INTRODUCTION
Ticks are among the most important vectors of human and animal diseases and surpass all other arthropods in the variety of the pathogenic organisms transmitted, including fungi, viruses, bacteria, and protozoa. Ixodes ricinus is the most widespread and abundant ixodid tick in Western Europe and frequently bites humans. It is an important vector of zoonotic diseases, including Lyme disease, tick borne encephalitis, ehrlichiosis and babesiosis. Despite its importance, our knowledge of pathogen transmission by ticks is incomplete, especially concerning protozoa-like piroplasms. Difficulties in rearing ticks as well as reproducing the parasitic life-cycles in the laboratory could partly explain this lack of data. It is essential to study the mechanism of tick infection and parasite infectiousness in order to understand the parasite–host–vector relationships and in order to develop new control strategies of the transmitted pathogens.
Babesia divergens is a bovine blood piroplasm transmitted by the tick I. ricinus, which can cause human babesiosis in immunocompromised patients. It appears to be the main confirmed zoonotic Babesia sp. in Europe (Gorenflot et al. 1998). B. microti, responsible for several hundred human cases reported each year in the United States (Kjemtrup and Conrad, 2000), is also present throughout Europe, but no verified cases of human disease have been reported on this continent, probably because its vector, I. trianguliceps, does not bite humans. However, recent field studies have demonstrated that I. ricinus could also be a vector of B. microti (Duh, Petrovec and Avsic-Zupanc, 2001; Gray et al. 2002) and some seroprevalence studies suggest that parasitic infections of humans may also occur in Europe (Foppa et al. 2002). In 2003, Herwaldt et al. reported the molecular characterization of a new Babesia species isolated from 2 human cases in Italy and Austria, which appeared to be closely related to B. divergens (Herwaldt et al. 2003). For cattle, B. divergens is the most pathogenic and widespread Babesia in northern temperate areas (L'Hostis et al. 1995).
Life-cycles of Babesia species are poorly known, even though in vitro cultivation of the blood stages was first described in 1982 (Väyrynen and Tuomi, 1982). Only asexual reproduction within the salivary glands of vector ticks and within the blood cells of vertebrates could be identified with certainty. The occurrence of sexual stages during the life-cycle of piroplasms has long been a matter of controversy. Koch in 1906 described the so called ‘Strahlenkörper’ (ray bodies) in the gut of female ticks as developing stages of the genera Theileria and Babesia (Koch, 1906). For B. divergens, strong evidence of a sexual cycle was given in 1990 by measurements and comparison of DNA levels of developmental stages of the parasite in the blood of the vertebrate host as well as in the gut, haemolymph and salivary glands of the tick vector (Mackenstedt et al. 1990). Each tick life-cycle stage (larva, nymph and adult) feeds only once, implying a persistence of the pathogen from one instar to the next to ensure transmission. Questions remain as to whether each instar can acquire and transmit B. divergens to vertebrate hosts. If there is transovarial transmission of the parasite it would appear that the engorging female tick is the only stage that can acquire babesial infection from its mammalian host but this would require experimental proof (Donnelly and Peirce, 1975; Friedhoff and Smith, 1981).
In order to investigate some of the mechanisms that control pathogen transmission as well as the relationship between them and tick vectors, an artificial feeding technique that allowed infection of ticks with known numbers of pathogens was needed. In the present study, a membrane technique for infecting I. ricinus with B. divergens from culture was devised and with it some of the parameters that govern tick transmission of the parasite were investigated.
MATERIALS AND METHODS
Parasite and tick maintenance
Ticks
A Babesia-free colony of I. ricinus ticks collected from Belle Ile en mer, a babesiosis-free island of Morbihan (France), was used in all the experiments. Ticks were reared and maintained in humidity chambers with a relative humidity (R.H.) of 80–90% at 22 °C. Every 4 months, all instars were fed on pathogen-free animals in order to maintain the colony: gerbils (Meriones unguiculatus) were used for larvae, rabbits for nymphs, and adults were fed either on rabbits or sheep. After moulting, larvae, nymphs and adult males and females were all reared in separate boxes.
