INTRODUCTION
The liver fluke, Fasciola hepatica is an economically important trematode parasite pathogen of livestock which frequently impacts on the health and welfare of cattle and sheep worldwide. Evidence from various sources suggests that the prevalence of infection has increased considerably in recent years for a variety of reasons – changing climate, changing farming practices and increased animal movements (van Dijk et al. Reference van Dijk, Sargison, Kenyon and Skuce2010). It is suggested that climate change is at least partly responsible for the increase in the prevalence of F. hepatica in the UK; moreover, warmer winters and wetter summers, which are predicted to occur in the UK over the next 50 years, combined with growing concerns about drug resistance (Charlier et al. Reference Charlier, Hostens, Jacobs, Van Ranst, Duchateau and Vercruysse2012) are likely to exacerbate the financial and welfare impact of fasciolosis on livestock production (Fox et al. Reference Fox, White, McClean, Marion, Evans and Hutchings2011). We also know that flukes modulate the host immune system and affect diagnosis and susceptibility to other pathogens including bovine tuberculosis (Claridge et al. Reference Claridge, Diggle, McCann, Mulcahy, Flynn, McNair, Strain, Welsh, Baylis and Williams2012).
The life cycle of F. hepatica includes many pre-parasitic stages which develop in the environment or the snail intermediate host, Galba truncatula, where they undergo a clonal expansion (Fig. 1). The infective stage is the metacercaria which is found encysted on herbage and following ingestion by grazing herbivores develops to patency in 10–14 weeks. Temperature and rainfall are the principle determinants affecting the life cycle and hence the prevalence and intensity of F. hepatica infection (McCann et al. Reference McCann, Baylis and Williams2010).

Fig.1. (A) Schematic showing the life cycle of F. hepatica. Eggs are produced by adults in the bile ducts of the definitive host (e.g. sheep) and are passed in the faeces. A key aspect of the life cycle that lends it to genetic manipulation is the ability of a single miracidium to hatch from an egg, infect the snail intermediate host and give rise to several hundred clonal cercariae that are subsequently released from the snail. These metacercariae encyst on pasture and are ingested by the definitive host; (B) Selected images from experimental infections: 1. Embryonated egg containing miracidium, 2. Free miracidum, 3. Redia from an infected snail, 4. Cercaria released from an infected snail.
F. hepatica is regarded by the WHO as a re-emerging zoonosis and of major concern in many developing countries. It has been estimated that 17 million people are at risk of infection and new hyper-endemic regions have been identified in Vietnam, Egypt and Iran, in addition to the well established hyper-endemic region in the altiplano of the South American Andes. The drug of choice to treat human fasciolosis, triclabendazole (TCBZ), is identical to one used to control fasciolosis in livestock. The emergence of anthelmintic resistance, in particular to TCBZ, by F. hepatica populations is an urgent concern to UK agriculture and a threat to human and animal health and welfare in endemic areas (González et al. Reference González, Esteban, Bargues, Valero, Ortiz, Náquira and Mas-Coma2011)
The majority of anthelmintic resistance studies focus on nematode species, where association of mutations in target genes are made with a resistance phenotype. The assumption that resistance is conferred primarily by single nucleotide polymorphisms (SNPs) or deletions in the coding regions of target genes has advanced our understanding for some parasite:drug interactions, such as benzimidazole resistance in Haemonchus contortus, but is proving to be a less effective approach for other parasite:drug combinations (Gilleard, Reference Gilleard2006). The potential for anthelmintic resistance to be complex and multigenic in nature, differing between parasite species and/or isolates of the same species, and involving yet unidentified genes as potential candidates, identifies the need for alternative approaches.
In this review, we discuss a novel genetic mapping approach to identify the major genetic loci involved in conferring TCBZ resistance in F. hepatica. In order to appreciate this approach it is first necessary to review the current knowledge of TCBZ resistance mechanisms in F. hepatica based on either candidate gene approaches or descriptive studies of parasites exposed to TCBZ in vivo or in vitro. Given that the approach we have taken relies on genome-wide markers to map resistance-conferring genes it is important to consider the availability of genomic and genetic resources for this parasite.
