Introduction
Ketamine, or ‘K’ as it is known by users, is a Schedule III drug in the United States and was reclassified as a class C drug in the UK last year amid concerns of its growing popularity amongst recreational drug users (Advisory Council on the Misuse of Drugs, 2006). Amongst nightclub goers in the UK, over the past 5 years ketamine lifetime prevalence has increased from 25.5% to 39.8%, whilst current use has increased from 3.9% to 16.0% (McCambridge et al. Reference McCambridge, Winstock and Hunt2007). Ketamine is primarily known as a ‘dance’ drug, used in nightclubs, illegal raves and warehouse parties (Mixmag, 2006). However, ketamine dependence has been reported anecdotally in the popular press (Lilly, Reference Lilly1978; Sputz, Reference Sputz1989; Turner, Reference Turner1994) and there have been a number of case reports in the medical literature (Ahmed & Petchovsky, 1980; Kamaya & Krishna, Reference Kamaya and Krishna1987; Jansen, Reference Jansen1990; Soyka et al. Reference Soyka, Krupinski and Volki1993; Hurt & Ritchie, Reference Hurt and Ritchie1994; Moore & Bostwick, Reference Moore and Bostwick1999; Pal et al. Reference Pal, Berry, Kumar and Ray2002; Lim, Reference Lim2003). Ketamine is from a class of compounds known as dissociative anaesthetics. It is primarily an N-methyl-d-aspartate receptor antagonist but also promotes striatal dopamine release in humans (Kegeles et al. Reference Kegeles, Martinez, Kochan, Hwang, Huang, Mawlawi, Suckow, Van-Heertum and Laurelle2002). In rats and non-human primates, ketamine is repeatedly self-administered (Marquis et al. Reference Marquis, Webb and Moreton1989; Winger et al. Reference Winger, Palmer and Woods1989) and, in rats, produces conditioned place-preference (Layer et al. Reference Layer, Kaddis and Wallace1993). An acute dose of this drug also increases ratings of subjective ‘high’ in healthy humans (Krystal et al. Reference Krystal, Karper, Seibyl, Freeman, Delaney, Bremner, Heninger, Bowers and Charney1994, Reference Krystal, Petrakis, Webb, Cooney, Karper, Namanworth, Stetson, Trevisan and Charney1998) who rate themselves as liking the effects of ketamine and wanting more of it after a single low dose (Morgan et al. Reference Morgan, Mofeez, Brandner, Bromley and Curran2004).
The desire to take a drug again and the degree to which its effects are perceived as pleasurable are thought to be governed by a complex interplay of several factors. According to the influential model of Robinson & Berridge (Reference Robinson and Berridge2003), initial drug exposure activates the mesolimbic dopamine system, producing positive reinforcement and the conscious experience of pleasure, i.e. drug ‘liking’. Over time, sensitization of the mesolimbic dopamine system results in increased incentive salience or drug ‘wanting’. The model suggests that the drug ‘wanting’ occurs outside of conscious awareness and is independent of drug ‘liking’. It is thought that in drug users, primary salience is attributed to the drug (Robinson & Berridge, Reference Robinson and Berridge1993), at the expense of other available rewarding stimuli in the environment (Goldstein & Volkow, Reference Goldstein and Volkow2002) and this results in increased drug ‘wanting’.
The attention-grabbing properties of drug stimuli have been shown experimentally using a modified ‘addiction Stroop’ task in which participants are typically asked to name the colour of words of an appetitive and drug-related nature. If participants are slower to colour name appetitive or drug-related words, this is interpreted as an attentional bias towards these stimuli. Such bias to processing of drug-related words has been demonstrated in people who are dependent on nicotine (Munafo et al. Reference Munafo, Mogg, Roberts, Bradley and Murphy2003), alcohol (Stetter et al. Reference Stetter, Ackermann, Bizer, Straube and Mann1995) and opiates (Franken et al. Reference Franken, Hendriks, Stam and van den Brink2004). However, results from this task have been somewhat inconsistent (for a review, see Weinstein & Cox, Reference Weinstein and Cox2006) and interpretation of findings is complicated by the involvement of a variety of processes (e.g. response inhibition) in Stroop performance, so it is difficult to attribute effects solely to attentional bias.
