INTRODUCTION
In temperate regions, it has been proposed that insectivorous birds indirectly monitor prey abundance by assessing habitat structure while adjusting territory size accordingly (Marshall & Cooper Reference MARSHALL and COOPER2004, Pasinelli Reference PASINELLI2000, Renken & Wiggers Reference RENKEN and WIGGERS1989, Seastedt & MacLean Reference SEASTEDT and MACLEAN1979). This constitutes the basis of the structural cues hypothesis, which proposes that birds adjust territory size using habitat structure as predictor of prey abundance within a given site over time (Seastedt & MacLean Reference SEASTEDT and MACLEAN1979, Smith & Shugart Reference SMITH and SHUGART1987). Canopy cover is considered a structural cue for the ovenbird (Seiurus aurocapillus, Smith & Shugart Reference SMITH and SHUGART1987) and the pileated woodpecker (Dryocopus pileatus, Renken & Wiggers Reference RENKEN and WIGGERS1989) during the breeding season.
In contrast to the generally short breeding season of birds in temperate regions, insectivorous birds in tropical forests typically defend all-purpose territories year-round and form long-term pair bonds with often low reproductive success (Stutchbury & Morton Reference STUTCHBURY and MORTON2001). Thus, for many tropical insectivores the capacity of a given territory to supply enough food for the mated pair and subsequent progeny throughout the year is of great importance. Given that there is considerable variation in territory size of tropical insectivorous birds within populations (Morton et al. Reference MORTON, DERRICKSON and STUTCHBURY2000, Terborgh et al. Reference TERBORGH, ROBINSON, PARKER, MUNN and PIERPONT1990), we asked if the structural cues hypothesis would apply to terrestrial insectivores that hold long-term territories in the tropical rain forest.
As a first step, here we examined the relationship between forest structure, as reflected in canopy openness and leaf area index (LAI), and non-breeding territory size in the neotropical insectivorous bird Henicorhina leucosticta (Cabanis). In addition to canopy openness, we also included LAI to describe the forest structure within the territories because arthropod abundance and biomass are positively associated with LAI in tropical lowland rain forests (Dial et al. Reference DIAL, ELLWOOD, TURNER and FOSTER2006). Therefore, we expect to find relatively smaller territories in sites with low levels of canopy openness and high LAI values, because these forest structural characteristics represent potentially higher prey abundance. We also explored possible differences between old-growth forest and abandoned agro-forest sites in terms of habitat structure and H. leucosticta territory size. Agro-forest systems modify the original forest structure, and structural parameters such as LAI change in relation to this disturbance (Dietz et al. Reference DIETZ, HÖLSCHER, LEUSCHNER, MALIK, AMIR, Tscharntke, Leuschner, Guhardja, Zeller and Bidin2007). Hence, an effect of previous land use on forest structure and consequently on H. leucosticta territory size is expected, if habitat structure is related to territory size. To our knowledge, this is the first study to investigate the relationship between forest structure and territory size in a tropical understorey insectivorous bird.
MATERIALS AND METHODS
Study area
The study was conducted from June to August of 2009 at La Selva Biological Station in the north-eastern Caribbean lowlands of Costa Rica (1600 ha, 10°25ʹN, 84°00ʹW, 30–150 m asl). Rainfall distribution is weakly seasonal with a wet season from May to December (Sanford et al. Reference SANFORD, PAABY, LUVALL, PHILLIPS, McDade, Bawa, Hespenheide and Hartshorn1994). Mean annual precipitation is ~4000 mm y−1 with at least 100 mm of rain mo−1 (Sanford et al. Reference SANFORD, PAABY, LUVALL, PHILLIPS, McDade, Bawa, Hespenheide and Hartshorn1994). We worked in two forest types classified according to their historical land use: old-growth forest, and abandoned agro-forest plantations (McDade & Hartsorn Reference MCDADE, HARTSHORN, McDade, Bawa, Hespenheide and Hartshorn1994). The agro-forest area was formerly planted with cocoa (Theobroma cacao L.), peach palm (Bactris gasipaes Kunth) and Cordia alliodora Oken, and was abandoned in the 1960s (Lieberman & Lieberman Reference LIEBERMAN and LIEBERMAN1987, McDade & Hartshorn Reference MCDADE, HARTSHORN, McDade, Bawa, Hespenheide and Hartshorn1994). The old-growth forest has had no documented history of recent human disturbance (Lieberman & Lieberman Reference LIEBERMAN and LIEBERMAN1987).