TCBZ RESISTANCE
Since its introduction in the 1980s the benzimidazole, TCBZ, has been adopted worldwide as the drug of choice for controlling F. hepatica infection in livestock and humans. Whilst other drugs can be used to target parasitic stages later in the fluke life cycle TCBZ remains the only drug available for the control of acute fasciolosis due to its efficacy against flukes less than four weeks old. However, this heavy reliance on TCBZ to treat sheep and cattle in particular, has resulted in resistance to TCBZ, which was first reported in fluke populations in Australia in 1995 and is now well established in the Netherlands, Ireland, Spain and all UK regions (Fairweather, Reference Fairweather2005; Brennan et al. Reference Brennan, Fairweather, Trudgett, Hoey, McCoy, McConville, Meaney, Robinson, McFerran, Ryan, Lanusse, Mottier, Alvarez, Solana, Virkel and Brophy2007). In the UK, our own studies in England and Wales showed that TCBZ resistance was evident on seven out of 25 farms analysed (Daniel et al. Reference Daniel, van Dijk, Jenkins, Akca, Mearns and Williams2012). The movement of livestock around the country facilitates the spread of TCBZ resistant (TCBZ-R) F. hepatica populations throughout the UK with the potential for considerable economic consequences to livestock production such as that recently reported by Sargison and others (Sargison et al. Reference Sargison, Wilson, Penny and Bartley2010; Sargison and Scott, Reference Sargison and Scott2011). In humans, TCBZ is the only drug used to treat fascioliasis, the zoonotic disease caused by infection with F. hepatica, and the first report of the failure of TCBZ to treat human F. hepatica infection has now emerged (Winkelhagen et al. Reference Winkelhagen, Mank, de Vries and Soetekouw2012).
Detecting TCBZ resistance
The issue of guidelines for the detection of anthelmintic resistance in nematodes has been promoted by the World Association for the Advancement of Veterinary Parasitology (WAAVP) and remains an active discussion; however, in the case of F. hepatica no gold standard exists for defining an isolate based on its drug sensitivity in vivo or in vitro. A comprehensive review of methods for detecting resistance falls outside the scope of this article but key considerations in this area involve the ongoing evaluation of methods applicable to the live host such as the faecal egg count reduction tests (FECRT) and coproantigen reduction test (Flanagan et al. Reference Flanagan, Edgar, Gordon, Hanna, Brennan and Fairweather2011a, Reference Flanagan, Edgar, Forster, Gordon, Hanna, McCoy, Brennan and Fairweatherb; Daniel et al. Reference Daniel, van Dijk, Jenkins, Akca, Mearns and Williams2012). Whilst these approaches are open to interpretation, multiple studies demonstrate TCBZ efficacy in vivo using treatment and necropsy approaches. These studies involve either a natural host, commonly sheep (for example see Coles et al. Reference Coles, Rhodes and Stafford2000, Reference Coles and Stafford2001; McConville et al. Reference McConville, Brennan, Flanagan, Edgar, Hanna, McCoy, Gordon, Castillo, Hernandez-Campos and Fairweather2009; Gordon et al. Reference Gordon, Zadoks and Skuce2012) or the rat ‘model host’ system (for example see Meaney et al. Reference Meaney, Allister, McKinstry, McLaughlin, Brennan, Forbes and Fairweather2007). In most cases the in vivo efficacy of drugs is established using a procedure of experimental infection (∼200 and ∼20 metacercariae for sheep and rats, respectively) followed by TCBZ treatment, typically at 12 weeks post-infection, to establish efficacy against adult stages. Necropsy is performed typically at 14 days post-treatment followed by recovery of surviving adult fluke for enumeration and descriptive studies. Variations to these protocols exist when drug efficacy against different stages of the parasite and/or TCBZ metabolites are under evaluation (Halferty et al. Reference Halferty, Brennan, Hanna, Edgar, Meaney, McConville, Trudgett, Hoey and Fairweather2008, Reference Halferty, Brennan, Trudgett, Hoey and Fairweather2009). An important consideration of the in vitro or in vivo descriptive studies reporting structural or morphological changes in TCBZ-R vs triclabendazole-susceptible (TCBZ-S) F. hepatica is the question of the long-term effect of these alterations over the course of infection. Even though steps were taken recently to demonstrate the validity of these changes in an in vivo system (Devine et al. Reference Devine, Brennan, Lanusse, Alvarez, Trudgett, Hoey and Fairweather2012), given that the flukes were still alive post-treatment these may merely reflect transient changes in fluke architecture in response to the drug from which the flukes may well recover. More recently, an in vitro tool for the diagnosis of TCBZ resistance was reported (Fairweather et al. Reference Fairweather, McShane, Shaw, Ellison, O'Hagan, York, Trudgett and Brennan2012), but this is proving difficult to take beyond a proof of concept due to the toxicity of DMSO required to dissolve TCBZ at the higher concentrations (Hartley, personal communication).
Mechanisms of TCBZ resistance
Understanding the basis of TCBZ resistance has been hampered because, despite extensive investigation, the mode of action of TCBZ remains unresolved. Extensive investigations into the mechanisms of TCBZ resistance in F. hepatica have been the focus of other reviews (Fairweather, Reference Fairweather2005, Reference Fairweather2009, Reference Fairweather2011) and are only outlined here.
