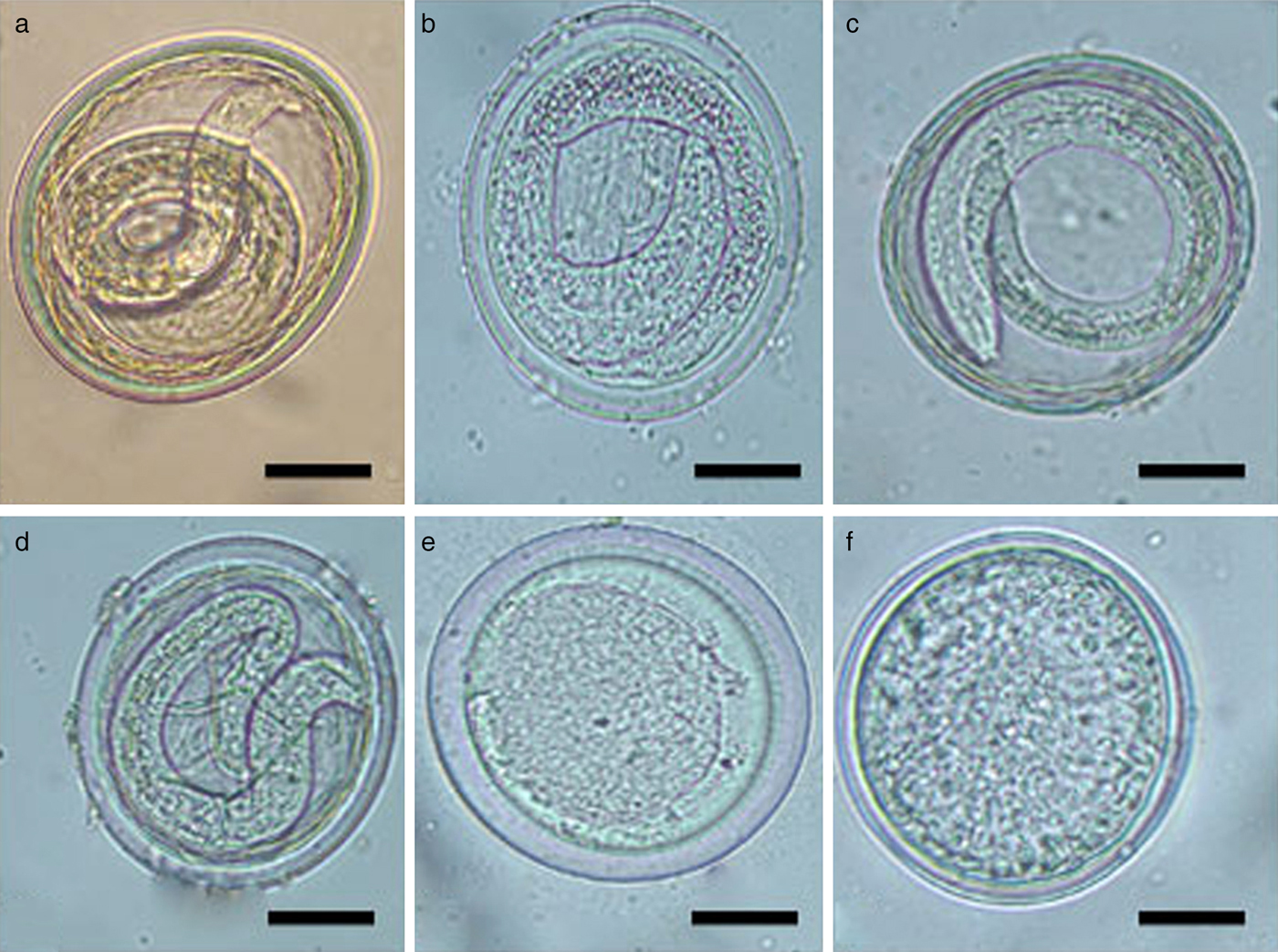
Fig. 1. Morphology of unembryonated eggs from Toxascaris leonina adult females obtained from necropsied cats and exposed to various disinfectants with incubation period 7–10 days. (a) An untreated egg (control). Scale bar: 25 μm. (b) An egg treated with 70% ethanol. Note the clearance of the eggshell and slight degeneration of the larval structure. Scale bar: 25 μm. (c) An egg treated with phenol. Both the eggshell and the larval structure appeared more or less normal. Scale bar: 25 μm. (d) An egg treated with TH4+. Note the thinning of the eggshell and compression of the contained larva. Scale bar: 25 μm. (e) An egg treated with Dettol. Note the great disappearance of the eggshell and cessation of embryonal development. Scale bar: 25 μm. (f) An egg treated with Virkon®S. Note the absolute decortication of the egg associated with a complete cessation and atrophy of the embryonal cells. Scale bar: 25 μm.
The effects on embryonated eggs
Concomitant with the effects of disinfectants on the unembryonated eggs, the tested disinfectants induced variable degrees of effects on the eggshells and/or larvae. Sodium hypochlorite elicited a marked decortication of treated eggs. The eggs of the phenol-treated group appeared more or less morphologically normal. TH4+ and 70% ethanol had no apparent effect on the larvae from treated eggs.
Dettol®-treated eggs showed a deformity in the shell 1 week post exposure. Virkon®S-treated eggs showed a complete degeneration of most of the larvae, and loss of their features 24 h post exposure (table 1 and fig. 2).