Study species
Henicorhina leucosticta is a common dweller of the lower understorey of mature tropical lowland rain forests and adjacent shady secondary growth (Stiles & Skutch Reference STILES and SKUTCH1989, Winker et al. Reference WINKER, KLICKA and VOELKER1996), from central Mexico to northern South America (Clements & Shany Reference CLEMENTS and SHANY2001). This species preys on arthropods, mostly insects, by searching mainly among the leaf litter trapped over palms, ferns and other understorey plants and also in the ground litter. Henicorhina leucosticta breeds from February to May and builds two types of nest (Skutch Reference SKUTCH1960, Stiles & Skutch Reference STILES and SKUTCH1989). Breeding nests are made during the breeding season, located well hidden and close to the ground (Skutch Reference SKUTCH1960). Dormitory nests are structures built year-round, used to sleep at night in slender saplings, palms or climber tangles, in higher positions (0.6–3 m) and each individual can have up to four dormitory nests in its territory (Skutch Reference SKUTCH1960). Territories are defended year-round by single individuals or monogamous pairs (Stutchbury & Morton Reference STUTCHBURY and MORTON2001). Both sexes sing and respond to playbacks of conspecific songs within their territory (Winker et al. Reference WINKER, KLICKA and VOELKER1996), and apparently react more aggressively towards local than unknown songs (Stutchbury & Morton Reference STUTCHBURY and MORTON2001). Females have repertoires composed of five to six song types which are shared with neighbouring females as a local dialect, whereas males have larger song repertoires of up to 30 song types, in which most song types are different from those in the repertoires of other local males (Stutchbury & Morton Reference STUTCHBURY and MORTON2001).
Song recording and editing
Songs of Henicorhina leucosticta were recorded in the field using an Olympus LS-10 digital recorder and a Sennheiser ME66/K6 microphone, at 48 kHz and 24-bit resolution. We made a sound file consisting of two parts of 1-min duration each, using Raven Pro 1.4 sound analysis software (Cornell Lab of Ornithology, Ithaca, New York). The first part was composed by a set of contact calls, and the second part by a local conspecific song recorded at La Selva, which was taken from the song repertoire of a randomly selected individual whose territory was not included in the present study. This sound file, hereafter referred to as ‘stimulus’, was made to elicit a territorial defence response.
Estimation of territory size through playbacks
Ten territories were studied and classified by forest type according to the land-use map of La Selva Biological Station for the year 2000 (Organization for Tropical Studies, http://www.ots.ac.cr/index.php?option=com_wrapper&Itemid=352). Five territories were located in the old-growth forest and five in abandoned agro-forest plantations. In each territory we observed the individuals for a 2-h period to mark the location of foraging and dormitory nest sites, the location of territorial disputes, and the position where calls or songs were emitted. The locations of each sighting were calculated using compass bearings, direct distances between these marks, and reference points of the geographic grid system of La Selva. We plotted the observations for each territory on a map and drew a polygon joining the external marks as the perimeter. We considered the polygon centroid as the centre of the territory. Interestingly enough, we found dormitory nests very close to the centre in eight territories.
Territory size was estimated using the playback technique following Falls (Reference FALLS1981), with some modifications as indicated below. We started a set of playbacks of the stimulus in the central point of the territory to estimate one radius length. We played back the stimulus successively in 20-m intervals, each time farther from the central point, and noted when the individual or pair stopped advancing towards the speakers. We marked the last point where the birds showed territorial defence movements as the territory boundaries. Considering habituation as a possibility, we played back the stimulus again inside the territory and the defensive behaviour was elicited again in all cases, thus habituation was discarded.