Several α− and β-tubulin isotypes are expressed in adult flukes but, in contrast to the situation with ruminant nematodes and benzimidazole drugs, there is a lack of evidence to support the binding of TCBZ to tubulin molecules or a role for tubulin mutations in TCBZ resistance (Ryan et al. Reference Ryan, Hoey, Trudgett, Fairweather, Fuchs, Robinson, Chambers, Timson, Ryan, Fetwell, Ivens, Bentley and Johnston2008). There is more convincing evidence of a role for the altered uptake, efflux and metabolism of TCBZ (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005). It has been shown in vitro that metabolism of TCBZ to the active metabolites TCBZ.SO and TCBZ.SO2 occurs to a greater extent in TCBZ-R than TCBZ-S flukes (Robinson et al. Reference Robinson, Lawson, Trudgett, Hoey and Fairweather2004; Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005). A role for drug metabolism was further supported by detailed electron microscopic analysis of adult flukes incubated in vitro with combinations of TCBZ and TBCZ.SO and inhibitors of the cytochrome P450 (Devine et al. Reference Devine, Brennan, Lanusse, Alvarez, Trudgett, Hoey and Fairweather2010a, Reference Devine, Brennan, Lanusse, Alvarez, Trudgett, Hoey and Fairweather2011) and flavin monooxygenase metabolic pathways (Devine, Reference Devine, Brennan, Lanusse, Alvarez, Trudgett, Hoey and Fairweather2010b). More recently, this has been demonstrated in an in vivo model system (Devine et al. Reference Devine, Brennan, Lanusse, Alvarez, Trudgett, Hoey and Fairweather2012). Drug efflux mechanisms have been highlighted as one of several potential contributory factors to TCBZ resistance (Alvarez et al. Reference Alvarez, Solana, Mottier, Virkel, Fairweather and Lanusse2005; Mottier et al. Reference Mottier, Alvarez, Fairweather and Lanusse2006) and are a current focus of anthelmintic resistance studies in other helminths (Prichard and Roulet, Reference Prichard and Roulet2007; James and Davey, Reference James and Davey2009). Possible transporters for drug efflux include the P-glycoproteins (Pgps), of which a homologue has been identified in F. hepatica, although this form is only expressed in immature flukes (Reed et al. Reference Reed, Spithill, Strugnell and Panaccio1998). A recent study reported single nucleotide polymorphisms (SNPs) in the nucleotide-binding domain of Pgp genes that differed between TCBZ-S and TCBZ-R adult F. hepatica from the field (Wilkinson et al. Reference Wilkinson, Law, Hoey, Fairweather, Brennan and Trudgett2012). Given the small number of flukes examined in the study (n=3 TCBZ-R and n=1 TCBZ-S), the significance of these SNPs remains to be determined. Overall these biochemical results suggest that any combination of several potential pathways may be responsible for TCBZ resistance (Brennan et al. Reference Brennan, Fairweather, Trudgett, Hoey, McCoy, McConville, Meaney, Robinson, McFerran, Ryan, Lanusse, Mottier, Alvarez, Solana, Virkel and Brophy2007).
GENOMIC, TRANSCRIPTOMIC AND PROTEOMIC RESOURCES FOR F. HEPATICA
The limitations of candidate gene-association studies and the value of employing genetic and genomic approaches to anthelmintic resistance have already been highlighted (Gilleard, Reference Gilleard2006). This latter approach, combined with an understanding of the population genetics of a parasite offers an attractive complementary approach to understanding how parasites develop resistance. The added advantage is that not only are genes identified in a non-biased way but their relative importance to the resistant phenotype can also be assessed. In the case of the ruminant nematode H. contortus, these studies are already yielding interesting insights into the biology of this parasite and drug resistance mechanisms in particular (Redman et al. Reference Redman, Sargison, Whitelaw, Jackson, Morrison, Bartley and Gilleard2012). In order for this approach to be relevant to F. hepatica it is important to review the genomic and genetic tools available and identify the need for further development of these resources.