B. divergens isolate
A B. divergens isolate named BOB was originally isolated in June 2004 in Objat (Corrèze, France) from a naturally infected bovine in the acute phase of the disease as previously described (Malandrin et al. 2004), except that isolation and cloning were performed in bovine red blood cells in the presence of 20% foetal calf serum (FCS, Cambrex; decomplemented for 30 min at 56 °C) instead of sheep red blood cells with 10% FCS. BOB 2 is a clone from the original isolate, which was then maintained in vitro only with bovine erythrocytes. Sequencing part of the 18S gene (see below) of the parasite strain confirmed the identity as B. divergens.
Blood samples used in parasite culture and skin feeding experiments
Ovine or bovine blood used in all experiments came from 3 sheep and a Babesia-free bovine (the status of these animals is regularly tested for parasite detection by indirect immunofluorescence detection tests with immune sera as well as blood culture tests on bovine erythrocytes) reared at the National Veterinary School of Nantes (France). Lithium heparinized vacutainer tubes were used to draw blood by venepuncture. Blood samples were immediately centrifuged for 10 min at 800 g in order to separate red blood cells, buffy coat and plasma. The pellet of red blood cells was then washed and diluted 1[ratio ]3 with RPMI 1640 (Cambrex) containing 50 μg/ml gentamicin (Cambrex) and 0·25 μg/ml amphotericin B (Cambrex). Erythrocytes were then maintained at 4 °C until use for parasite culture or as negative control for experimental infections. The same concentrations of gentamicin and amphotericin B were added to the remaining plasma, which was frozen at −20 °C until use. For control experiments, non-infected erythrocytes, cleared of RPMI by a centrifugation step of 10 min at 800 g, were mixed with 1[ratio ]3 (v/v) of homologous plasma. For infection with parasitized blood, infected erythrocytes from in vitro culture (see below) were also obtained by centrifugation and diluted 1[ratio ]3 with the same homologous plasma. In each case, blood was pre-warmed at 37 °C, and used immediately for artificial feeding.
In vitro culture of B. divergens
The parasite was maintained in vitro as previously described for culture in sheep erythrocytes (Chauvin et al. 2002), except that 20% foetal calf serum (Cambrex; decomplemented) (instead of 10%) was used in the case of bovine erythrocytes. Briefly, parasites were maintained in 75 cm2 tissue culture flasks at 37 °C in 5% CO2-air, in a final volume of 40 ml containing 30 ml of RPMI 1640 (Cambrex) with 50 μg/ml gentamicin (Cambrex) and 0·25 μg/ml amphotericin B (Cambrex), either 8 ml of FCS or 4 ml of ovine serum, and 1 ml of bovine or ovine red blood cells pelleted by a centrifugation step of 10 min at 800 g. Parasitaemia was evaluated daily by May-Grünwald Giemsa-based staining (Diff-Quick, Dade Behring) of thin smears and maintained routinely at 2–3% by dilution with fresh red blood cells during medium change.
Artificial feeding of I. ricinus on gerbil and rabbit skins
Animal skins
Gerbil or rabbit skins were obtained from animals previously used for rearing tick colonies in the laboratory. Pieces of animal skin were obtained by skinning slaughtered animals. The skin was cut into 10 cm diameter pieces and treated as described by Musyoki et al. (2004) with slight modification: skin was first sterilized in 70% alcohol, rinsed in 2 changes of sterile distilled water, 2 changes of sterile PBS 1X (Phosphate Buffered Saline) and finally sterilized again in a solution of 50 μg/ml gentamicin (Cambrex), 0·25 μg/ml amphotericin B (Cambrex), 0·25 μg/ml streptomycin (Sigma) and 0·25 U/ml penicillin (Sigma). The skins were then stored at −20 °C prior to use.
Artificial feeding
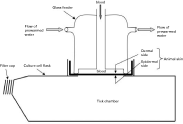
As described in Fig. 1, ticks were placed in plastic cell culture boxes pierced at the top in order to accommodate the glass feeder. Three or 8 ml of restored ovine or bovine blood were put into a 4 or 7 cm diameter glass feeder, respectively, designed for mosquitoes feeding (Bonnet et al. 2000). The feeder apparatus was closed with a slightly stretched Parafilm® membrane at the top and with a gerbil or a rabbit skin membrane at the bottom. Gerbil skins were used to feed larvae, and either gerbil or rabbit skins to feed nymphs and adults. In order to attract the ticks and to preserve parasites, constant temperature (37 °C) was maintained by a water-jacket circulation system through the glass feeder. The culture box containing ticks was arranged under the feeding apparatus and the blood was presented to the ticks until repletion with 2 changes per day. At each blood change, the upper dermal side of the skin, in contact with the blood, was washed with sterile water followed by RPMI containing gentamycin and amphotericin B as used for parasite culture. To feed adult ticks, an equal number of males and females was used. Glass feeders were autoclaved prior to use and all manipulations were done under sterile conditions. The rearing was performed in a climatized incubator at 22 °C, 80–90% R.H. and an ambient CO2 level.