Playbacks were made using Radioshack 40–1441 speakers held at a height of 1 m, at a constant amplitude level comparable to natural song. The playback points were located on the territory maps, and the minimum linear distance between the central point and the territory boundary was used as a radius to calculate circular areas for each one. We used ArcView GIS 3.3 (ESRI, California) to calculate territory areas and make a map of the territories. Territories were considered to be circular for ease of measurement in the field, actual territory shapes were unknown. However, since a circular area is a function of its radius and territory radii were directly measured in the field, we report the results concerning forest structure variables using territory radius as the indicator of territory size. All the playback points of a given territory were tested on a continuous session in the same day, without losing visual or acoustic contact with the target bird or birds. Two birds from different territories were colour banded. Two pairs of territories were adjacent, one territory of one pair was held by a marked individual, in the other case a territorial dispute between neighbours provoked by the experimental playbacks was observed, thus facilitating the boundaries determination.
Assessment of forest structure using hemispherical photography
We took digital hemispherical photographs across sites separated by 20 m following linear transects from the central point of the territory to the boundary. We took from three to eight photos in each territory depending on the magnitude of its radius, and 50 photos in total. All photographs were taken at 1.5 m height using a Nikon Coolpix 5000 digital camera with an 8-mm Nikon fish-eye lens. We oriented the camera so the top of each photograph was aligned with the geographic North. Photographs were taken only under overcast conditions to avoid direct beams of sunlight from obscuring the contrast between forest cover and open sky (Rich Reference RICH1990).
Photographs were analysed with Gap Light Analyzer version 2.0 software (Forest Ecology Lab, Simon Fraser University, Burnaby, BC, Canada) using the default threshold level to distinguish pixels between sky and non-sky classes. Canopy structure parameters, such as gap fraction or the complementary canopy cover, can be extracted from the photograph. We used the images to calculate the percentage of canopy openness (PCO) and leaf area index (LAI), in order to characterize the overall structure of the canopy and lower vegetation within the territories. PCO measures the percentage of open sky seen from a point beneath the forest canopy (Frazer et al. Reference FRAZER, CANHAM and LERTZMAN1999). LAI is a measure of canopy foliage content, defined as the total one-side leaf area per unit ground surface area (Asner et al. Reference ASNER, SCURLOCK and HICKE2003, Bréda Reference BRÉDA2003). LAI estimation through hemispherical photography is widely used as a non-destructive method in which LAI is determined from gap fraction measurements by inverting a light interception model (Bréda Reference BRÉDA2003, Chason et al. Reference CHASON, BALDOCCHI and HUSTON1991, Jonckheere et al. Reference JONCKHEERE, NACKAERTS, MUYS and COPPIN2005, Stenberg et al. Reference STENBERG, LINDER, SMOLANDER and FLOWER-ELLIS1994). Gap Light Analyzer in addition divides the canopy and calculates partial LAI values extracted from two concentric rings within the image (Stenberg et al. Reference STENBERG, LINDER, SMOLANDER and FLOWER-ELLIS1994). The complete image represents a 180° field of view, the zenith is the vertical axis in the centre of the image at 0°, and the horizons at the edges are 90° from the zenith. We used the LAI 4 Ring estimate, which is the effective LAI integrated over zenith angles 0° to 60° because it gives a less biased LAI estimate than Ring 5 (Chason et al. Reference CHASON, BALDOCCHI and HUSTON1991, Stenberg et al. Reference STENBERG, LINDER, SMOLANDER and FLOWER-ELLIS1994).
Statistical analyses
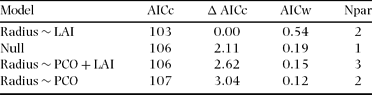
We tested for differences in territory radius between forest types using the Mann–Whitney U-test (Zar Reference ZAR1996), and Student's t-tests were used to analyse PCO and LAI differences between forest types. Since we found no difference in PCO or LAI between forest types, we pooled the data to examine further relationships between PCO, LAI and territory size. We decided to use median values of PCO and LAI to circumvent the effects of extreme values of some of the territories. Lastly, we examined the relationships between these two forest structural variables and territory size using linear regression models. We used the Akaike's Information criterion with a small sample size correction (AICc) for model selection, then we calculated Δ AICc and the Akaike's weights (Burnham & Anderson Reference BURNHAM and ANDERSON2002). The model with the lowest AICc value was then used to examine territory size variation in relation to forest structure characteristics. All tests were done using R version 2.7.1 statistical software (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Territory size
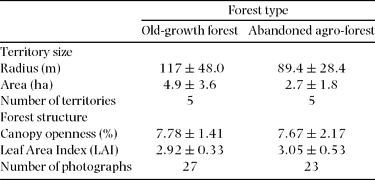
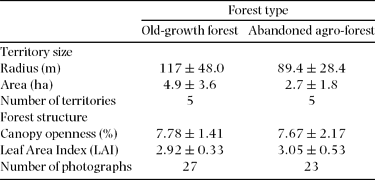
Territory radius did not show differences between forest types (U = 17, P > 0.05) (Table 1). When all territories were pooled, Henicorhina leucosticta mean territory radius length was 103 ± 37.8 m (mean ± SD) (n = 10) and mean circular territory area was 3.8 ± 2.8 ha (mean ± SD) (n = 10). Half of the territories were defended by pairs, and only one territory was defended by a single individual. The rest of the territories were occupied by three or four individuals (pairs with immatures).
