Introduction
South African lovegrass (Eragrostis plana Nees), known as “tough lovegrass” (capim-annoni) in Brazil, is a C4 perennial grass native to South Africa that has become increasingly problematic in southern Brazilian grasslands (Barbosa Reference Barbosa2016). The management of this species is extremely difficult because of its wide dispersion and invasion of native pasture areas in Brazil. The botanical similarity between E. plana and native grass species makes selective control of E. plana difficult (Goulart et al. Reference Goulart, Nunes, Kupas and Merotto Junior2012). In recent years, farmers have reported cases of herbicide failures, even in burndown operations with glyphosate, in which plants often regrow after herbicide application. The efficacy of glyphosate on E. plana is influenced by growth stage. It is believed that glyphosate does not translocate completely into all tillers, especially the older ones. Instead, minimum amounts are distributed across some tillers, resulting in sublethal doses on a per-tiller basis, allowing several tillers to regrow (Bastiani et al. Reference Bastiani, Lamego, Langaro, Salas-Perez, Rouse and Burgos2018; Corrêa et al. Reference Corrêa, Silveira, Morais, Trentin, Perez and Natividade2014).
Herbicides can be wiped directly onto the foliage of weeds from the moistened cords of a “wiper applicator.” This technique of wiping on herbicides can eliminate spray drift and allow selective use of nonselective, systemic herbicides (Harrington and Ghanizadeh Reference Harrington and Ghanizadeh2017). A wiper applicator was developed in Brazil exclusively to control E. plana in native pasture, where up to 80% weed control can be achieved without causing injury to the native pasture grass, which is shorter than E. plana (Perez Reference Perez2010). Cases of unsatisfactory control of E. plana have been reported, whether using the wiper applicator or conventional sprayer. Control failures are due to various factors, including variable plant size, advanced growth stage, herbicide applications under drought periods, and insufficient coverage owing to extremely dense grass canopy (Kim et al. Reference Kim, Marshall, Brain and Caseley2011; Ziska Reference Ziska2016). There may be other unknown factors that contribute to the herbicide control failures of E. plana. One known factor is the limited translocation of glyphosate to the tillers. Knowledge of the processes endowing tolerance to herbicides is necessary to develop effective and efficient weed management strategies.
In several cases, the differences in the susceptibility of crops or weeds to glyphosate are due to differences in herbicide absorption and/or translocation. This was observed in a perennial ryegrass (Lolium multiflorum Lam.) resistant population that had a lower proportion of phloem vessels than xylem, which reduced the amount of herbicide translocated with photoassimilates across the plant (Galvani et al. Reference Galvani, Rizzardi, Carneiro and Bianchi2012). Recently, it was confirmed that impaired absorption and translocation endowed glyphosate resistance in Palmer amaranth (Amaranthus palmeri S. Watson), in which a susceptible population had 10% and 20% more herbicide absorbed and translocated, respectively, compared with the resistant population (Palma-Bautista et al. Reference Palma-Bautista, Torra, Garcia, Bracamonte, Rojano-Delgado, Alcantara-De La Cruz and De Prado2019). Shrubby false buttonweed (Spermacoce verticillata L.) may also survive glyphosate application due to minimal absorption and translocation (Fadin et al. Reference Fadin, Tornisielo, Barroso, Ramos, Dos Reis and Monquero2018).
The leaf cuticle and plasma membrane are among the barriers limiting glyphosate entry and mobility in plants (Travlos et al. Reference Travlos, Cheimona and Bilalis2017). In most cases, the various salts of glyphosate cannot cross these barriers efficiently without appropriate adjuvants (Hess and Chester Reference Hess and Chester2000; Satchivi et al. Reference Satchivi, Wax, Stoller and Briskin2000). Sprayable ammonium fertilizer is a popular adjuvant, because it prevents precipitation of glyphosate in the spray tank, decreases surface tension, and increases herbicide spreading and penetration into the leaf (Nalewaja and Matysiak Reference Nalewaja and Matysiak2000). In particular, the addition of ammonium sulfate (AMS) to the glyphosate spray solution improves glyphosate efficacy (Pline et al. Reference Pline, Hatzios and Hagood2000; Salisbury et al. Reference Salisbury, Chandler and Merkle1991; Soltani et al. Reference Soltani, Nurse, Shropshire and Sikkema2016). While salts in the water, such as calcium and magnesium, form bonds or complexes with glyphosate, thereby reducing its efficacy (Wills and Mcwhorter Reference Wills and Mcwhorter1985), AMS can sequester these salts, allowing optimum glyphosate activity.
The addition of AMS can improve glyphosate activity when weeds are water stressed (Satchivi et al. Reference Satchivi, Wax, Stoller and Briskin2000). Plants growing under drought conditions for a prolonged period develop thicker cuticles or leaf pubescence, which reduces herbicide absorption. Further, reduced photosynthesis and transport of photoassimilates under drought stress contribute to reduced herbicide translocation (De Ruiter and Meinen Reference De Ruiter and Meinen1998). Adjuvants like AMS may alleviate this problem in E. plana by enhancing absorption and/or translocation of glyphosate under drought stress.
We conducted two main experiments to: (1) determine whether AMS increases glyphosate efficacy on E. plana under water stress and (2) evaluate the effect of AMS on the absorption and translocation of glyphosate in E. plana compared with a highly sensitive plant, junglerice [Echinochloa colona (L.) Link]. To support these experiments, we also performed two preliminary studies to compare the efficacy of various glyphosate salts on E. plana at different growth stages and determine the optimum dose of glyphosate for E. plana control.