Another paradigm that has been used to assess attentional bias to drug stimuli is the dot-probe task. Participants view two pictures simultaneously presented on the left and the right sides of a screen. The pictures then disappear and one of them is immediately replaced by a neutral probe stimulus to which the participant must respond as quickly as possible. Participants' response time is reduced if the probe replaces a picture to which they have been attending. Attentional bias to drug stimuli has been observed using the dot-probe task in opiate- (Lubman et al. Reference Lubman, Peters, Mogg, Bradley and Deakin2000) and nicotine- (Ehrman et al. Reference Ehrman, Robbins, Bromwell, Lankford, Monterosso and O'Brien2002) dependent individuals. The dot-probe paradigm also appears to discriminate between problematic and more ‘recreational’ substance use. Thus attentional biases have been shown with this task in heavy but not social drinkers (Townshend & Duka, Reference Townshend and Duka2001) and heavy but not moderate caffeine drinkers (Yeomans et al. Reference Yeomans, Javaherian, Tovey and Stafford2005). Findings of one study suggested that performance on the dot-probe paradigm was predictive of relapse rates in opiate-dependent individuals (Marissen et al. Reference Marissen, Franken, Waters, Blanken, van den Brink and Hendriks2006). Indeed, recent theoretical accounts have suggested that this increased attentional bias to drug-related stimuli may be one of the core processes underlying drug dependence (Franken, Reference Franken2003).
The dot-probe task can be used to differentiate the initial orienting of attention, perhaps a more automatic process, from the final capture of attention, a process likely to be under conscious control. Robinson & Berridge (Reference Robinson and Berridge2003) have suggested that the ‘wanting’ of a drug is largely an automatic process. However, based on the notion that maintained attention is a motivational process and that drug addiction constitutes a disorder of motivation, Bradley et al. (Reference Bradley, Field, Mogg and De Houwer2004) predicted that addicts should also show attentional bias in the maintenance of attention. The presentation duration of the pictures in the dot-probe can be manipulated to examine the relative contribution of these different attentional processes, as initial, more automatic shifts of attention are thought to occur at much shorter durations (50–200 ms; Allport, Reference Allport and Posner1989) than deliberate, intentional shifts. Based on the work of Bradley et al. (Reference Bradley, Field, Mogg and De Houwer2004) we included two stimulus presentation intervals, short (200 ms) and long (2000 ms). Bias at the short presentation interval would indicate a fast, automatic shift in attention to drug stimuli or initial attentional capture. Bias at the long interval is indicative of a bias in the conscious maintenance of attention, or its final capture by the stimuli. In addition, we further modified the task to include a non-drug incentive condition, photographs of money, to explore attentional biases to secondary reinforcers in drug users and also to examine whether biases to universal reinforcers like money occur in healthy individuals as well as drug users.
Although, to date, ketamine dependence has only been reported anecdotally, given that ketamine use is increasing, it seemed important to investigate this population. This study forms part of a larger longitudinal investigation, still underway. However, based on preliminary suggestions of ketamine dependence, the present study set out to investigate: (a) whether attentional biases to drug stimuli occur in ketamine users in a similar manner to other drug users; (b) if such biases do occur, are they related to the extent of drug use and do they persist on cessation of use; (c) whether they can differentiate this potentially ketamine-dependent population from recreational ketamine users.
Method
Participants
Participants were recruited via an existing subject database and using a ‘snowball’ sampling technique (Solowij et al. Reference Solowij, Hall and Lee1992) and were selected to be in one of five groups:
(1) Frequent ketamine users (using the drug more than four times per week);
(2) Infrequent ketamine users (using the drug less than four times per week but at least once per month);
(3) Ex-ketamine users (abstinent for a minimum of 3 months);
(4) Poly-drug users who were matched with the current ketamine-using groups for other drug use;
(5) Non-drug users who did not take illicit recreational drugs.