Table 1. Territory size and forest structure of Henicorhina leucosticta territories by forest type (mean ± SD) at La Selva, Costa Rica, July 2009.
Forest structural variables
We did not observe differences in PCO between the territories found in old-growth forest and abandoned agro-forest sites (t = −0.29, P = 0.77) (Table 1). In addition, territories located in old-growth forests did not differ in LAI relative to those within abandoned agro-forest sites (t = 1.03, P = 0.31) (Table 1).
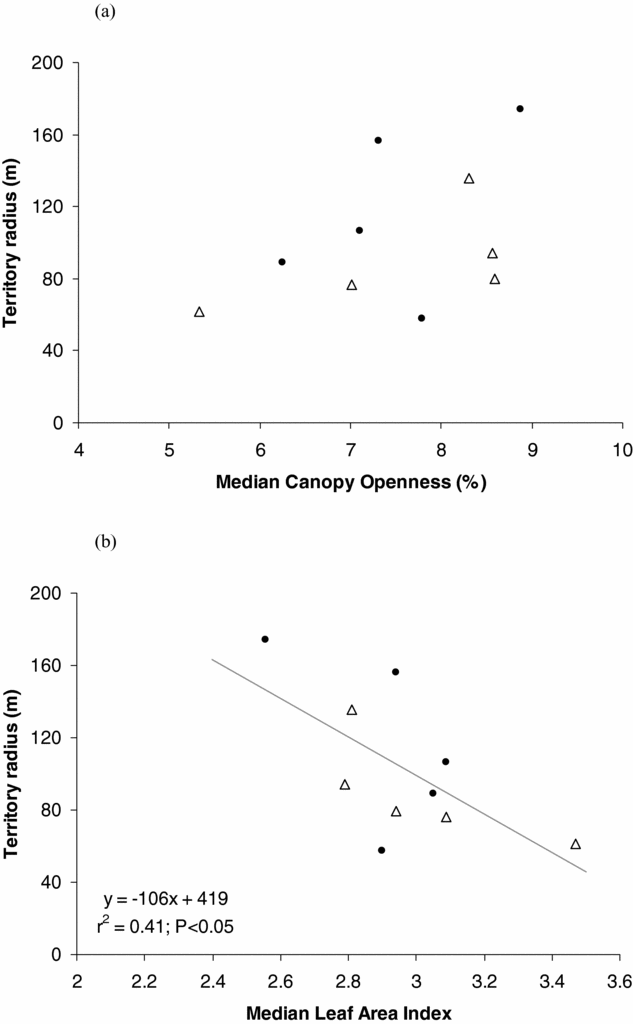
Most territories showed considerable variation in both PCO and LAI evident along their radii. The linear regression model that only included LAI best explained H. leucosticta territory size variation (Table 2). We found that territory radius was negatively correlated with median LAI (r = −0.64, P = 0.04, n = 10), and median LAI explained 41% of the H. leucosticta territory size variation (Figure 1).
Table 2. Linear regression models set of Henicorhina leucosticta territory radius in relation to forest structural variables at La Selva, Costa Rica, July 2009. Models are ranked using Akaike's Information Criterion with a small sample size correction (AICc). Null = territory radius model without forest structure parameters. LAI = leaf area index, PCO = percentage of canopy openness. The Npar column gives the number of parameters used in each model.