I. ricinus infection and B. divergens transmission
Experimental infection
Daily thin blood smears were taken from the culture, which was diluted with fresh red blood cells to obtain a parasitaemia of around 5–8% of infected erythrocytes for experimental infections. Blood was changed twice a day in feeders and replaced with parasitized erythrocytes and plasma as described above. Following tick feedings, only fully engorged ticks that spontaneously detached were retained for the study and were placed in a humidity chamber.
In order to check the ingestion of parasites by the ticks, some engorged nymphs and adult females were dissected the day post-repletionem (p.r.). The whole intestine was removed and dissected in sterile PBS 1X in order to detect the parasite in the bloodmeal by microscopical examination of smears stained with Giemsa-based coloration. Remaining engorged larvae and nymphs were then allowed to moult to nymphs and adults (around 2–3 months p.r.) and at least 1 month after moulting, salivary glands were dissected in order to detect the parasite by PCR amplification. Engorged females were allowed to lay eggs during about 10–30 days, some eggs were then analysed for the presence of parasitic DNA by PCR amplification and the remaining ones were allowed to transform into larvae, which were also subjected to DNA extraction for parasite detection by PCR amplification. Larvae, the issue from female adults exposed to infection with B. divergens by skin feeding were examined in order to evaluate parasite persistence over moulting. Approximately 150 larvae were applied by brush to each of 2 non-infected gerbils. The animals were maintained over trays of water from which detached engorged ticks were harvested. Salivary glands of nymphs that developed from these larvae were then analysed for infection as described below.
PCR detection of B. divergens in I. ricinus
Salivary glands from I. ricinus adults and nymphs were dissected under a magnifying glass in sterile PBS 1X. All dissection material was cleaned with DNA off (Eurobio) and rinsed with sterile water between each sample. Individual pairs of adult female salivary glands or pools of 2 pairs for nymphs were frozen at −80 °C in 150 μl of PBS 1X until DNA extraction. DNA extraction was performed with Promega extraction kit (Promega) according to the manufacturer's instruction except for a 10-min longer isopropanol precipitation step followed by a centrifugation step of 15 min at 16000 g. Finally the DNA was rehydrated in 50 μl of rehydration solution for adults and 35 μl for pools of nymph's salivary glands. For the eggs and larvae, about 50 of them were crushed in 150 μl of PBS 1X, frozen at −80 °C, and DNA extracted as described for salivary glands. DNA was then rehydrated in 300 μl of rehydration solution before use for PCR amplification. Control DNA samples were extracted using the same protocol from salivary glands of nymphs and adults as well as from eggs resulting from respectively, larvae, nymphs and adults feeding on non-infected cattle blood. PCR-positive controls were obtained with genomic DNA of BOB 2 prepared from culture samples with a minimum parasitaemia of 10%: B. divergens merozoite preparations were obtained by Percoll gradient centrifugation with a density of d=1·08 in PBS (Amersham) and recovered for DNA extraction according to the protocol of the Promega extraction kit.
In each case, PCR amplifications were performed on 10 μl of extracted DNA samples. PCR was performed in a final volume of 30 μl containing 0·33 mM dNTPs (Eurobio), 4 mM MgCl2, 1X PCR buffer, 1·5 U Taq polymerase (Eurobio) and 0·5 μM of each of primers BAB GF2 (GYYTTGTAATTGGAATGATGG) and BAB GR2 (CCAAAGACTTTGATTTCTCTC) designed for the 18S rRNA gene of B. divergens (AC number: AY046576). Amplification was performed in a programmable thermal cycler PT100 (MJ Research) with a cycling programme as follow: 5 min denaturation step at 94 °C, 40 cycles of 1 min at 94 °C, 1 min at 60 °C, 1 min at 72 °C, and a final elongation step of 10 min at 72 °C. The 559 bp amplification product was visualized on an ethidium bromide-stained 1·8% agarose gel (Eurobio). In the case of a negative result and in order to validate the DNA extraction, samples were subject to a second PCR amplification with primers designed to amplify Ixodes sp. DNA (unpublished data).