TCBZ has been reported to induce a broad range of effects, with several potential modes of action (Brennan et al. Reference Brennan, Fairweather, Trudgett, Hoey, McCoy, McConville, Meaney, Robinson, McFerran, Ryan, Lanusse, Mottier, Alvarez, Solana, Virkel and Brophy2007; Chemale et al. Reference Chemale, Perally, LaCourse, Prescott, Jones, Ward, Meaney, Hoey, Brennan, Fairweather, Trudgett and Brophy2010). Investigating the nature of resistance to this anthelmintic therefore requires a broad analysis of these helminths, using all available genomic, transcriptomic and proteomic datasets. To date, several studies have been carried out using proteomic tools to investigate the secretome of F. hepatica (Jefferies et al. Reference Jefferies, Campbell, van Rossum, Barrett and Brophy2001; Morphew et al. Reference Morphew, Wright, LaCourse, Woods and Brophy2007; Robinson et al. Reference Robinson, Menon, Donnelly, Dalton and Ranganathan2009), including the response to TCBZ (Chemale et al. Reference Chemale, Perally, LaCourse, Prescott, Jones, Ward, Meaney, Hoey, Brennan, Fairweather, Trudgett and Brophy2010) and ‘virulence’-associated proteins (reviewed by McVeigh et al. Reference McVeigh, Maule, Dalton and Robinson2012). One study integrated transcriptomic and proteomic data to analyse the potential differences in expression during migration of F. hepatica parasites in the liver (Robinson et al. Reference Robinson, Menon, Donnelly, Dalton and Ranganathan2009), but it was limited by the lack of available mRNA and EST sequences from Wellcome Trust Sanger Institute (WTSI; Table 1). In-depth sequencing of the F. hepatica and F. gigantica transcriptomes using next-generation sequencing technologies has only recently been carried out (Young et al. Reference Young, Halls, Jex, Cantacessi and Gasser2010, Reference Young, Jex, Cantacessi, Halls, Campbell, Spithill, Tangkawattana, Tangkawattana, Laha and Gasser2011; Paterson et al. unpublished data; Table 1). Young and colleagues interrogated the transcriptomic data using Gene Ontology (GO) and metabolic pathways to identify key biological processes and pathways, and used data from closely related helminths to investigate potential Fasciola species-specific genes (Young et al. Reference Young, Halls, Jex, Cantacessi and Gasser2010, Reference Young, Jex, Cantacessi, Halls, Campbell, Spithill, Tangkawattana, Tangkawattana, Laha and Gasser2011). In-depth analysis of the Fasciola species transcriptome has been limited to date to specific gene families; for example, the SCP/TAPS proteins (Cantacessi et al. Reference Cantacessi, Hofmann, Young, Broder, Hall, Loukas and Gasser2012), the glutathione transferase superfamily (Morphew et al. Reference Morphew, Eccleston, Wilkinson, McGarry, Perally, Prescott, Ward, Williams, Paterson, Raman, Ravikumar, Khalid Saifullah, Abbas Abidi, McVeigh, Maule, Brophy and LaCourse2012) and cathepsins (Morphew et al. Reference Morphew, Wright, LaCourse, Porter, Barrett, Woods and Brophy2011). As these analyses develop they are likely to reveal interesting insights into parasite biology, including resistance mechanisms, and will provide platforms for the discovery of novel diagnostics, vaccines and drugs to support intervention strategies in the future.
Table 1. Currently available nucleotide sequence for F. hepatica
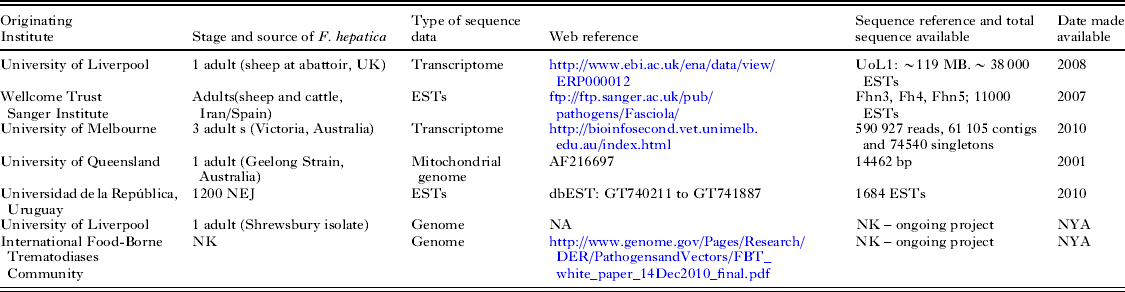
NEJ, Newly excysted juveniles; EST,expressed sequence tags; bp, base pairs; NA, Not Available; NK, Not Known, NYA, Not Yet Available.
Recently there has been increased interest in microRNAs (miRNAs) and their involvement in anthelmintic resistance (Devaney et al. Reference Devaney, Winter and Britton2010). One study investigating miRNAs in F. hepatica and F. gigantica identified 16 and 19 miRNAs, respectively in the two helminth datasets (Xu et al. Reference Xu, Ai, Fu, Nisbet, Liu, Chen, Zhou and Zhu2012). Only a small number of ESTs (WTSI) were used for analysis of potential targets of the miRNAs and the genome of the related helminth, Schistosoma japonicum, was used as a reference - both of which are likely to have limitations.