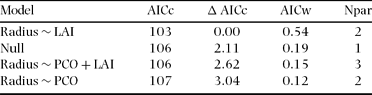
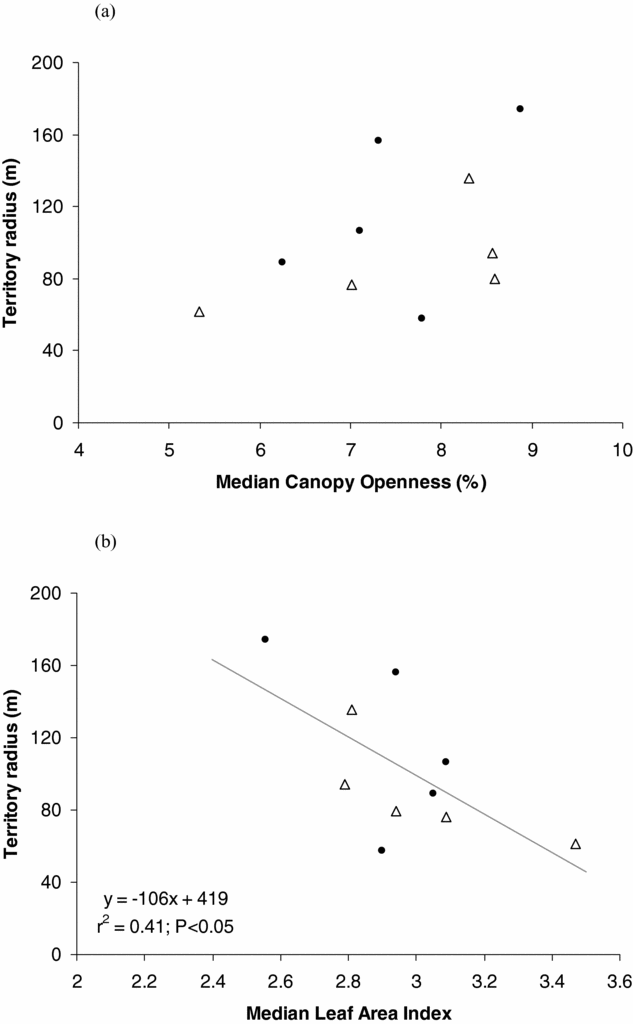
Figure 1. Relationship of Henicorhina leucosticta territory radius with median percentage of canopy openness (a) and median leaf area index (b) at La Selva, Costa Rica, July 2009. Closed circles and open triangles correspond to territories located in old-growth forest and abandoned agro-forest respectively.
DISCUSSION
Playback technique and territory size
We observed that Henicorhina leucosticta kept reacting vigorously to the stimulus and did not seem to habituate. When playbacks are used as an experimental tool, habituation can be an issue (Thompson et al. Reference THOMPSON, GROVES, TEYLER, ROEMER, Peeke and Herz1973). Simpson (Reference SIMPSON1984) did not find evidence of habituation in the Carolina wren (Thryothorus ludovicianus). Thryothorus ludovicianus and H. leucosticta have a comparable singing style in the sense that each song is a succession of repeated short phrases (Mann et al. Reference MANN, DINGESS, BARKER, GRAVES and SLATER2009, Stiles & Skutch Reference STILES and SKUTCH1989). A resistance to habituation of repeated conspecific songs could be associated with this singing style (Simpson Reference SIMPSON1984). Both a strong reaction to playbacks and low or lack of habituation to repeated songs, are characteristics that make H. leucosticta especially suitable for using the playback technique to estimate its territory size. The territory boundaries were very clear in all cases, nevertheless we recommend the measurement of a number of radii per each territory and then average these to improve the accuracy of this procedure for future use.
Robinson et al. (Reference ROBINSON, BRAWN and ROBINSON2000) reported a territory size range (0–2 ha) for H. leucosticta in Panama, which is somewhat lower than our mean territory size at La Selva. However, further comparisons are not easy since they used the spot-mapping technique, while we used field playbacks instead. Even estimations of territory size using the same technique could give different results for the same species, such as the musician wren (Cyphorhinus arada) whose territory was 22.7 ha in Brazil (Stouffer Reference STOUFFER2007), but only 11 ha in Peru (Terborgh et al. Reference TERBORGH, ROBINSON, PARKER, MUNN and PIERPONT1990) using in both cases the spot-mapping method. Use of a different technique to measure territory size may account for the difference observed between the study of Robinson et al. (Reference ROBINSON, BRAWN and ROBINSON2000) and ours. However, many ecological factors, such as habitat structure, may affect territory size differentially in two distinct locations.