Materials and Methods
The Effect of Formulation and Growth Stage on the Activity of Glyphosate on Eragrostis plana
Plants were grown in the greenhouse (environmental conditions not controlled) at Federal University of Pelotas, Capão do Leão, RS, Brazil from January to March 2017. Seeds were collected from a native pasture area (no E. plana management implemented in this area) of Federal University of Pelotas, Capão do Leão, RS, Brazil (31.806°S, 52.489°W) in March 2016. In January 2017, seeds were sown in pots (23-cm height; 26-cm diameter) containing 8 kg of sandy loam soil (pH 5.3, 15% clay, and 1.24% organic matter) at various times to attain the following growth stages simultaneously: 5 to 6 leaves, full tillering (20 to 30 tillers per plant), and panicle initiation. Seedlings were thinned to four plants per pot and were kept at field capacity and fertilized as needed. Plants in the aforementioned stages were treated with glyphosate (see details in Table 1) at a sublethal dose of 540 g ae ha−1. Four glyphosate formulations were tested (Table 1), along with a nontreated check, with four replications (one replication = four plants per pot). Glyphosate was applied at a constant pressure of 200 kPa using a CO2-operated backpack sprayer connected to a 2-m spray boom fit with four flat-fan TeeJet® AIXR110015 nozzles (Spraying Systems, North Avenue and Schmale Road, Wheaton, IL 60187) spaced 0.5 m apart and calibrated to deliver a spray volume of 150 L ha−1. We decided to use a sublethal dose of glyphosate to allow better separation of glyphosate salts according to their efficacy. Also, no AMS was added to glyphosate in this experiment.
Table 1. Glyphosate formulations tested for Eragrostis plana control at the Federal University of Pelotas, Capão do Leão, RS, Brazil, 2017. a

a All formulations of glyphosate were from Monsanto, 800 North Lindbergh Boulevard, St Louis, MO 63167.
At 7, 14, 21, 28, and 35 d after application (DAA), the herbicide effect on E. plana was evaluated visually on a scale of 0% to 100%, with 0% being the absence of visible symptoms and 100% being plant death. Shoots were collected at 35 DAA and oven-dried at 60 C to determine final shoot dry mass. The data were subjected to ANOVA, and when significant (P ≤ 0.05), the means were compared using a Tukey’s test at 5% probability (P ≤ 0.05). Data were analyzed separately by growth stage, because the plants did not reach the targeted growth stage at the same time. This experiment was not repeated in time.
Dose Response to Glyphosate
Based on data from the previous experiment, we performed a dose–response assay of glyphosate at full tillering, which was the most tolerant growth stage. The experiment was conducted in the greenhouse (environmental conditions not controlled) at Federal University of Pelotas, Capão do Leão, RS, Brazil from April to June 2017. The climate of the region, according to the climatic classification of Wilhelm Köppen (Köppen and Geiger Reference Köppen and Geiger1930) is Cfa (C: hot mesothermal climate, with average cold months between 3 and 18 C; f: average monthly rainfall of not less than 60 mm, always humid; and a: temperature of the hottest month above 22 C), with average temperature of 16.7 C during the time when the experiment was conducted.
The seeds used and plant establishment and growth conditions were the same as described in the previous experiment. Glyphosate potassium salt (Roundup® Transorb R, Monsanto, 800 North Lindbergh Boulevard, St Louis, MO 63167) was applied when E. plana had 20 to 30 tillers per plant, using a backpack sprayer calibrated as described previously. Glyphosate doses were 0, 22.5, 45, 90, 180, 360, 720, and 1,440 g ae ha−1. No AMS was included in this experiment.
Shoots were collected at 42 DAA and oven-dried at 60 C to determine final shoot dry mass. Data for dry mass were fit with a nonlinear log-logistic regression model, according to Equation 1:
where c is the lower limit of y, d the upper limit, e is the x value that reduces the value of y 50% between d and c, and b is the relative slope. The data were analyzed using the drc package in R Core Team software (Knezevic et al. Reference Knezevic, Streibig and Ritz2007). This experiment was not repeated in time.
Effect of AMS and Water Stress on Glyphosate Efficacy
This study was conducted in a greenhouse at the Altheimer Laboratory Complex of the Department of Crop, Soil, and Environmental Sciences, University of Arkansas, Fayetteville, AR, USA, from July to December 2017. Seeds of E. plana from the same location as described before (efficacy of glyphosate formulations experiment) were sown in pots containing 8 kg (23-cm height; 26-cm diameter) of field soil (Captina silt loam [fine-silty, siliceous, active, mesic Typic Fragiudults]).
The greenhouse was maintained at 32/25 ± 3 C day/night temperature with supplemental light for 14 h. Plants were kept at field capacity until the beginning of the water-deficit treatments at the full tillering stage (20 to 30 tillers per plant). The water-deficit treatment was initiated 4 d before herbicide application. Water-stressed plants were kept at 50% water-holding capacity and compared with plants grown at 100% of the water-holding capacity of the pot (Cw). The Cw was determined using the weight of the soil after water saturation (Cfm) and the weight of the soil after drying for 24 h at 105 C (Cdm), using the equation Cw = (Cfm − Cdm)/Cfm × 100 (Santos et al. Reference Santos, Bottcher, Kiyota, Mayer, Vicentini, Brito, Creste, Landell and Mazzafera2015). At the beginning of the water-stress treatment, soil in all pots was saturated, drained, and weighed. The pots were then weighed every day, and the water lost by evapotranspiration was replenished (considering 1 g = 1 ml) to maintain Cw at 50% and 100%. Plants were kept under these conditions for 4 d, after which the herbicide treatments were applied. Drought stress was terminated at 4 d after herbicide application (Figure 1).

Figure 1. Schematic diagram of the timeline of water-stress duration and herbicide application on Eragrostis plana at full tillering (20–30 tillers per plant).
The experiment was arranged in a completely randomized design, using a factorial arrangement of treatments with four replications (one replication = four plants per pot) and conducted twice. This experiment consisted of two factors: Factor A was the soil water condition (50% and 100% of water-holding capacity) and Factor B was herbicide by adjuvant treatment [no herbicide; glyphosate alone [312 g ha−1]; and glyphosate [312 g ha−1] + AMS [2,805 g ha−1]). We used a glyphosate dose of 312 g ha−1 (LD50 determined in the dose–response experiment) to observe the benefit of adding an adjuvant, if any. Glyphosate treatments using potassium salt (Roundup® Power Max II, Monsanto) were applied in the spray chamber at a constant pressure of 276 kPa. The spray boom was fit with two flat-fan TeeJet® 80067 nozzles (Spraying Systems), spaced 0.5 m apart and delivering 187 L ha–1.