Such studies have highlighted the fact that to elucidate the nature of complex gene families, such as the cathepsins (McVeigh et al. Reference McVeigh, Maule, Dalton and Robinson2012), to identify species-specific sequences of interest and to map genes associated with TCBZ resistance, a F. hepatica genomic resource is essential. To date, no publically available Fasciola species genome sequence datasets are available, although several groups, including ourselves, are currently sequencing the F. hepatica and F. gigantica genomes (Table 1).
GENETIC RESOURCES FOR F. HEPATICA
Population genetic analyses are a vital component of anthelmintic resistance studies, facilitating our understanding of the origin, evolution and, most importantly, spread of resistance genes in populations (Gilleard and Beech, Reference Gilleard and Beech2007). An understanding of the geographical variation of F. hepatica will also allow interpretation of candidate gene-association studies. Given that Fasciola species undergo parthenogenesis and can both self- and cross-fertilize, they are an interesting and complex group of parasites in which to study population genetic structure and gene flow. This is compounded by the fact that there is a clonal expansion in the snail intermediate host and infection can occur in multiple mammalian definitive hosts, which may impact on the levels of gene flow, aggregation of transmission and parasitic load. All these factors affect the effective population size and the degree of inbreeding which in turn impacts on the spread of resistance genes.
Ribosomal and mitochondrial DNA markers
A number of randomly amplified genetic markers or protein electrophoresis methods has been developed and employed over the last 15 years to facilitate characterization and differentiation of F. hepatica and/or F. gigantica isolates (Li and Quiros Reference Li and Quiros2001; Vilas et al. Reference Vilas, Paniagua, Outeiral and Sanmartín2002; Vargas et al. Reference Vargas, Vega and González2003; Ramadan and Saber, Reference Ramadan and Saber2004; Aldemir, Reference Aldemir2006; McGarry et al. Reference McGarry, Ortiz, Hodgkinson, Goreish and Williams2007; Alasaad et al. Reference Alasaad, Li, Lin, Martín-Atance, Granados, Díez-Baños, Pérez and Zhu2008). The ability of these markers to determine population sub-structuring and/or genetic diversity remains either untested or is limited (Ellsworth et al. Reference Ellsworth, Rittenhouse and Honeycutt1993; Backeljau et al. Reference Backeljau, De Bruyn, De Wolf, Jordaens, Van Dongen, Verhagen and Winnepenninckx1995; Beveridge, Reference Beveridge1998; Vázquez-Prieto et al. Reference Vázquez-Prieto, Vilas, Mezo, González-Warleta, Ubeira and Paniagua2011). Ribosomal and mitochondrial DNA of Fasciola species offer a more promising region to exploit for studying heterogeneity.
Amplification of the entire 28S rDNA (∼4 kb) gene revealed few polymorphisms within F. hepatica and F. gigantica isolates from different geographical locations (Marcilla et al. unpublished data cited in Marcilla et al. Reference Marcilla, Bargues and Mas-Coma2002) however, a 618 bp region was subsequently developed as a PCR-restriction fragment length polymorphism assay (PCR-RFLP) (Marcilla et al. Reference Marcilla, Bargues and Mas-Coma2002). Similarly the complete mitochondrial genome of F. hepatica (∼14·5 kb) was sequenced from two geographically distinct isolates (Australia and Salt Lake City, Table 1) but showed limited (<1%) intra-specific variation (Le et al. Reference Le, Blair and McManus2001). More recently a PCR-RFLP of the ribosomal ITS-1 sequence was used to determine heterogeneity of Fasciola species in Asian countries (Ichikawa and Itagaki, Reference Ichikawa and Itagaki2010). Other ribosomal regions, such as the ITS-2 rDNA, have been sequenced and compared amongst isolates of F. hepatica (Erensoy et al. Reference Erensoy, Kuk and Ozden2009); used to investigate the origin of triploidy in Fasciola species (Itagaki and Tsutsumi, Reference Itagaki and Tsutsumi1998) and to differentiate between F. hepatica, F. gigantica and intermediate forms of Fasciola species from sheep and cattle (Itagaki and Tsutsumi, Reference Itagaki and Tsutsumi1998; Ali et al. Reference Ali, Ai, Song, Ali, Lin, Seyni, Issa and Zhu2008). Detection of SNPs in fragments of a 28S rDNA gene region identified two basic lineages of F. hepatica and showed a distinct difference between north-eastern and south-eastern European isolates (Walker et al. Reference Walker, Prodöhl, Fletcher, Hanna, Kantzoura, Hoey and Trudgett2007; Teofanova et al. Reference Teofanova, Kantzoura, Walker, Radoslavov, Hristov, Theodoropoulos, Bankov and Trudgett2011). However, no explicit lineages or genetic structuring were identified in these isolates based on the β-tubulin 3 gene (Teofanova et al. Reference Teofanova, Kantzoura, Walker, Radoslavov, Hristov, Theodoropoulos, Bankov and Trudgett2011). Analysis of SNPs in NAD1 and COX1 genes from twenty locations across eastern Europe and western Asia revealed little genetic structuring between the two populations (eastern Europe and western Asia) possibly indicating high gene flow between them due to the migration of the definitive host (Semyenova et al. Reference Semyenova, Morozova, Chrisanfova, Gorokhov, Arkhipov, Moskvin, Movsessyan and Ryskov2006).