The study was approved by the institutional ethics committee. All subjects were paid for participation.
Procedure
At the start of the testing session participants gave informed consent, a drug history was taken and then urine samples were analysed (Medscreen, London, UK). Hair samples were collected to verify participants' reports of drug use and confirmed their inclusion in their respective groups (TrichoTech, Cardiff, UK). The participants then participated in a semi-structured interview about various aspects of their drug use including the ‘Cut down, Annoyed, Guilty, Eye-opener’ questionnaire (CAGE; Bush et al. Reference Bush, Shaw, Cleary, Delbanco and Aronson1987), a short screening instrument for drug or alcohol dependency. The participants then completed the dot-probe task along with some other assessments that formed part of a larger longitudinal study.
Dot-probe task
Stimuli were 10 colour photographs of drug- (ketamine, i.e. a white powder, Fig. 1a) related stimuli and 10 colour photographs of money-related stimuli (Fig. 1b). Each drug or money stimulus was paired with a photograph of another scene matched as closely as possible for content but lacking any drug-related cues. An additional 20 picture pairs (neutral and unrelated to drugs or money) were used as filler trials. Ten practice picture pairs were also used.

Fig. 1. (a) Example pair of drug-related dot-probe stimuli. (b) Example of money-related dot-probe paired stimuli.
Each trial started with a fixation cross shown centrally for 1000 ms. This was then replaced by a pair of pictures presented for either 200 or 2000 ms. The 10 practice trials were followed by two blocks of 80 experimental trials, with a short break between blocks. Of the total 160 experimental trials there were 80 critical trials. These were composed of 40 drug-neutral picture trials and 40 money-neutral picture trials. The drug-neutral and money-neutral picture pairs appeared twice for 200 ms and twice for 2000 ms. Within each stimulus duration condition the picture appeared once on the left and once on the right, with both a probe and a target appearing behind the two types of picture (drug/money and neutral). The probe was an asterisk. The side upon which the probe appeared was counterbalanced across the 10 critical drug-neutral trials, the 10 money-neutral trials and the 20 filler trials.
After the visual probe task, participants completed the picture-rating task. This consisted of 80 trials where participants were asked to rate each picture on a seven-point anchored rating scale that was displayed at the bottom of the screen. The rating scale ranged from −3 (very unpleasant) to +3 (very pleasant). The picture remained upon the screen until participants had made a response.
Statistical analysis
Data were analysed using spss version 10.0 (SPSS, Inc., Chicago, IL, USA). A bias score was calculated for the dot-probe data by subtracting the time taken to respond to the probe when it replaced an incentive picture (either drug or money) from the time taken to respond to the probe when it replaced a neutral matched picture. Reaction times greater than 2.5 standard deviations from the mean were excluded, as were reaction times to incorrect responses. These data were then subjected to a 2×2×5 repeated-measures analysis of variance (ANOVA) with stimulus type (drug, money) and stimulus duration (long, short) as the within-subjects factors and group (frequent ketamine user, infrequent ketamine user, ex-ketamine user, poly-drug control and non-drug user) as the between-subjects factor. Demographic, drug use and CAGE data were analysed with one-way ANOVAs or, where data were non-parametric, Kruskal–Wallis tests. Dichotomous data were analysed with χ2 analyses.
Results
Demographics
A total of 150 participants completed the study: 30 frequent, 30 infrequent and 30 ex-ketamine users and 30 poly-drug and 30 non-drug-using controls. There were no differences in age and gender across the groups. There were significant differences in years in education between the groups [F(4, 145)=4.07, p=0.004]. This reflected fewer years in education in the frequent ketamine users compared with the infrequent ketamine users (p=0.018) and the non-drug users (p=0.04). There was also a significant difference in pre-morbid intelligence quotient as indexed by the spot the word test (Baddeley et al. Reference Baddeley, Emslie and Nimmo-Smith1993) [F(4, 145)=7.29, p<0.001]. This reflected higher scores in the poly-drug users compared with frequent ketamine users (p=0.01) and non-drug users (p=0.028) and higher scores in the infrequent ketamine users compared with frequent users (p=0.04) (Table 1).