Glyphosate efficacy (weed control) was evaluated at 7, 14, 21, 28, and 60 DAA, and shoot dry mass was determined at 60 DAA, as described previously. Weed control data were best described with the nonlinear, sigmoidal, three-parameter Gompertz regression defined by Equation 2:
where y is the weed control (%), a is the asymptote, b is the growth rate, c is the inflection point, and x is the glyphosate dose. The number of days to reach 50% of weed control (IC50), was calculated from the above equation. The a parameter estimates the maximum weed control (MWC).
For shoot dry mass, we considered the factors to be runs (Factor A), water-stress treatments (Factor B), and herbicide treatments (Factor C), and then data were subjected to ANOVA. There was no interaction between treatment factors and runs, so the runs were combined. These data were initially tested for normality and homogeneity of variance (there was no need for data to be transformed) and submitted to ANOVA. When significant differences were observed, the water-stress treatments were compared using Student’s t-test (P ≤ 0.05), and herbicide treatment means were compared using Tukey’s test (P ≤ 0.05), with SAS System software v. 9.0 (SAS Institute, 100 SAS Campus Dr, Cary, NC 27513).
AMS Effect on Absorption and Translocation of Glyphosate in Eragrostis plana
Eragrostis plana plants were grown in the greenhouse at the Altheimer Laboratory Complex of the Department of Crop, Soil, and Environmental Sciences, University of Arkansas, Fayetteville, AR, USA, from September 2017 to January 2018. As the pattern of glyphosate absorption and translocation has not been reported for E. plana, we used E. colona as a highly glyphosate-sensitive reference plant. Seeds of E. plana were collected from a native pasture area of the Federal University of Pelotas, Capão do Leão, RS, Brazil, as described earlier. Echinochloa colona seeds were collected from a rice field in east Arkansas, USA.
Experimental units were pots with 8 kg of field soil from the Arkansas Main Research Station at Fayetteville, as described previously. Plant material and growth conditions were the same as described in the efficacy experiment, except that only one plant was grown per pot. Experimental units were arranged in a completely randomized design, using a factorial arrangement of treatments with four replications. The experiment was conducted twice. The absorption experiment consisted of two factors: Factor A was the addition of AMS (glyphosate alone and glyphosate + AMS) and Factor B was sampling time [24, 72, and 144 h after herbicide treatment [HAT]). Four replications of nontreated plants were used to account for background radioactivity. We used 720 g ae ha−1 glyphosate potassium salt (Roundup® PowerMax II, Monsanto) with 2,805 g ae ha−1 AMS as recommended, which controlled E. plana 100% (Figure 2). Use of the full dose of glyphosate (720 g ae ha−1) was based on the methodology described by Nandula and Vencill (Reference Nandula and Vencill2015). Sublethal rates may be applicable in certain situations but do not provide the same physiological response as the full rate. Eragrostis plana survives a full dose of glyphosate in the field in many cases; hence, a full dose is needed to study glyphosate absorption and translocation in this species. Each treatment was spiked with radioactive [14C]glyphosate as a tracer. The radiolabeled herbicide was applied to the second and third youngest, fully expanded leaves (Figure 3) using a 50-μl microsyringe in six 2-μl droplets containing 12,500 dpm μl−1. Thus, each plant received a total of 300,000 dpm [14C]glyphosate. Plants were harvested at 24, 72, and 144 HAT. At each harvest time, plants were removed from the pots, the roots were rinsed, and the plant was fractionated into six parts (above the treated leaf [ATL], treated leaf [TL], culm of main tiller [CMT], roots of main tiller [RMT], other tillers [OTL], and roots of other tillers [ROT]), as illustrated in Figure 3.

Figure 2. Weed control (A) and shoot dry mass (B) of Eragrostis plana at 42 d after glyphosate treatment, Federal University of Pelotas, Capão do Leão, RS, Brazil, 2017. Treatment means (n = 4) and standard errors (error bars) are plotted with the regression curve. Data were best described with a nonlinear log-logistic, four-parameter regression:
![]() $y = c + \left\{ {\left( {d - c} \right)/\left[ {1 + {{\left( {\frac{x}\over{e}} \right)}^b}} \right]} \right\}$
. Glyphosate as a potassium salt formulation was applied at full tillering growth stage (20–30 tillers per plant) at 150 L ha−1 spray volume.
$y = c + \left\{ {\left( {d - c} \right)/\left[ {1 + {{\left( {\frac{x}\over{e}} \right)}^b}} \right]} \right\}$
. Glyphosate as a potassium salt formulation was applied at full tillering growth stage (20–30 tillers per plant) at 150 L ha−1 spray volume.

Figure 3. Plant parts of Eragrostis plana (A) and Echinochloa colona (B) used to evaluate glyphosate translocation. Legend: (1) above treated leaf, (2) treated leaf, (3) culm of main tiller, (4) roots of main tiller, (5) other tillers, (6) roots of other tillers.
The treated leaves were rinsed with 10 ml of deionized water to remove the unabsorbed glyphosate. The rinsate from the treated leaf of each sample was mixed with 15 ml of scintillation cocktail (Ultima Gold™, PerkinElmer, Waltham, MA, USA) and quantified using a liquid scintillation spectrometer (LSS; Packard Tri-Carb 2100TR liquid scintillation spectrometer, Packard Instrument, Downers Grove, IL, USA). Absorption of glyphosate was calculated as the percentage of total radioactivity recovered (rinsate + radioactivity inside the plant) as described by Equation 3:
 $$\eqalign{	 \hbox\scale 90%{Absorption}\;(%) \cr	 \quad= {\scale 90%{\hbox{Total of radioactivity in tissues} \over \hbox{Total of radioactivity in tissues} + \hbox{radioactivity from leaf rinsate}}} \cr	 \qquad{\scale 90%\times 100}}$$
$$\eqalign{	 \hbox\scale 90%{Absorption}\;(%) \cr	 \quad= {\scale 90%{\hbox{Total of radioactivity in tissues} \over \hbox{Total of radioactivity in tissues} + \hbox{radioactivity from leaf rinsate}}} \cr	 \qquad{\scale 90%\times 100}}$$
To evaluate glyphosate translocation, a factorial (two by three by six) arrangement of treatments was used. Factor A was the addition or absence of AMS; Factor B was harvest time (24, 72, and 144 HAT); and Factor C was plant section (Figure 3). Plant sections were dried at 60 C for 48 h and then oxidized in a biological oxidizer (OX700™, R. J. Harvey Instrument, Tappan, NY, USA). The 14CO2 evolved during sample combustion was trapped in a scintillation vial containing 15 ml of scintillation cocktail (Carbon-14 Cocktail, R. J. Harvey Instrument) and quantified using an LSS, as previously described. For the roots of the main tiller (RMT), other tillers (OTL), and roots of tillers (ROT), the tissues were ground in a coffee grinder (BCG211OB, KitchenAid, Benton Harbor, MI, USA). Three subsamples (1 g) were oxidized as described earlier. Considering an efficiency of 85.2% for the biological oxidizer and 95.6% for the LSS counter, the total amount recovered in the translocation experiment was the same in both species at 83.67% for E. plana and 84.0% for E. colona.