Despite its widespread popularity as a genetic marker there are limitations to using mitochondrial DNA such is its potential to undergo genetic recombination and positive selection, thus reducing its value as a neutral marker of population diversity (Galtier et al. Reference Galtier, Nabholz, Glémin and Hurst2009).
Microsatellite markers
In order to study the spread of anthelmintic resistance, biparentally inherited markers such as microsatellites offer a more comprehensive approach (Johnson et al. Reference Johnson, Webster, Adam, Buckland, Dawson and Keller2006). To date, few microsatellite markers have been identified for F. hepatica and have been limited in their application (Hurtrez-Boussès et al. Reference Hurtrez-Boussès, Durand, Jabbour-Zahab, Guégan, Meunier, Bargues, Mas-Coma and Renaud2004; Dar et al. Reference Dar, Amer, Courtioux and Dreyfuss2011). The most extensive study to date used a combination of microsatellites (n=4) and allozymes (n=8) to analyse populations of F. hepatica (n=587) from sheep and cattle in north west Spain (Vilas et al. Reference Vilas, Vásquez-Prieto and Paniagua2012). Existing studies have concentrated on the adult stage of the F. hepatica life cycle, often with only small sample sizes and have identified a need for greater analysis and development of genome-wide microsatellites, to unravel the population genetic structure of F. hepatica. From our genomic data we have developed a reliable new panel of ∼15 markers (Cwiklinski et al. unpublished data) to genetically profile adult parasites which we are using to determine the current population genetic structure of F. hepatica in the UK. In addition, a sub-panel of microsatellite markers (8 polymorphic loci; Cwiklinski et al. unpublished data) has been optimized to genotype batches of 50 metacercariae, miracidia and eggs. This approach offers significant promise for population genetic and molecular epidemiological studies of F. hepatica in animals and humans in the future, avoiding the need to passage cercariae through a host to produce adults for analysis as has been described for Schistosoma haematobium (Dabo et al. Reference Dabo, Durand, Morand, Diakite, Langand, Imbert-Establet, Doumbo and Jourdane1997) and Opisthorcis viverrini (Jex et al. Reference Jex, Young, Sripa, Hall, Scheerlinck, Laha, Sripa and Gasser2012; Laoprom et al. Reference Laoprom, Sithithaworn, Andrews, Ando, Laha, Klinbunga, Webster and Petney2012).
Application of population genetic markers to TCBZ resistance in F. hepatica
The application of genetic markers to explore the development and spread of resistance genes in F. hepatica is limited to just a few studies on a small number of parasites. A 510 bp fragment of the 28S rDNA gene was sequenced for adult F. hepatica, from sheep in northwest Spain, which were either fully susceptible to albendazole (ALB), clorsulon (CLOR) and TCBZ (n=10); ALB/CLOR resistant (n=5); ALB/TCBZ resistant (n=5); or ALB/CLOR/TCBZ resistant (n=5). SNPs were detected but their significance in resistance remains unknown (Vara-Del Río et al. Reference Vara-Del Río, Villa, Martinez-Valladares and Rojo-Vázquez2007). An alternative RFLP approach using three loci of the mitochondrial genome was used to profile genetically small numbers of adult F. hepatica from sheep and cattle from several geographical locations and from two laboratory isolates; Fairhurst (TCBZ-S, n=18 fluke) and Oberon (TCBZ-R, n=18 fluke) (Walker et al. Reference Walker, Prodöhl, Fletcher, Hanna, Kantzoura, Hoey and Trudgett2007). The RFLP study was extended to F. hepatica (n=422) from cattle (n=29) displaying either a TCBZ-S or -R phenotype (Walker et al. Reference Walker, Johnston, Hoey, Fairweather, Borgsteede, Gaasenbeek, Prodöhl and Trudgett2011). Whilst it is tempting to speculate about how these studies impact on our understanding of the mechanisms and spread of TCBZ resistance (Walker et al. Reference Walker, Johnston, Hoey, Fairweather, Borgsteede, Gaasenbeek, Prodöhl and Trudgett2011; Vilas et al. Reference Vilas, Vásquez-Prieto and Paniagua2012) more comprehensive analyses are required to further our understanding of how resistant genes emerge and once established, how they flow both within and amongst different mammalian hosts from different geographical locations.