Table 1. Group characteristics across key demographic variables in the study
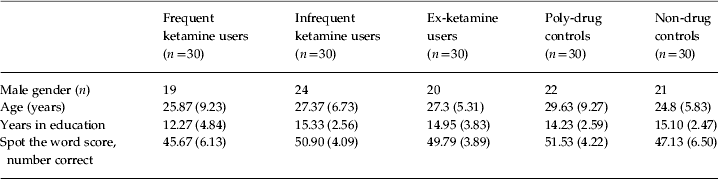
Values are given as mean (standard deviation).
Drug use data
Ketamine use
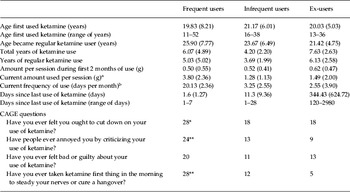
There were no significant differences between the three ketamine-using groups in age of first use of ketamine, the age that they became a regular user of the drug and the amount per session taken when they first used ketamine (Table 2). There were significant differences between the two currently ketamine-using groups (frequent and infrequent) in both the amount of ketamine currently taken (Z=1.81, p=0.003), the current frequency of ketamine use (Z=3.87, p<0.001) and days since last use of ketamine (Z=2.84, p<0.001). Of the frequent ketamine-using group, 19 subjects classed themselves as daily users. Eight members of the ex-ketamine group reported a history of daily ketamine use with a peak frequency of use in this group being a mean of 11.21 (s.d.=9.34) days per month. Three members of the infrequent ketamine-using group classed themselves as having a history of daily ketamine use. There were significant differences in the total years of ketamine use [F(2, 89)=7.45, p<0.001] and years of regular ketamine use [F(2, 89)=3.74, p=0.027] between the groups. This reflected a longer period of use of ketamine in the ex-users compared with the infrequent ketamine-using group for both total years (p=0.001) and years of regular use (p=0.023).
Table 2. Subjective estimates of ketamine use amongst the three ketamine groups and number of ‘Yes’ responses on the CAGE questionnaire
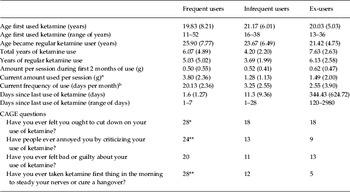
CAGE, Cut down, Annoyed, Guilty, Eye-opener.
Values are given as mean (standard deviation).
a Amount used just before stopping in ex-users.
b Frequency of use just before stopping for ex-users.
* p<0.05, ** p<0.01.
Other drug use
Only drugs that were reported as used more than once per month by any of the respondents were included in this analysis (Table 3). There were no significant differences between the four drug-using groups for any of their subjective estimates of drug use. There were additionally no differences in alcohol use across all five groups.
Table 3. Subjective estimates of recreational drug use amongst the four recreational drug-using groups: frequent ketamine users, infrequent ketamine users, ex-ketamine users and poly-drug-using controlsFootnote a
Values are given as mean (standard deviation).
a In order to be classed as a regular user participants had to use the drug more than ten times per year.
Other occasional drug use, i.e. drugs taken less than 10 times per year by participants, included the following: lysergic acid diethylamide (LSD), psilocybin, crack, heroin, 2-(4-bromo-2,5-dimethoxy-phenyl)ethanamine (2CB), 2,5-dimethoxy-4-iodophenethylamine (2Ci), phencyclidine (PCP), methamphetamine, nitrous oxide, dimethyltryptamine (DMT), γ-hydroxybutyrate (GHB) and mescaline.