Eragrostis plana and E. colona were considered separate experiments. The interaction between treatment factors and runs was not significant for each species, so the runs were combined. The interaction among treatment factors of each experiment was analyzed, and when it was significant, Student’s t-test (P ≤ 0.05) was used to separate means using the R Core Team software (R Foundation for Statistical Computing, Vienna, Austria, http://R-project.org).
Results and Discussion
The Effect of Formulation and Growth Stage on the Activity of Glyphosate on Eragrostis plana
The potassium salt of glyphosate had the fastest activity when applied to plants with 5 to 6 tillers, showing the highest control (55%) at 7 DAA (Table 2), while ammonium and isopropylamine salt were the slowest-acting formulations at this growth stage. Potassium salt remained the best treatment (99% control) at 21 DAA, comparable only to diammonium salt of glyphosate (88% control). Isopropylamine salt was the slowest-acting formulation, with less than 50% activity at 28 DAA. Isopropylamine salt was also the least effective among the glyphosate formulations, as it attained only 72% control at 35 DAA when the experiment was terminated. Diammonium and potassium salt formulations controlled E. plana 100% up to 35 DAA in plants with 5 to 6 tillers. At full tillering, the glyphosate formulations did not differ in activity up to 21 DAA. At 3 wk after application, E. plana control ranged from 30% to 47% across formulations. None of the formulations killed E. plana 100% at 35 DAA (salts ranging from 33% to 57% of control), showing a large reduction in efficacy relative to efficacy on younger plants. Regardless of formulation, glyphosate was more effective at panicle initiation than at full tillering, although the ratings cannot be compared statistically across growth stages (because each growth stage was considered to be a separate experiment). At panicle initiation, potassium and isopropylamine salt formulations had the highest activity up to 28 DAA, when these treatments reached maximum activity at 98% to 99% control. All other formulations reached peak activity at 35 DAA, resulting in >90% control across formulations, except for diammonium salt, which showed numerically lower activity (85%).
Table 2. Comparative efficacy of glyphosate formulations on Eragrostis plana control at three growth stages in the greenhouse at the Federal University of Pelotas, Capão do Leão, RS, Brazil, 2017.
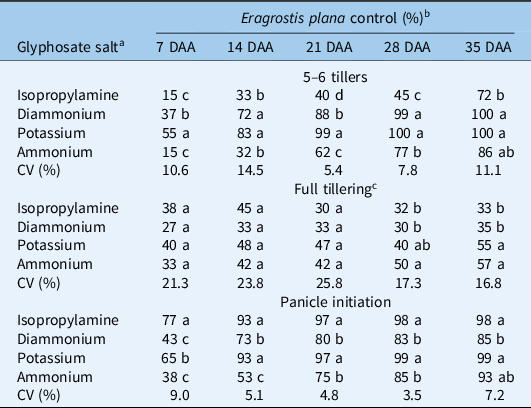
a Glyphosate was applied at rate of 540 g ae ha−1 and 150 L ha−1 of spray volume.
b DAA, days after glyphosate treatment. Different lowercase letters indicate statistical differences among glyphosate salts by Tukey’s test (P ≤ 0.05), n = 4 replicates.
c Growth stage where plants had 20–30 tillers per plant.
All formulations of glyphosate reduced the dry shoot biomass equally compared with the nontreated check at all application timings (Table 3). The degree of biomass reduction was greater when smaller plants were treated, with 79% to 88% reduction at 5 to 6 tillers and 43% to 57% reduction at full tillering. At these stages, the plants were actively growing; thus, the nontreated plants accumulated significantly more biomass from the time of herbicide application, while treated plants ceased growing. At panicle initiation, biomass reduction was generally significant across formulations, but the magnitude of biomass reduction was lower compared with that of younger plants. This is understandable, as desiccating a fully grown, large bunchgrass like E. plana does not eliminate the high amount of biomass already accumulated at the time of herbicide application. The plant at this stage has reached peak growth; therefore, the nontreated check plants would have minimal additional biomass accumulation in the monthlong period after treatment. Overall, glyphosate was least effective on actively tillering E. plana.
Table 3. Comparative efficacy of glyphosate formulations on shoot dry mass accumulation in Eragrostis plana at three growth stages in the greenhouse, Federal University of Pelotas, Capão do Leão, RS, Brazil, 2017.
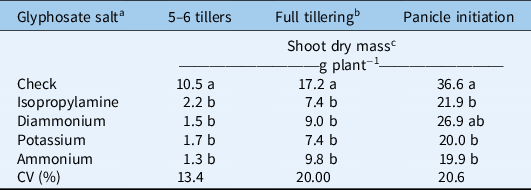
a All glyphosate salts were applied at rate of 540 g ae ha−1 and 150 L ha−1 of spray volume.
b Growth stage where plants had 20–30 tillers per plant.
c Different lowercase letters indicate statistical differences among glyphosate salts by Tukey’s test (P ≤ 0.05), n = 4 replicates.