Some PCR amplification products obtained on each sample (i.e. salivary glands, eggs and larvae) were subjected to direct sequencing by using primers BAB GF2 and BAB GR2 in order to verify the identity of the DNA product with BOB2.
RESULTS
Artificial feeding of I. ricinus
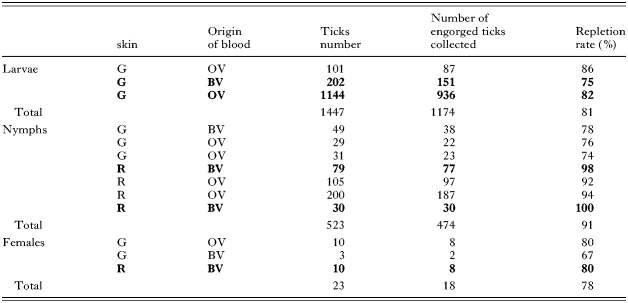
A total of 1447 larvae, 523 nymphs and 23 females (and 23 males for reproduction) I. ricinus were used in skin feeding experiments. Feeding experiments ran for 6 days for larvae, 12 for nymphs and 20 for adults. For each instar, the time-course of feeding experiments is presented in Fig. 2. In all cases, attachment rate to the membrane was around 90–100%. The repletion rate (percentage of engorged I. ricinus to repletion) for the different life stages is presented in Table 1, regardless of whether the blood was infected or not. Larvae repletion rate was not affected by the use of either bovine or ovine blood. Concerning nymphs, a significantly better result was obtained with rabbit skins than with gerbil skins with repletion rates of 94% versus 76%, respectively (χ2 test, P<10−7). The time-course of the experiment was similar whatever the feeding condition used (rabbit or gerbil skins, bovine or ovine blood, parasitized blood or non-infected blood (see Fig. 2), with a peak of engorged ticks between days 5 and 10. With respect to adult ticks, the experimental conditions between the 3 experiments were too different to reach any conclusion, but it seems that gorging was achieved quicker in the case of rabbit skin (day 17 versus day 20, Fig. 2). Mean body mass of the engorged females was 161 mg (62–288), 72% of the 18 engorged ticks laid eggs between days 8 and 32 p.r. and 77% of them hatched to give living larvae between days 90 and 136 p.r. (Data not shown.)

Fig. 1. Diagram of the feeding apparatus used in experimental feeding of Ixodes ricinus ticks.

Fig. 2. Daily percentages of engorged (A) larvae, (B) nymphs and (C) adult females of Ixodes ricinus via experimental feeding on animal skin membrane.
Table 1. Repletion rates of Ixodes ricinus larvae, nymphs and adult females by skin feeding (Larvae were engorged on gerbil skin (G) (Nymphs and adults were engorged either on gerbil (G) or rabbit (R) skins. All feedings were performed either on bovine blood (BV) or ovine (OV) blood. Experiments performed in the presence of in vitro Babesia divergens parasitized bovine or ovine blood are indicated in bold.)

Transmission of B. divergens to I. ricinus ticks
The parasitaemias to which the feeding ticks were exposed ranged from 5 to 8%. B. divergens parasites were specifically labelled, using a sheep anti-Babesia serum, in the bloodmeal of engorged nymphs and adults examined the day of the repletion, proving that the parasite had passed through both gerbil or rabbit skins during the bloodmeal of the ticks (data not shown). Tick gut content, May Grünwald Giemsa-stained, showed a mixture of intact erythrocytes containing intact Babesia, haemolysed erythrocytes, partially digested parasites, as well as exoerythrocytic parasites different from those observed in culture, which may have been sexual stages (Fig. 3).

Fig. 3. May Grunwald Giemsa stain of Babesia divergens in the gut of Ixodes ricinus (A) nymph and (B) adult female at repletion, after artificial feeding on parasitized blood. A: clumping of extracellular forms with raylike protusions (R), B: binuclear (N) form with protrusion (R), which may result from the fusion of 2 gametes.