CAGE
Amongst the three ketamine groups there were significantly more ‘Yes’ responses (following a χ2 analysis) in the frequent users compared with the ex- and infrequent users to all of the CAGE questions (Table 2) except feeling bad or guilty about their drug use. All participants in the frequent group responded ‘Yes’ to at least two questions, i.e. met CAGE criteria for problematic substance use.
Dot-probe task
Error rates were at floor levels across all groups.
Reaction time data
Time to respond to the probe when it replaced a drug or money picture was subtracted from time to respond to the probe when it replaced a matched neutral picture to calculate a bias score (in ms). A 2×2×5 repeated-measures ANOVA, with picture content (drug or money), duration (short, long) and group (frequent, infrequent, ex-user, poly-drug, non-drug) yielded a significant group×duration interaction [F(4, 145)=4.82, p=0.001] and a significant duration×picture content interaction [F(1, 145)=12.53, p=0.001]. The first interaction, as depicted in Fig. 2, reflects a greater bias score at the short duration in the frequent ketamine users compared with all other groups for both drug (p<0.02) and money pictures (p<0.01) but no difference between the groups at the long duration.

Fig. 2. Bias scores across picture content and duration (long or short stimulus onset asynchrony; SOA).![]() , Frequent ketamine users;
, Frequent ketamine users;![]() , infrequent ketamine users;
, infrequent ketamine users;![]() , ex-ketamine users; □, poly-drug users; ■, non-drug users. Values are means, with standard errors represented by vertical bars.
, ex-ketamine users; □, poly-drug users; ■, non-drug users. Values are means, with standard errors represented by vertical bars.
The duration×picture content interaction reflects a significantly greater bias score for drug pictures at the long duration compared with the short duration [t(149)=3.03, p=0.003] but no significant difference in bias to money pictures across the two durations. Amongst the frequent users, bias scores were correlated with participants' estimates of their drug use. Positive correlations emerged between years of regular ketamine use and the drug picture bias score (r=0.778, p<0.001) and money picture bias score (r=0.602, p<0.001) at the short duration, along with negative correlations between years of regular ketamine use and drug picture bias score (r=−0.687, p<0.001) and money picture bias score (r=−0.634, p<0.001) at the long duration. Amongst the ex-users there was a trend for a negative correlation between duration since last use of ketamine and bias to drug pictures at the long duration (r=−0.408, p=0.028).
Rating data
As seen in Fig. 3, all groups rated neutral pictures similarly. Further, all groups rated money as equally pleasant. Participants' ratings of drug and money pictures were compared with their ratings for the neutral matched pictures in each condition. A 2×2×5 repeated-measures ANOVA with picture content (drug, money), type (target, neutral) and group (frequent, infrequent, ex-user, poly-drug, non-drug) was conducted. A significant content×type×group interaction emerged [F(4, 145)=18.58, p<0.001] along with significant interactions of content×group [F(4, 145)=21.85, p<0.001], type×group [F(4, 145)=13.07, p<0.001] and content×type [F(1, 145)=133.56, p<0.001]. There were also significant main effects of group [F(4, 155)=14.68, p<0.001], content [F(1, 145)=64.47, p<0.001] and type [F(1, 145)=74.02, p<0.001].

Fig. 3. Mean ratings of pleasantness (−3 to 3) across group, picture content and picture type. —◆—, Drug target; – -■– -, drug neutral; - -▲- -, money target; – –×– –, money neutral.
The three-way interaction reflects a significant difference between the ratings of the target drug pictures only [F(4, 145)=38.85, p<0.001] but no differences in the ratings of the target money pictures or the matched neutral pictures across groups. As can be seen from Fig. 3, at the 0.05 level, there were no significant differences in ratings of drug pictures between frequent and infrequent ketamine users, or infrequent and ex-ketamine users, or poly-drug users and ex-ketamine users; however non-drug users rated drug pictures as significantly less positive than any of the other groups.
Discussion
‘… My ears zone in when I hear someone mention K. It's annoying because people are always saying “OK” …’ (participant 24A, current study).