Dose Response to Glyphosate
The goal of this experiment was to estimate how much glyphosate is needed to achieve a targeted level of control. Because the efficacy of glyphosate varies by growth stage, the amount of glyphosate needed to attain approximately 90% control would differ across growth stages. The log-logistic model with four parameters best fit the dry shoot biomass data. The log-logistic model has been extensively used to describe herbicide effects, mainly to determine the amount of herbicide that would cause 50% growth reduction or 50% control (GR50) in weeds. Complete weed control was attained with 720 g ae ha−1 (Figure 2), for which the LD50 and LD90 were reached at doses of 199 and 461 g ha−1, respectively (Figure 2; Table 4). The GR50 for E. plana at full tillering was 312 g ha−1 of glyphosate (Figure 2; Table 4).
Table 4. Log-logistic parameters from the dose–response regression fit to the weed control and shoot dry mass data of Eragrostis plana. a

a Abbreviations: GR50, glyphosate dose that caused 50% of shoot dry mass reduction; LD50, glyphosate dose that caused 50% of weed control; LD90, glyphosate dose that caused 90% of weed control. ±, standard error.
n = 4 replicates.
Effect of AMS and Water Stress on Glyphosate Efficacy
The interaction effect between water-stress and herbicide treatments on weed control and shoot dry mass was significant (P ≤ 0.05). Weed control data across time was fit with regression equations to show the differential progression on E. plana control (Figure 4). The regression parameters also provided estimates of the maximum weed control (MWC) and number of days to reach 50% of control (IC50). Glyphosate was less effective on drought-stressed plants than on well-watered ones, regardless of adjuvant treatment, except with glyphosate + AMS, which controlled E. plana equally regardless of drought stress at 21 DAA (Table 5). Treatments with glyphosate alone or glyphosate + AMS had equivalent levels of E. plana control across the two water-stress conditions at 28 DAA (Figure 4), which resulted in the same shoot dry mass at 60 DAA (Figure 5).

Figure 4. Weed control of Eragrostis plana with time after glyphosate treatment under well-watered (WW) and drought-stressed (DS) conditions, University of Arkansas, Fayetteville, AR, USA, 2018. AMS, ammonium sulfate. Treatment means (n = 8) and standard errors (error bars) are plotted with the regression curve. Data were best described with the nonlinear, sigmoidal, three-parameter Gompertz regression function: y = a*exp{−exp[−b*(x − c)]}. Glyphosate was applied at full tillering growth stage (20–30 tillers per plant) at a 150 L ha−1 spray volume.
Table 5. Maximum weed control (MWC) and number of days to reach 50% weed control (IC50) of Eragrostis plana, estimated from the regression equations fit to the dose–response data on weed control from 7 to 60 d after treatment, University of Arkansas, Fayetteville, AR, USA, 2018. a

a MWC and IC50 were best described with the nonlinear, sigmoidal, three-parameter Gompertz regression function: y = a*exp{−exp[−b*(x − c)]}, n = 8 replicates.±, standard error.

Figure 5. Shoot dry mass of Eragrostis plana at 60 d after glyphosate treatment, University of Arkansas, Fayetteville, AR, USA, 2018. Treatment means (n = 8) with standard errors (error bars) are shown. Glyphosate was applied to the plants at full tillering growth stage (20–30 tillers per plant) using a 150 L ha−1 spray volume. Different lowercase letters indicate statistical differences among herbicides treatments by Tukey’s test (P ≤ 0.05), and uppercase letters indicate differences between water conditions by Student’s t-test (P ≤ 0.05). AMS, ammonium sulfate.
The addition of AMS clearly enhanced glyphosate efficacy (Figure 4), reaching IC50 in 13.5 d compared with 33.5 d for glyphosate alone (Table 5). Visually, the addition of AMS to glyphosate increased efficacy compared with glyphosate alone, except at 14 DAA under drought stress.
Although no differences in shoot dry mass were observed between glyphosate alone and glyphosate + AMS at 60 DAA (Figure 5), there was a significant difference in control between glyphosate + AMS (98% to 99%, respectively, when drought stressed or well watered) and glyphosate alone (63% and 76%, respectively, when drought stressed or well watered) (Figure 4).
AMS Effect on Absorption and Translocation of Glyphosate in Eragrostis plana
The previous experiment showed a clear advantage of mixing AMS with glyphosate to control E. plana. We hypothesized that AMS increased the absorption and translocation of glyphosate in E. plana. The beneficial effect of AMS was apparent whether the plants were drought stressed or well watered; thus, we used only well-watered plants to study the effect of AMS on glyphosate absorption and translocation.
AMS did not increase the absorption of [14C]glyphosate in E. plana (Table 6). The [14C]glyphosate absorption leveled off at 72 HAT, with the highest absorption attained at 33% at 144 HAT. The main effects of AMS addition and time of harvest on [14C]glyphosate translocation were significant in E. plana (Table 7). The interaction effect of these factors was also significant. Without AMS, [14C]glyphosate translocation was higher (19%) early on (24 HAT) than with AMS (7%). With AMS, glyphosate translocation increased significantly with time, resulting in a higher proportion of absorbed glyphosate translocated at the end of the experiment (63%) compared with the glyphosate-alone treatment (53%).
Table 6. The main effects of ammonium sulfate (AMS) and time of harvest on the total absorption of [14C]glyphosate in Eragrostis plana and Echinochloa colona, University of Arkansas, Fayetteville, AR, USA, 2018.

a 720 g ae ha−1 of potassium salt of glyphosate (cold herbicide) was applied at full tillering growth stage (20–30 tillers per plant in E. plana and 8–12 tillers per plant in E. colona) at 150 L ha−1 spray volume. Each plant received a total of 300,000 dpm of [14C]glyphosate. Different lowercase letters or asterisks (*) indicate statistical differences among harvest times or addition of AMS by Student’s t-test (P ≤ 0.05) for each species, n = 8 replicates.
Table 7. The interaction effect of ammonium sulfate (AMS) addition and harvest time on total translocation of [14C]glyphosate out of the treated leaf of Eragrostis plana, University of Arkansas, Fayetteville, AR, USA, 2018.