Transtadial persistence of B. divergens
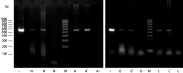
The presence of B. divergens after moulting of the artificially infected instar (nymphs and larvae) was checked for PCR of DNA from the salivary glands. Parasite DNA could be amplified from all tested females (n=9) and from all tested pools of nymphal (n=10) salivary glands with a band at the expected size of 559 bp (Fig. 4). Amplifications performed on the remaining carcass (whole body except salivary glands) of each tested adult and nymph were also positive (data not shown), indicating that some parasite DNA persisted in the body of the ticks after moulting.

Fig. 4. PCR detection of Babesia divergens in Ixodes ricinus ticks after feeding on B. divergens parasitized bovine blood by skin feeding. M, 100 base-pairs DNA molecular weight marker; +, positive control on babesial genomic DNA from in vitro culture; Lanes N, N′, salivary glands of nymphs exposed to infection as larvae; Lane N, salivary glands of nymphs moulted from larvae fed on uninfected bovine blood control; Lanes A, A′, salivary glands of female adults exposed to infection as nymphs; Lane A, salivary glands of female adult after nymph feeding on uninfected bovine blood control; Lanes E, E′, eggs laid by female adults exposed to infection; Lane E, eggs laid by female adults fed on uninfected bovine blood control; Lane L, L′, larvae hatched from female adults exposed to infection; Lane L, larvae hatched from female adults feeding on uninfected bovine blood control.
Larval I. ricinus infected with B. divergens via skin feeding as adults, were used to check for the persistence of the parasite in this vector beyond 1 instar without any additional supply of parasite. PCR analysis performed on the nymphs that moulted from these infected larvae fed on non-parasitized gerbils showed that parasite DNA was still present in salivary glands, indicating that the parasite persists from the adult to the nymph (data not shown).
The resulting 559-bp sequence of PCR products obtained in each condition showed 100% homology with that of the 18S rRNA gene sequence obtained from in vitro culture of BOB2 as well as with those published for B. divergens (Herwaldt et al. 2003; Accession number: AY046576).
Transovarial transmission of B. divergens
Positive PCR detection of parasite DNA performed on pools of eggs (n=7) resulting from the laying of adults infected by skin feeding demonstrated the transovarial transmission of B. divergens. PCR amplifications performed 3 months later on the resulting larvae were also positive, indicating that the parasite DNA remained in the larvae (Fig. 2). In both cases, sequencing of the PCR products showed that we recovered DNA from that of B. divergens Accession number AY046576.
DISCUSSION
Despite the great medical and veterinary importance of ticks, relatively few studies have been reported on in vitro membrane feeding systems for gorging these vectors, due to their long, complex, and poorly understood feeding pattern (Joyner and Purnell, 1968; Kemp et al. 1975; Wetzel, 1979; Kuhnert et al. 1995; de Moura et al. 1997). To our knowledge, this is the first report concerning membrane feeding of I. ricinus. Among the previous reports on other tick species, most of the systems used required the use of olfactory stimuli for tick attachment to the membrane, especially when artificial membranes were used. Here, no specific attractants were needed and the skin alone with a blood temperature maintained at 37 °C was enough for attachment of all tick instars. As well, the cleaning of the membrane twice a day by rinsing, the use of a fungistat and bacteriostat were enough to avoid skin decay. In the present system, it was essential for the blood to be above the ticks for the infection trials because of the rapid sedimentation of the red blood cells. For nymphs and adults, a rabbit skin gave better results than that of gerbil skin. However, no difference was detected with respect to the use of bovine or ovine blood for the gorgement, and the presence of parasites in the bloodmeal seemed to have no influence on the repletion rates of the ticks. Concerning adult females, comparison with those engorged directly on ears of naïve live rabbits during the same period (n=95), shows that the repletion rate was better on skin membranes (n=23) than on animals (78% versus 36%, χ2 test, P<10−3), and that weight gain was also significantly higher with skin feeding (mean of 161 mg versus 115 mg (56–218) than on rabbit; (Mann Whitney test, P=0·002). Opposite results were reported for Amblyomma variegatum with weight achieved by membrane-fed ticks lower than that achieved by ticks fed on cattle (Voigt et al. 1993). No difference was observed between Rhipicephalus appendiculatus fed either on membrane or on cattle (Musyoki et al. 2004). In contrast, it seems that the percentage of engorged ticks that are able to lay eggs was significantly higher with animal feeding (94%) than with membrane feeding (72%) (two-sided Fisher's exact test, P=0·016). However, more data involving a larger number of ticks are required in order to validate such conclusions.