Fig. 2. Morphology of embryonated eggs from Toxascaris leonina adult females obtained from necropsied cats and exposed to some disinfectants. (a) An intact egg, not treated with disinfectants, showing a well-defined eggshell and fully formed larva. Scale bar: 25 μm. (b) An egg treated with sodium hypochlorite. Note the complete disappearance of the outer layer of eggshell with a still intact larva. Scale bar: 25 μm. (c) An egg 24 h post treatment with Dettol. Note a slight thinning of the eggshell together with more or less normal larval structure. Scale bar: 25 μm. (d) An egg 7 days post treatment with Dettol. Note the prominent destruction (arrow) of the eggshell. Scale bar: 25 μm. (e) An egg 7 days post treatment with Dettol. Note a prominent projection (arrow) of the eggshell. Scale bar: 50 μm. (f) An egg treated with Virkon®S. A distinct thinning/disappearance of the eggshell associated with a marked degeneration of the larva can be seen, denoting the effect of the disinfectant. Scale bar: 25 μm.
Discussion
The present study examined the effects of various commercially available disinfectants on embryonation, larval development and eggshell structure of T. leonina eggs. Although the disinfectants tested serve as bactericidal, virucidal and fungicidal agents, their effect on T. leonina eggs had not yet been investigated. In our study, differences among the various disinfectants in their effects on T. leonina eggs were observed.
Sodium hypochlorite and ethanol have considerable efficacy against infective eggs of T. canis and, therefore, veterinarians recommend these disinfectants for dog kennels, cages and dog houses (Morrondo et al., Reference Morrondo, Díez-Morrondo, Pedreira, Díez-Baños, Sánchez-Andrade, Paz-Silva and Díez-Baños2006; Verocai et al., Reference Verocai, Tavares, Ribeiro, Correia and Scott2010) due to their effectiveness, low cost and ready availability. Although it would seem that they might be effective against T. leonina eggs, since both T. canis and T. leonina belong to the family Ascarididae, sodium hypochlorite was not effective in reducing T. leonina larval development in the current study. However, it did cause a marked decortication in T. leonina eggs. On the other hand, ethanol did induce a significant reduction in larval development. On the contrary, Oh et al. (Reference Oh, Kim, Ahn and Shin2016) showed that sodium hypochlorite suppressed the development of Ascaris suum eggs within 5 min of exposure, whereas ethanol did not inhibit embryonation of decorticated eggs even 1 h post exposure.
Ethanol is known to be effective against most bacteria, viruses and fungi, with a few reports on inhibition of sporulation (Yasuda-Yasuki et al., Reference Yasuda-Yasuki, Namiki-Kanie, Hachisaka, Chambliss and Vary1978; McDonnell & Russell, Reference McDonnell and Russell1999). The antimicrobial activity of ethanol is optimal at a concentration of 60–90%. Ethanol causes proteins in the cell wall to denature, leading to membrane damage, interrupted metabolism and, eventually, cell lysis (Morton, Reference Morton and Bloch1983). Similarly, Dettol® (chloroxylenol) is mainly used as an antibacterial, acting on cell surfaces (Russell & Furr, Reference Russell and Furr1977). In the current study, Dettol® suppressed embryonal development, with a significant reduction in larval development. This might be due to the germicidal effect of Dettol® on T. leonina eggshells. TH4+ is a widely used disinfectant with bactericidal, virucidal and fungicidal action. While demonstrating a significant reduction in T. leonina larval development, TH4+ was not as effective as ethanol, Dettol® or Virkon®S in reducing larval development in our study.
In the current study, phenol-exposed T. leonina eggs did not show a significant reduction in larval development and the eggs appeared more or less normal externally, despite some embryonal degeneration. This might be attributed to phenol, a protoplasmic poison, inducing coagulation of protoplasmic organelles with irreversible cell damage (Sharma, Reference Sharma1997; McDonnell & Russell, Reference McDonnell and Russell1999).
Virkon®S is a unique cleaning and disinfecting agent used in all animal and industrial purposes as a bactericide, virucide and fungicide (Møretrø et al., Reference Møretrø, Vestby, Nesse, Storheim, Kotlarz and Langsrud2009). Interestingly, in this study, Virkon®S caused complete embryonic death together with an absolute cessation of larval development in mature T. leonina eggs, suggesting that it has lethal effects on both eggshell and embryonic cells. To the best of our knowledge, this is the first report revealing both the ovicidal and larvicidal activities of the commercial disinfectant Virkon®S against T. leonina eggs.
Okoshi & Usui (Reference Okoshi and Usui1968) determined that the development of T. leonina eggs was affected by temperature and climatic conditions. When eggs were exposed to −15°C, they remained viable for 40 days, and when exposed to 25°C, approximately all eggs completed development to the infective stages. Thus, the effectiveness of any particular disinfectant against T. leonina is likely to be influenced by these factors as well.
In conclusion, our study shows that several types of available and commercial disinfectants could be used to stop embryonation and/or larval development of T. leonina eggs. However, in practice, Virkon®S or Dettol® might outperform others, based on the results of this current study. Their usage is highly recommended against other potentially zoonotic parasites of pets, particularly helminths such as T. cati and T. canis. In the future, multidisciplinary studies should be undertaken to determine the pharmacology of Virkon®S as an antiparasitic agent.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
All procedures of the current study were approved by the Committee of the Ethics of Scientific Research in the Faculty of Veterinary Medicine, Beni-Suef University, Egypt.