a 720 g ae ha−1 potassium salt of glyphosate (cold herbicide) was applied at full tillering growth stage (20–30 tillers per plant) at 150 L ha−1 spray volume. Each plant received a total of 300,000 dpm of [14C]glyphosate. Different lowercase letters indicate statistical differences among harvest times; uppercase letters indicate differences between AMS treatments by Student’s t-test (P ≤ 0.05), n = 8 replicates.
AMS increased glyphosate absorption in E. colona by 6% (Table 6) and continued to rise significantly, reaching 44% at the end of the experiment (144 HAT). In E. colona, the interaction between AMS treatment and harvest time on the translocation of [14C]glyphosate out of the treated leaf was not significant (Table 8). Averaged across AMS treatments, the highest percentage of [14C]glyphosate translocation occurred at 72 HAT (44%). Averaged over harvest times, the addition of AMS increased the translocation of glyphosate in E. colona. Thus, AMS improved the translocation of glyphosate in both highly tolerant (E. plana) and highly sensitive (E. colona) species, contributing to increased glyphosate activity.
Table 8. Effect of ammonium sulfate (AMS) addition on total translocation out of treated leaf of [14C]glyphosate in Echinochloa colona, University of Arkansas, Fayetteville, AR, USA, 2018.

a 720 g ae ha−1 of potassium salt of glyphosate (cold herbicide) was applied at full tillering growth stage (8–12 tillers per plant) at 150 L ha−1 spray volume. Each plant received a total of 300,000 dpm of [14C]glyphosate. Different lowercase letters indicate statistical differences among harvest times or between AMS treatments by Student’s t-test (P ≤ 0.05), n = 8 replicates.
The distribution of [14C]glyphosate in E. plana tissues at 24 and 72 HAT was as follows: treated leaf (TL) > culm of main tiller (CMT) > roots of main tiller (RMT) ≥ other tillers (OTL) ≥ roots of other tillers (ROT) ≥ above treated leaf (ATL) regardless of AMS treatment (Table 9). At the end of the study (144 HAT), the amount of [14C]glyphosate remaining in the TL was equivalent to that in the CMT. However, more [14C]glyphosate was translocated to the CMT when glyphosate was applied with AMS than when applied alone. The addition of AMS increased the quantity of glyphosate that translocated out of the TL, this being concentrated in the CMT (Table 9). At 144 HAT (the end of the study), the [14C]glyphosate translocated to the CMT was 9% higher when glyphosate was mixed with AMS than when applied alone. The quantity of glyphosate translocated to the roots was the same across harvest times. No [14C]glyphosate was detected in the other tillers regardless of the AMS treatment. Likewise, zero to minimal (1% to 2%) amounts of glyphosate were detected in the ROT with or without AMS.
Table 9. Effect of ammonium sulfate (AMS) addition on the distribution of [14C]glyphosate in Eragrostis plana, University of Arkansas, Fayetteville, AR, USA, 2018. a

a AMS rate was 2,805 g ha−1. HAT, h after treatment.
b 720 g ae ha−1 of potassium salt of glyphosate (cold herbicide) was applied to the plants at full tillering growth stage (20–30 tillers per plant) at 150 L ha−1 spray volume. Each plant received a total of 300,000 dpm of [14C]glyphosate. The different lowercase letters indicate statistical differences among plants parts; uppercase letters indicate differences between harvest times; asterisks (*) indicate statistical differences between AMS addition by Student’s t-test (P ≤ 0.05), n = 8 replicates.
As was see with E. plana, the concentration of [14C]glyphosate in E. colona (Table 10) was highest in the treated leaf (TL), followed by culm of main tiller (CMT) > roots of main tiller (RMT) ≥ other tillers (OTL) ≥ roots of other tillers (ROT) ≥ above treated leaf (ATL) regardless of AMS treatment. At 24 and 72 HAT, when glyphosate was sprayed alone, the [14C]glyphosate remaining in the TL was higher than when glyphosate was applied with AMS. The addition of AMS favored a higher translocation of [14C]glyphosate out of the treated leaf, mainly accumulating in the CMT at 24 and 72 HAT. The addition of AMS did not affect the distribution of [14C]glyphosate at 144 HAT. As observed with E. plana, AMS did not change the quantity of glyphosate translocated to the roots of E. colona at any time. Traces of [14C]glyphosate ranging from 1% to 2% were quantified in the tillers and roots of tillers, regardless of the addition of AMS.
Table 10. Effect of ammonium sulfate (AMS) addition on the distribution of [14C]glyphosate in Echinochloa colona, University of Arkansas, Fayetteville, AR, USA, 2018. a

a AMS rate was 2,805 g ha−1. HAT, h after treatment.
b 720 g ae ha−1 potassium salt of glyphosate (cold herbicide) was applied at full tillering growth stage (8–12 tillers per plant) at 150 L ha−1 spray volume. Each plant received a total of 300,000 dpm of [14C]glyphosate. Different lowercase letters indicate statistical differences among plants parts; uppercase letters indicate differences between harvest times; and asterisks (*) indicate statistical differences between AMS treatment means by Student’s t-test (P ≤ 0.05), n = 8 replicates.