Several methods have been used to infect ticks with different pathogens, including feeding ticks on infected animals (Joyner et al. 1963; Donnelly and Peirce, 1975; Purnell et al. 1975; Lewis and Young, 1980; Rudzinska et al. 1983; Mackenstedt et al. 1990; Mosqueda et al. 2004), injecting pathogens through the cuticule (Kocan et al. 1986; Rehacek et al. 1994; Rechav et al. 1999), feeding ticks on artificial or animal skin membrane (Howarth and Hokama, 1983; Voigt et al. 1993; Waladde et al. 1993; Abbassy et al. 1994; Burkot et al. 2001; Musyoki et al., 2004), or using capillary tubes filled with infectious suspensions (Purnell, 1970; Gern et al. 1990; Rechav et al. 1999; Macaluso et al. 2001). Only the last 3 methods allowed the control of the amount of pathogen given to the ticks and among them, only the last 2 could reproduce the natural oral route of the infection, an essential parameter due to the complexity of parasite developmental stages in the gut of the tick. However, infection of ticks by capillary tube feeding implies a pre-feeding on a host followed by force removal of the ticks, leading to difficult manipulations and feeding conditions further from reality than membrane feeding. For all these reasons, we chose to use the membrane skin feeding technique described to evaluate the in vitro transmission of B. divergens to I. ricinus. The positive result obtained in the case of PCR detection performed on the remaining carcass of each positive salivary gland indicated that some parasite DNA persisted in the body of the ticks after moulting. Such a result clearly indicates the importance of performing PCR detection on salivary glands instead of whole ticks, in order to define whether or not the acari are vectors of the pathogen of interest, either in experimental or in field studies.
As a rule, it is admitted that, in the Babesia genera, the engorging female tick is the only stage that can acquire parasite infection from its mammalian host with the exception of Babesia from rodents, which are acquired by engorging larvae and transmitted by nymphs (Friedhoff and Smith, 1981). To our knowledge, only 3 studies involving tick infection on B. divergens-infected animals have been reported (Joyner et al. 1963; Donnelly and Peirce, 1975; Lewis and Young, 1980). The studies of Donnelly and Peirce (1975) on cattle showed that only adult females could acquire B. divergens, which was then transovarially transmitted to larvae (Joyner et al. 1963; Donnelly and Peirce, 1975). The transovarial transmission of the parasite demonstrated in this study, which is characteristic of the Babesia genera, confirmed the infection of the adult stage. However, from our results, it seems that I. ricinus larvae and nymphs can also acquire B. divergens, which persists transtadially in the subsequent nymphal and adult stages. These can probably transmit to the vertebrate host, as the parasite was detected in their salivary glands. In the study by Donnelly and Peirce (1975), ticks were infected on splenectomized calves with a parasitaemia in the range of 5 to 30%. Parasitaemia used in the present study ranged from 5 to 8%, and so this level of parasitaemia does not explain the difference in the results. However, reported failure of the larvae and nymphs to acquire infection could be explained by the small number of experiments reported as well as by the difficulties of controlling parasitaemia during the whole feeding period of the ticks on infected animals (especially at the exact site where the ticks take the blood). The authors noted that not all females of a group of ticks fed on the same infected bovine host would become infected. Further, as larvae or nymphs take smaller volumes of blood than adults, the probability of ingesting parasites is even lower than for adult females. However, if the results presented here, based on parasite DNA detection by PCR, lead to strong evidence that the ticks are able to acquire the parasite by membrane feeding, conclusion on acquisition and transmission of B. divergens by larvae and nymphs could only be confirmed by future in vivo experiments.