F. HEPATICA ISOLATES AND ANTHELMINTIC RESISTANCE STUDIES
A number of in vivo and in vitro studies has identified biological differences in isolates and genetic analysis showing that there is considerable heterogeneity of fluke populations in the field, both of which are important considerations when interpreting experimental studies. The wider liver fluke research community relies on a small number of providers of F. hepatica metacercariae (for example, Baldwin Aquatics, Oregon, US) and, as identified in a recent review, few isolates of F. hepatica are available for drug efficacy studies (Fairweather, Reference Fairweather2011). A summary of the isolates currently available for resistance studies can be found in Table 2. Historically, studies on TCBZ resistance in F. hepatica have relied on four laboratory isolates, the TCBZ–S Fairhurst and Cullompton isolates and the TCBZ-R, Sligo and Oberon isolates (Table 2). All of these isolates have been maintained in the laboratory for more than a decade and the serial passage required for their maintenance, in particular the clonal expansion in the snail, plus the self fertilization of adult parasites, is likely to render the isolate more homogenous with time and potentially less relevant to the field. To ensure the laboratory isolate is representative of the original population requires active steps to derive metacercariae from multiple miracidial:snail infections using eggs recovered from multiple hosts. Little reference is made to the production of metacercariae in any study and studies of population genetics on laboratory isolates are scarce. The observation of a single haplotype in 18 adult fluke of the Fairhurst isolate is consistent with increased homogeneity over time (Walker et al. Reference Walker, Prodöhl, Fletcher, Hanna, Kantzoura, Hoey and Trudgett2007) as is the lack of heterogeneity observed in the Shrewsbury fluke isolates (Cwiklinski, unpublished data). Whilst in vivo infection and drug-treatment studies serve to confirm an isolate's phenotype, the question of their relevance in a field context still remains. The maintenance of the widely used TCBZ-S Cullompton isolate has resulted in an isolate which is aspermic, where there is no meiosis and which is also triploid (Fletcher et al. Reference Fletcher, Hoey, Orr, Trudgett, Fairweather and Robinson2004); a situation which contrasts with diploid flukes found in naturally infected hosts (Fletcher et al. Reference Fletcher, Hoey, Orr, Trudgett, Fairweather and Robinson2004; Reblánová et al. Reference Reblánová, Špakulová, Orosová, Králová-Hromadová, Bazsalovicsová and Rajský2011; Beesley, unpublished data). The fact that a fluke isolate cannot be archived at any point necessitates serial passage but it is important that due consideration of this process is given by academic and commercial suppliers of metacercariae.
Table 2. A summary of the provenance of F. hepatica isolates available for TCBZ resistance studies

a References are cited for studies reporting resistance status, if no published studies available then personal communications cited. TCBZ-S, triclabendazole susceptible; TCBZ-R, triclabendazole resistant; CLOR-R(+IVM) , clorsulon resistant (given as combination with ivermectin); Diploid, 2n=20; Triploid, 3n=30; week, week; pi, post infection; ND, Not determined.
MAPPING TCBZ RESISTANCE GENES IN F. HEPATICA
Currently underway in our laboratory is a project which employs genome-wide SNPs to map TCBZ resistance genes in genetically manipulated F. hepatica (see Fig. 2). The advantage of this approach is that no assumption is made about the mechanisms of TCBZ resistance and our findings will be directly relevant in the current UK field situation. The approach controls for the complex reproductive biology and demography of F. hepatica that would complicate a study of field isolates alone. A draft genome sequence for F. hepatica has been produced in order to support SNP discovery and the application of genome-wide SNPs to map regions of the genome associated with resistance is underway. In order to dissect the spread of genes associated with TCBZ resistance we are taking an experimental approach by crossing TCBZ-S and TCBZ-R clones of F. hepatica and mapping TCBZ-R genes through subsequent F1 and F2 populations (Fig. 2).

Fig. 2. Schematic representation of the experimental approach used to map TCBZ resistance in F. hepatica. The experiment is divided into three phases: (1) the production of clonal infections; (2) the production of F1 recombinants and (3) the production and phenotyping of F2 recombinants. Concurrent production of a genomic scaffold of F. hepatica supports discovery of SNPs in parental clones to allow association of SNPs in the pooled genotyping of phenotyped F2 recombinants either exposed or unexposed to TCBZ. The identification of F1 recombinants is performed using genome-derived microsatellites as neutral markers of parental clones. Metacercariae from self-fertilization or parthenogenesis, which display parental markers only, are shown for completeness but are not used for subsequent production of F2 recombinants. TCBZ, triclabendazole; TCBZ-S, triclabendazole susceptible; TCBZ-R, triclabendazole resistant.