Practical Implications
This research demonstrated that for a difficult-to-control species like E. plana, glyphosate formulation and growth stage matters. In this case, potassium salt is best, and the greatest advantage with potassium salt occurs when glyphosate is applied during the early tillering stage. Like all other plant species, the most vulnerable stage is at the onset of the reproductive stage; in grasses that would be at panicle initiation. The majority of related studies had shown no differences between glyphosate formulations. This is expected when the target species are more susceptible to glyphosate than E. plana. For example, glyphosate diammonium or glyphosate-isopropylamine salts applied POST at three rates did not differ in control of common ragweed (Ambrosia artemisiifolia L.), ivyleaf morningglory (Ipomoea hederacea Jacq.), large crabgrass [Digitaria sanguinalis (L.) Scop.], or pitted morningglory (Ipomoea lacunosa L.) (Richardson et al. Reference Richardson, Bailey, Armel, Whaley, Wilson and Hines2003). In addition, the absorption and translocation of [14C]glyphosate formulated as isopropylamine or trimethylsulfonium salts were similar in velvetleaf (Abutilon theophrasti Medik.) and giant foxtail (Setaria faberi Herrm.). In this case, the absence of differences between these formulations may be because both formulations, upon ionization, yield the same active acid glyphosate. Also, the efficacy of isopropylamine, diammonium, and potassium salts of glyphosate does not differ on yellow nutsedge (Cyperus esculentus L.), I. lacunosa, broadleaf signalgrass [Brachiaria platyphylla (Munro ex C. Wright) Nash], and A. palmeri, even when some salts affected the pattern of absorption and translocation (Mueller et al. Reference Mueller, Main, Thompson and Steckel2006). Differences in absorption and translocation could be attributed to differences in leaf anatomy (Oliveira et al. Reference Oliveira, Dario, Alves and Gandolfo2015) but do not always equate to differences in efficacy of control. The potassium and ammonium salt formulations resulted in higher leaf wetting when applied to hairy beggarticks (Bidens pilosa L.), while the isopropylamine salt formulation resulted in lower wettability of southern sandbur (Cenchrus echinatus L.) leaves (Oliveira et al. Reference Oliveira, Dario, Alves and Gandolfo2015). In waterhemp [Amaranthus tuberculatus (Moq.) Sauer], the initial absorption of glyphosate was higher with the isopropylamine salt formulation compared with the diammonium salt formulation at 2 HAT (Li et al. Reference Li, Smeda, Sellers and Johnson2005). However, the initial observable differences in absorption and translocation did not result in differentiation of efficacy between formulations at 72 HAT. Thus, the interaction among glyphosate formulations and leaf surfaces can help predict the efficacy of formulations (Travlos et al. Reference Travlos, Cheimona and Bilalis2017).
Anecdotal evidence suggests that plants are most sensitive to herbicides when young (i.e., seedlings), and herbicide efficacy declines with plant age. Controlling weeds when small also brings an economic advantage, because it is a stage when weeds have minimal or no effect on crop yield. It is also common knowledge that plants are most sensitive to stress (i.e., drought, heat, herbicides) at the onset of the reproductive stage. Thus, herbicide application in the crop season is mostly terminated before the reproductive phase. For a difficult grass weed in a pasture ecosystem (such as E. plana), it appeared that the best timing for glyphosate application is during the onset of reproduction. At the reproductive stage, plants tend to translocate sugar to roots and reproductive organs, thus also transporting most of the glyphosate molecules. If more glyphosate is translocated basipetally, then more will reach the meristematic tissues and the tillers, resulting in better control. Our previous study showed that E. plana sprayed at panicle initiation translocated more [14C]glyphosate out of the treated leaf (mainly to the main culm) compared with the tillering stage (Bastiani et al. Reference Bastiani, Lamego, Langaro, Salas-Perez, Rouse and Burgos2018). This timing is suitable for pastures if there is height differentiation between the pasture grass and the weed that allows application by a “wipe-on” technique when the weeds are taller. This timing can also be used when one is doing a complete rejuvenation of pasture grass after E. plana infestation is reduced to a minimum or completely eradicated.
The current study informs us that one should start chemical management of E. plana at the early tillering stage (or maybe before tillering, but we have not tested this timing). This is the second-best timing compared with panicle initiation. At early tillering stage, the best formulation was potassium salt. Differential performance of glyphosate salts could be explained by the interaction between glyphosate formulations and leaf surfaces, as the leaf surfaces change with time. Young plants are typically more easily controlled compared with older plants, because tissues in the former are less developed, allowing greater absorption of herbicides (Degreeff et al. Reference Degreeff, Varanasi, Dille, Peterson and Jugulam2018). When plants are small, the concentration of the herbicide per plant would be higher compared with when plants are large; hence, foliar herbicides are more effective on seedlings than large plants. Further, the translocation pattern of photosynthates (sugar) changes during the life cycle of the plant; hence, herbicides like glyphosate (which moves in a similar pattern as photosynthates) could be translocated to various tissues, resulting in a dilution of glyphosate reaching the target site and reduced efficacy (Fadin et al. Reference Fadin, Tornisielo, Barroso, Ramos, Dos Reis and Monquero2018). The early timing may be possible if the desired pasture grass is slightly dormant or at a stage when it can recover from herbicide injury.
At full tillering, when E. plana is most difficult to control, the GR50 and LD50 for E. plana were 312 and 199 g ae ha−1 of glyphosate, respectively. Based on control failures reported by farmers, we expected a GR50 higher than this, as well as a dose greater than 720 g ae ha−1 to achieve 100% control. Eragrostis plana is apparently less tolerant to glyphosate when grown in the greenhouse than when growing in pastures. In our collective experience, greenhouse-grown plants are more sensitive to herbicides than field-grown plants. Some species with innate tolerance to glyphosate such as Ipomoea grandifolia (Dammer) O’Donell and Benghal dayflower (Commelina benghalensis L.) have a GR50 around 616 and >1,440 g ha−1, respectively in greenhouse experiments (Lacerda and Victoria Filho Reference Lacerda and Victoria Filho2004), almost 2-fold above the values we obtained in this study. On the other hand, velvetbean [Mucuna pruriens (L.) DC], a plant also known to have high tolerance to glyphosate, had a GR50 of 404 g ha−1 in a greenhouse experiment (Rojano-Delgado et al. Reference Rojano-Delgado, Cruz-Hipolito, De Prado, Luque De Castro and Franco2012). Thus, the GR50 values obtained from greenhouse-grown plants are usually less than the observed tolerance level of such species to the same herbicide in the field. For E. plana, the capacity to regrow after herbicide application and its high tillering capacity are the main factors linked to failures observed in the field (Supplementary Figure S1). Regrowth was observed even at 5 to 6 tillers in a previous experiment (Supplementary Figure S1).
Under drought conditions, the activity of glyphosate on E. plana is greatly reduced; thus, herbicide should not be applied in the dry season. It is common knowledge that drought stress reduces herbicide uptake, translocation, metabolism, and efficacy. Plants grown under drought conditions can develop thicker cuticles or leaf pubescence, which inhibits herbicide absorption; drought reduces photosynthesis and transport of photoassimilates; and transport via the xylem or phloem is reduced, thereby also reducing herbicide translocation (Patterson Reference Patterson1995). The addition of AMS may help in this situation. However, research is lacking on the utility of adjuvants to improve the efficacy of herbicides under drought.