Habitat structure in old-growth forest and abandoned agro-forest
Abandoned agro-forest had canopy openness and LAI values comparable to those of the old-growth forest. The agro-forest we studied has been abandoned for more than 40 y (Lieberman & Lieberman Reference LIEBERMAN and LIEBERMAN1987). Secondary forests in humid lowlands of Costa Rica recovered a similar structure to the original forest in 30–50 y after abandoning farming plots (Ewel Reference EWEL1980). Forest structure should recover faster in abandoned agro-forest sites than in comparison with a more extremely disturbed site (i.e. after farming), because some of the original forest trees remain in the agro-forest (Holl et al. Reference HOLL, LOIK, LIN and SAMUELS2000). In addition, LAI increases at a faster rate relative to other forest structural characteristics in the wet tropical lowlands after a given disturbance (Dietz et al. Reference DIETZ, HÖLSCHER, LEUSCHNER, MALIK, AMIR, Tscharntke, Leuschner, Guhardja, Zeller and Bidin2007, Ewel Reference EWEL1980). Therefore, it is expected to have similar levels of LAI and canopy openness in the old-growth forest and the abandoned agro-forest after that period of recovery. In congruence with this lack of structural differences between old-growth forest and abandoned agro-forest, forest type did not have a detectable effect on H. leucosticta territory size, an expected result if territory size is related to forest structure and the two forest types had similar structure.
The LAI values we obtained in both forest types were about half the landscape LAI of 6.00 found in a previous study at La Selva (Clark et al. Reference CLARK, OLIVAS, OBERBAUER, CLARK and RYAN2008), in which LAI was measured directly by harvesting foliage. A key assumption in the estimation of LAI with hemispherical photography is that the leaves are randomly distributed through the canopy (Chason et al. Reference CHASON, BALDOCCHI and HUSTON1991, Stenberg et al. Reference STENBERG, LINDER, SMOLANDER and FLOWER-ELLIS1994). In contrast, the leaves in the forest canopies are grouped in branches and trees. The deviation of the canopy foliage from a random distribution has been accepted as the main reason for constantly obtaining LAI underestimations when using indirect methods such as hemispherical photography (Bréda Reference BRÉDA2003, Chason et al. Reference CHASON, BALDOCCHI and HUSTON1991). However, the spatial variability of effective LAI, as estimated by indirect methods, is reliable and a correction factor can be used to obtain accurate LAI values (Chason et al. Reference CHASON, BALDOCCHI and HUSTON1991). For our purpose, we were more interested in the relative spatial variation of forest structural variables among territories than on exact LAI values, therefore our LAI estimations are functional to analyse H. leucosticta territory size in relation to forest structural characteristics.
Habitat structure and territory size
Canopy openness and LAI were found to be highly variable within territories. High variability of these canopy structural variables across a territory reflects the small-scale spatial heterogeneity at the plant community-level characteristic of tropical rain forests (Wirth et al. Reference WIRTH, WEBER and RYEL2001), exposing a wide overlap of microhabitat conditions inside H. leucosticta territories.
Studies concerning the structural cues hypothesis have found a negative relationship between canopy cover and territory size (Renken & Wiggers Reference RENKEN and WIGGERS1989, Smith & Shugart Reference SMITH and SHUGART1987). Conversely to our expectation, median canopy openness was not included in the best model describing the relationship between H. leucosticta territory size and forest structure. Median LAI alone best explained territory size variation, showing a negative relationship in which territory size decreased as median LAI increased. Even though these two forest structure variables are negatively related (Kabakoff & Chazdon Reference KABAKOFF and CHAZDON1996), they are not redundant and describe different biological aspects of the forest structure.
LAI is positively associated with total arthropod biomass and abundance, as well as with the abundance of 13 out of 14 arthropod groups in tropical lowland rain forests (Dial et al. Reference DIAL, ELLWOOD, TURNER and FOSTER2006). Dial et al. (Reference DIAL, ELLWOOD, TURNER and FOSTER2006) also demonstrated that total leaf area explained 85% of the variability in arthropod abundance and arthropod density did not vary with height from the ground level to the canopy's highest trees. In addition, plant and arthropod biomass are positively associated in tropical rain forests (Sayer et al. Reference SAYER, SUTCLIFFE, ROSS and TANNER2010). The fact that arthropod abundance correlates well with tropical rain forest structure, and the existence of a negative relationship between LAI and territory size, is compatible with the idea that LAI could be used by H. leucosticta as a forest structural cue to indirectly assess prey abundance.