A draft F. hepatica genome has been generated by a combination of Roche 454 and Illumina sequence data and assembled into contigs and scaffolds. Annotation is underway, using automated pipelines to generate gene models based on the F. hepatica transcriptome and schistosome protein sequence. Even in this rough form, this draft genome will greatly improve the sequence resources available to the research community (Table 1), although generating improved functional annotation will likely be a community effort requiring significant investment.
To ensure use of isolates representative of the current UK field situation and to avoid issues associated with laboratory isolates we have identified TCBZ-R field isolates from south Wales and northwest England, UK where high levels of TCBZ resistance have been reported (Daniel et al. Reference Daniel, van Dijk, Jenkins, Akca, Mearns and Williams2012). Following confirmation of their TCBZ resistance status in experimental infections in sheep (in collaboration with Ridgeway Research), we have exploited the life-cycle of F. hepatica to generate clonal lines of TCBZ-R and TCBZ-S isolates. Metacercariae from single TCBZ-resistant miracidial:snail infections have been used to infect sheep concurrently infected with a clone of the TCBZ-S Shrewsbury isolate (Table 2). Key to the success of this approach is the ability to distinguish F1 and F2 progeny that have undergone genetic recombination. These experiments are complicated by the fact that flukes can both self- and cross-fertilize, although there is evidence that cross fertilization predominates (Hanna et al. Reference Hanna, Edgar, Moffett, McConnell, Fairweather, Brennan, Trudgett, Hoey, Cromie, Taylor and Daniel2008). A sub-panel of 8 microsatellites (Cwiklinski, unpublished data) has been used to profile parental clones genetically and is being employed as neutral markers to track the genotypes of the recombinant progeny and allow subsequent identification of F1 and F2 recombinants. A snail infected with one miracidium can produce up to 600 cercariae, all genetic clones of the infecting miracidium, allowing a proportion of cercariae to be genotyped to identify F1 recombinants but leaving sufficient metacercariae to infect a host for the purposes of producing F2.
Ultimately, the project relies on comparing the frequency of SNP alleles derived from the resistant parental clone and linked to the TCBZ resistance locus (or loci) in TCBZ exposed parasites (only resistant parasites) relative to untreated controls (a mixture of resistant and susceptible parasites). Unlinked SNPs (neutral loci) will show no difference in frequency between TCBZ and control parasites. Pooled genotyping on a number of replicates followed by analysis of allele frequencies to associate SNPs (and hence genomic regions) to TCBZ-resistance will be performed on F2 populations. Once SNPs are identified they will be mapped to the draft F. hepatica genome sequence to identify contigs and scaffolds (and ultimately genes) associated with TCBZ resistance.
FUTURE PERSPECTIVES
The identification of genomic regions associated with TCBZ resistance will enable future work to investigate the biochemical function of candidate resistance genes and to perform finer-scale disequilibrium mapping of resistance in field samples. The genome-wide scan for SNPs linked to TCBZ resistance will highlight many genomic scaffolds and may determinate whether these scaffolds are physically linked, i.e. whether there is a single TCBZ resistance locus or several loci, and independent validation for the association between genomic regions and TCBZ resistance. Whilst an initial analysis of the predicted coding sequences lying within the contigs can be performed to determine whether they contain any obvious candidates for TCBZ resistance, the more detailed molecular analysis of candidate resistance loci will form the basis of future work. This newly available genomic resource, together with the recent developments in functional genomics tools such as RNAi and luciferase reporter gene activity assays (McGonigle et al. Reference McGonigle, Mousley, Marks, Brennan, Dalton, Spithill, Day and Maule2008; Rinaldi et al. Reference Rinaldi, Morales, Cancela, Castillo, Brindley and Tort2008) will promote studies at the molecular level to further facilitate our understanding of these important parasites.
ACKNOWLEDGEMENTS
The authors would like to thank Dr James LaCourse for his contribution to the writing of the BBSRC project to map TCBZ resistance, Miss Katherine Allen for her work maintaining the snail colonies and fluke isolates at the University of Liverpool and Mrs Catherine Hartley for her work with the egg hatch assay and parasite recovery. We would like to thank Ms Paula Martin, Mr Oliver Gladstone and staff at Ridgeway Research for their support and advice with experimental infection and parasite maintenance. We would like to thank Roger Daniel and Rowan Wood at the AHVLA for their support in the identification of TCBZ-R field isolates.
FINANCIAL SUPPORT
The work described here was generously supported by the BBSRC grant number BB/I002480/1. Ms Nicola Beesley is a PhD student funded by the Institute of Infection and Global Health, University of Liverpool.