The addition of AMS, an inorganic salt, to the spray solution has been reported for years to improve the efficacy of glyphosate (Pline et al. Reference Pline, Hatzios and Hagood2000; Soltani et al. Reference Soltani, Nurse, Shropshire and Sikkema2016). It has been reported also that certain ions in the water (e.g., Ca2+, Mg2+) could react with the phosphate group in the glyphosate molecule, consequently inactivating some glyphosate molecules and reducing the effectiveness of the herbicide (Wills and Mcwhorter Reference Wills and Mcwhorter1985). The water used in our experiments (Supplementary Table S1) did not contain enough salts to be considered hard water, indicating that the beneficial effect of AMS was unlikely related to inactivation of salts in the water. AMS may enhance glyphosate efficacy through mechanisms other than overcoming antagonistic salts. Glyphosate plus AMS applied using deionized water reduced the fresh weight of quackgrass [Elymus repens (L.) Gould] in the greenhouse more than glyphosate sprayed alone (De Ruiter and Meinen Reference De Ruiter and Meinen1998). Therefore, the role of AMS in enhancing the efficacy of glyphosate is still not understood. Some researchers reported increased absorption and/or translocation of glyphosate with AMS (Satchivi et al. Reference Satchivi, Wax, Stoller and Briskin2000). Satchivi and colleagues reported increased glyphosate absorption in A. theophrasti and S. faberi upon the addition of 1% AMS to either isopropylamine or trimethylsulfonium salt formulations. Glyphosate activity under drought stress conditions was also improved by the addition of AMS (Satchivi et al. Reference Satchivi, Wax, Stoller and Briskin2000), which was confirmed by our experiments.
While the addition of AMS did not improve glyphosate uptake in E. plana, AMS improved glyphosate uptake in E. colona in our study, as it also improved glyphosate uptake in A. theophrasti and S. faberi (Satchivi et al. Reference Satchivi, Wax, Stoller and Briskin2000). The addition of AMS to both glyphosate formulations increased the quantity of glyphosate translocated out of the treated leaf but did not increase the quantity of glyphosate translocated to the roots. In which case, we would expect increased desiccation but no effect on regrowth capacity of grasses, because the meristems are at the base, close to the roots. Glyphosate translocation varies across species, as our data showed on E. plana (58%) and E. colona (30%). As E. plana is considered glyphosate tolerant, we expected low levels of glyphosate translocation compared with the glyphosate-sensitive E. colona. The GR50 for E. colona was low (48.45 g ha−1) (Supplementary Table S2), confirming high sensitivity to glyphosate. This could explain the low amount of glyphosate translocation. Extremely low tolerance to glyphosate of E. colona means that glyphosate kills the cells and tissues fast (rapid and complete desiccation), preventing continued translocation of glyphosate to other plant organs. It takes more glyphosate to kill a tolerant plant like E. plana; therefore, the plant can continue to translocate glyphosate for as long as the cells and tissues are alive.
The addition of AMS to glyphosate increased translocation out of the treated leaf and increased concentration of glyphosate in the culm of the primary tiller of E. plana, which did not occur in E. colona. The accumulation of glyphosate in the primary culm of E. plana may be the main reason for improved performance of glyphosate with AMS. The regrowth of E. plana observed in the field (Supplementary Figure S1) and also in this experiment consists of new tillers growing from the base of the primary tiller. Increased concentration of glyphosate in the primary culm would prevent the development of new ones.
Based on the higher percentages of glyphosate absorption and translocation in E. plana, relative to E. colona (a highly sensitive species), we can deduce that herbicide failures in the field are unlikely to be due to low absorption. Failure to control E. plana in the field can be explained by the absence or lack of [14C]glyphosate translocation to the secondary and younger tillers. These findings confirm the theory first proposed by Corrêa et al. (Reference Corrêa, Silveira, Morais, Trentin, Perez and Natividade2014) and later confirmed by Bastiani et al. (Reference Bastiani, Lamego, Langaro, Salas-Perez, Rouse and Burgos2018), wherein plants of E. plana exhibit a mechanism of early termination of dependence among tillers. According to this theory, glyphosate does not translocate up the culm of tillers or a large enough amount does not translocate to result in complete control of the weed. To achieve better control of the whole bunch in the field, each tiller must be exposed to a lethal dose of glyphosate. An additive that enhances translocation of glyphosate from leaves to the base of the tillers is necessary to prevent regrowth. Anything that impedes proper coverage and glyphosate translocation will contribute to control failure.
In summary, the potassium salt of glyphosate had the fastest activity across growth stages of E. plana, while isopropylamine salt was the slowest-acting formulation, which may be explained by the higher leaf wettability attained with potassium salt compared with isopropylamine salt. Younger plants were typically more easily controlled than older plants at the full tillering stage. Eragrostis plana was most vulnerable to glyphosate at the onset of the reproductive stage (panicle initiation), when the plants would start translocating sugar to perennating organs (meristematic tissues at the base) and reproductive organs. In this manner, more glyphosate could be carried to sensitive tissues, improving the efficacy of glyphosate. Plants are generally vulnerable to herbicide treatment (or other stresses) at the onset of reproduction. AMS improved the efficacy of glyphosate by increasing translocation out of the treated leaf and consequently increasing the concentration of glyphosate in the primary culm, which would limit the development of new tillers. Drought-stressed E. plana cannot be controlled with glyphosate, and AMS increased the level of control compared to glyphosate alone. These data can be used to plan an effective management program for E. plana in coordination with the developmental stage of desired pasture grass species.
Acknowledgments
The authors are grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES, finance code 001) for a scholarship to MOB and to the National Council for Scientific and Technological Development (CNPq) for a fellowship to FPL (process no. 305816/2016-0). This research was funded by Embrapa Pecuária Sul; the University of Arkansas Agricultural Research Station, Weed Physiology Laboratory, Fayetteville, AR, USA; and partially by the Federal University of Pelotas (UFPel). No conflicts of interest have been declared.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2020.97


