This study is the first to document and research a group of frequent ketamine users. The main finding was of an attentional bias to incentive stimuli in the frequent ketamine-using group for stimuli presented for a short interval (200 ms). This attentional bias was strongly correlated with subjective estimates of drug use. Whilst there were no group differences in bias to incentive stimuli presented at the long interval, bias was negatively correlated in the frequent users with number of years of ketamine use. There was no evidence of an attentional bias in any of the other four groups (infrequent ketamine users, ex-ketamine users, poly-drug users, non-drug users) at the long or short stimulus presentation interval to either drug or money stimuli. Both frequent and infrequent ketamine users rated drug stimuli as more pleasant than neutral stimuli but equivalent to money, whereas the other three groups rated drug stimuli as less pleasant than money.
By demonstrating an attentional bias to incentive stimuli in frequent ketamine users, this study provides further support for recent theoretical accounts of drug dependence (Franken, Reference Franken2003; Robinson & Berridge, Reference Robinson and Berridge2003) and demonstrates that ketamine evokes a similar change in processing as other drugs of abuse. In line with Robinson & Berridge, attentional biases in this group of ketamine users seem to occur only at the short stimulus presentation interval. This may provide support for the notion that these processes are automatic and not under conscious control. However, given that the ketamine users may show elevated bias to these stimuli, it is possible that focusing on neutral stimuli requires disengaging from the incentive stimuli, a process which would require they have conscious control, therefore this may not be an entirely automatic mechanism. Furthermore, the short interval may represent initial orienting to the stimulus and the long interval final attentional capture. Nevertheless, that such processes are only evident in the ketamine-dependent individuals also supports the notion that drug ‘wanting’ is something that develops over time and is possibly mediated by the sensitization of the mesolimbic dopamine system. The observed correlation at the same interval between attentional bias and years of ketamine use also emphasizes this point. The negative correlations observed at long intervals between drug use and bias to both types of incentive stimuli may reflect the conscious shift of attention to avoid craving, although this is inconsistent with other craving models (e.g. Tiffany, Reference Tiffany1990). Conscious ratings of the pleasantness of drug stimuli, akin to conscious hedonic processes (drug ‘liking’), were greater in both frequent and infrequent ketamine users when compared with other groups. Again, this is in line with theories that these conscious processes are independent of the attribution of excessive salience to drug stimuli.
There was no difference between the attentional bias to drug stimuli and the attentional bias to money stimuli in the frequent ketamine-using group. Goldstein & Volkow (Reference Goldstein and Volkow2002) have suggested that in drug users, drug stimuli become powerfully wanted over other natural reinforcers in the environment. It is perhaps difficult to evaluate this claim with these data as money is clearly not a ‘natural’ reinforcer and is indeed intimately linked with the purchase and availability of drugs which could explain the absence of difference. However, other data suggest that instead of the notion of drugs becoming wanted over other reinforcers, there may in fact be some ‘motivation spill-over’ (Robinson & Berridge, Reference Robinson and Berridge2003) to non-drug rewards, for example some cocaine addicts have been found to be hypersexual (Washton & Stone-Washton, Reference Washton and Stone-Washton1993) and hyper-responsive to monetary rewards (Bechara et al. Reference Bechara, Dolan and Hindes2002). Another potential explanation for the equal bias to money incentive stimuli was that, whilst the ‘ketamine’ stimuli were ambiguous white powders, money can unambiguously act as a secondary reinforcer. It may be that this lack of ambiguity served to cancel out the extra influence of being a primary reinforcer. Further studies should perhaps aim to directly compare ‘natural’ appetitive stimuli, such as food and sex-related pictures; however, there are problems inherent in controlling for the degree of valence each picture has for each individual.