Greater foliage content in the canopy represents an advantage to H. leucosticta in terms of its foraging and nest building behaviour. Since LAI measures foliage area per unit of ground area (Asner et al. Reference ASNER, SCURLOCK and HICKE2003, Cournac et al. Reference COURNAC, DUBOIS, CHAVE and RIÉRA2002), H. leucosticta territories with higher LAI are more likely to have greater leaf fall and subsequently greater leaf litter accumulation over the understorey plants. In fact, leaf litter collections are used as a method to estimate LAI (Chason et al. Reference CHASON, BALDOCCHI and HUSTON1991, Dufrêne & Bréda Reference DUFRÊNE and BRÉDA1995, Veneklaas Reference VENEKLAAS1991). Fallen leaves are intercepted by branches, climber tangles, ferns and small palms in the understorey and constitute a very important reservoir of arthropods for H. leucosticta and many other understorey insectivorous birds. These leaves trapped over the understorey plants are known as the aerial leaf litter (Gradwohl & Greenberg Reference GRADWOHL and GREENBERG1982). Comparably, highest arthropod abundance corresponded with the greatest leaf litter depth at La Selva (Lieberman & Dock Reference LIEBERMAN and DOCK1982). Additionally, H. leucosticta builds dormitory nests year-round, each individual having a number of these dormitory nests within its territory (Skutch Reference SKUTCH1960), and frequently searches for skeletons of partially decayed leaves and small brown leaves within the aerial leaf litter. Therefore, high LAI within a forest area could be associated with more potential prey microhabitats to forage in as well as more nest material sources for H. leucosticta, resulting in smaller territories that guarantee the food and nest building requirements in the long term for this species.
Our results showed that LAI explained a considerable part of H. leucosticta territory size variation. However, territory size of tropical understorey insectivores is probably the result of the interaction between habitat structure and other factors, such as interspecific competition (Robinson & Terborgh Reference ROBINSON and TERBORGH1995, Wilson Reference WILSON1975), population density (Robinson et al. Reference ROBINSON, BRAWN and ROBINSON2000) and predation risk (Lima Reference LIMA1998). Here we emphasize the role of forest structure on territory size and its possible function as a predictor of arthropod abundance for these species. Habitat structural cues could be useful for species that defend long-term territories especially after territory switching or first-time territory acquisition by juveniles, because food supply within the new territory is unknown. Variable rates of territory switching have been reported in the bay wren Thryothorus nigricapillus (Levin Reference LEVIN1996), buff-breasted wren Thryothorus leucotis (Stutchbury & Morton Reference STUTCHBURY and MORTON2001) and dusky antbird Cercomacra tyrannina (Morton et al. Reference MORTON, DERRICKSON and STUTCHBURY2000). However, more research is needed to understand the territory dynamics of tropical understorey insectivores.
In conclusion, we found that territory size is related to forest structure in a neotropical understorey insectivorous bird. These results are congruent with the structural cues hypothesis, given that median LAI is negatively related to territory size and LAI is positively associated with arthropod abundance in tropical rain forests. The mechanism we propose by which forest structure and territory size are connected gives rise to new research questions based on an integrative ecological approach. We urge the next step to be the measurement of aerial leaf litter mass and its respective arthropod abundance within the territories of H. leucosticta, and other tropical insectivores with similar foraging behaviour, in order to investigate the interactions between these understorey micro-habitat characteristics and forest canopy structure variables, such as LAI, in relation to territory size over the long term.
ACKNOWLEDGEMENTS
We thank the Organization for Tropical Studies and La Selva Biological Station staff. Federico Bolaños, Tomás de Camino and Erin Marnocha provided helpful comments. Johel Chaves, and two anonymous reviewers gave substantial feedback on previous versions of the manuscript. David Martínez gave advice in the use of R. Iria Chacón and Graziella Direnzo helped in the field. This study received financial support from the CRUSA Foundation.