Moreover, if our results have suggested that all tick instars may represent a risk of infection with B. divergens, it is important to note that, in the field, infection of ticks implies that they feed on reservoir hosts of the parasite, which is the case for nymphs and adults on big animals like cattle but not necessarily for larvae that feed on smaller animals like rodents, which are resistant to B. divergens infection. Some field studies have suggested that cattle are most readily parasitized by adult ticks and that such instars may be more important for parasite transmission (Gray, 1980; L'Hostis and Chauvin, 1999). In addition, and although occurring in large numbers, larvae tend to have a very aggregated distribution and therefore may infect a small number of hosts. Consequently, this instar is probably of limited importance for the epidemiology of bovine babesiosis caused by B. divergens (Gray, 1980). Such observations imply that the parasite must be able to survive throughout all the stages of a generation in order to be transmitted to a susceptible host. Here, we demonstrated by detecting B. divergens DNA in the nymphal stage that had fed on non-parasitized blood as infected larvae, that the parasite persists beyond more than 1 moult. A similar result was not obtained with B. microti, which does not persist in the tick beyond 1 instar (Gray et al. 2002). Concerning this experiment, even though tested, we were not able to detect any parasite in the gerbils used for gorging larvae (the test was done by in vitro culture of gerbil blood in sheep red blood cells). One possible explanation of such a result could be the fact that we used non-splenectomized gerbils for this. Indeed, and to our knowledge, the only study that reported B. divergens transmission to gerbils by ticks was that of Lewis and Young (1980) in which splenectomized gerbils were used.
As previously reported for another intraerythrocytic parasite, Theileria parva (Young et al. 1996), our results showed that B. divergens remained viable when drawn in heparinized blood and that its transmission to the tick appeared to be efficient in such conditions. Culture of B. divergens in blood from its natural bovine host was successfully achieved here and parasitaemia up to 20% was obtained without problem in this system. The demonstrated parasite transmission to the ticks implies that B. divergens infective stages are produced in vitro in the described culture conditions. This is an important feature as it is very difficult to obtain sexual stages infective for the vector for other closely related vector-transmitted haemoprotozoan such as Plasmodium falciparum (Meuwissen and Ponnudurai, 1988) and because it is suspected that parasites artificially maintained in vitro may lose their infectiousness capacity (Stewart et al. 1986).
The observation of the tick gut content after repletion showed the complexity of the stages found at any given time. Such diversity came from the fact that the Babesia were ingested over a period of time and the development of the parasites was not synchronous. Figure 3A shows parasites with ray-like protrusions as well as some multi-nucleated bodies, which could be ‘Strahlenkörper’ adhering to each other, similar to those observed with B. bigemina (Stewart et al. 1986; Gough et al. 1998; Mosqueda et al. 2004). The binuclear parasite form with a vacuolated cytoplasm presented in Fig. 3B could correspond to the resulting fusion of 2 gametes and is also very similar to those observed for B. bigemina (Mehlhorn and Schein, 1984; Stewart et al. 1986; Gough et al. 1998) or B. canis (Mehlhorn et al. 1980). Concerning the parasite stages present in the salivary glands of host-seeking ticks, it seems to be established that, for Babesia species, sporozoite development begins only when the infected tick attaches to a vertebrate host (Mehlhorn and Schein, 1984). Concerning B. divergens, our results demonstrated that the parasites are also already located in the salivary glands before attachment and feeding begin. It will be now of interest to know if the stimulus of feeding permits the development of the parasite into an infective sporozoite form, and if all artificially infected tick instars are capable of retransmitting the parasite to a vertebrate host as previously described with ticks infected on animals (Joyner et al. 1963; Donnelly and Peirce, 1975).
Many questions regarding B. divergens epidemiology, biology, and transmission by ticks remain unanswered. In order to understand the zoonotic potential of B. divergens as well as to improve control mechanisms of the cattle disease, it will be necessary to elucidate the transmission process by ticks. Having a suitable artificial method to feed ticks and to infect them under controlled conditions will now enable us to study the development of the pathogen inside ticks and to discover the mechanisms of its transmission. In addition, there is the potential to study the transmission of several other pathogens transmitted by I. ricinus. At last, benefits offered by an effective in vitro feeding system for ticks would also include applications in research on substances injected into hosts by the vector as well as those ingested from hosts by the vector.
This project was supported by research funds from the Institut National de la Recherche Agronomique and the Ecole Nationale Vétérinaire de Nantes. We are indebted to Dr N. Pieniazek for permitting to use primers BAB GF2 and BAB GR2. We acknowledge A. M. Marchand and I. Perray for technical assistance in ticks maintenance and E. and C. Lebigre (DVM) from ‘La maison de la nature’ in Belle Ile en mer for help in collecting breeding ticks. Particular thanks are also due to Dr P. David for his critical reading of the manuscript.