Despite all groups rating the monetary stimuli as equally pleasurable as the drug stimuli, there was no evidence of an attentional bias to monetary stimuli in any group except the frequent ketamine users. Previous research has found a bias to food stimuli in hungry (Mogg et al. Reference Mogg, Bradley, Hyare and Lee1998) and fasting (Placanica et al. Reference Placanica, Faunce and Soames Job2002) individuals. Thus such biases can occur during particular non-pathological motivational states. However, perhaps in such cases only ‘natural’ reinforcers can elicit sufficient levels of incentive salience to produce attentional bias, except in individuals who have an already sensitized dopaminergic system. This may suggest that both the drug and money biases in the frequent ketamine users are indicative of some pathological mechanism. In hindsight, subjectively rated indices of craving for both money and drug may have been useful measures to include in the study to correlate with the ‘objective’ craving measure of attentional bias.
Some of the ex-ketamine group had been daily ketamine users; however, there was no evidence of any persisting attentional bias in this group. There was also an indication that, for the ex-ketamine group, the longer they had been abstinent from the drug, the less their ‘conscious’ bias towards drug pictures. These are similar to the findings of Marissen et al. (Reference Marissen, Franken, Waters, Blanken, van den Brink and Hendriks2006) who found upon cessation of heroin use, attentional biases decreased. In the latter study, attentional bias before abstinence was predictive of relapse rates. Other studies have also found attentional bias on a Stroop task to be related to treatment outcome in alcohol-dependent individuals (Cox et al. Reference Cox, Hogan, Kristian and Race2002) and treatment-seeking status in cocaine users (Vadhan et al. Reference Vadhan, Carpenter, Copersino, Hart, Foltin and Nunes2007). This would be interesting to examine in the ketamine-using population and suggests the potential clinical utility of the dot-probe task as a diagnostic tool.
All of the frequent ketamine group responded positively to at least two of the CAGE questions, indicating problematic substance abuse. Although this is a crude measure, it is thought to be a reliable screening instrument for problematic substance use. In addition, over half of the frequent ketamine-using group reported daily use, with some individuals taking up to 9 g per day. Although anecdotal, the background data on drug use in this group highlight some of the issues that may surround frequent ketamine use. Some members of the ketamine group had started to use the drug when they were 11 years old. In the literature an anaesethetic dose of ketamine is 0.5 mg/kg to 1.5 mg/kg, whilst over a period of a day, some of the frequent users in this study were reporting using doses of 9 g, which equates to about 130 mg/kg per day. On such doses, they report being able to function quite normally, say they experience no anaesthesia and no psychotic effects. This combined with the reports of dose increases of on average about 600% speaks to the tolerance they have developed to the drug. Tolerance is a major factor in the development of dependence on drugs (Nutt et al. Reference Nutt, Lingford-Hughes and Daglish2003) and taken together these findings speak to the potential for dependence that may be associated with ketamine.
As far as we are aware, this is the first large-scale study to document a frequent ketamine-using population. These frequent ketamine users in the main differed from the less frequent users in that the majority of them were squatters or travellers. They were aware of the dependence-forming properties of the drug, reflected in that ketamine is known as ‘kiddie smack’ by this population (smack is slang for heroin). From the semi-structured interview it transpired that most had not sought treatment for their ketamine problem but those that had reported that drug services had seemed unaware of issues surrounding ketamine use.
This study was inevitably subject to some limitations. As ketamine was taken only intranasally by the participants of this study, then the ketamine-related pictures were all white powders, hence that they also could have been perceived as cocaine or amphetamine. However, the groups were well matched for all other drug use thus making it likely that any differences would have arisen as a result of ketamine use, and by inference that the pictures were being interpreted as ketamine-related. A further shortcoming of the study was that, whilst participants were asked to remain abstinent from ketamine for at least 24 h, as some of the participants in the frequent group were daily ketamine users, they may have used ketamine on the day of testing. Although we verified drug use by urinanalysis, ketamine and its metabolite, norketamine stay in the urine for 2–3 days, thus a positive urine screen did not necessarily mean that they had taken ketamine that day. Future in-patient studies would circumvent this problem.
In summary, this study demonstrated an attentional bias to incentive stimuli in frequent ketamine users that was associated with degree of use of the drug, supporting incentive theories of drug abuse. There was no difference between bias to drug or money stimuli in frequent ketamine users, which may be indicative of a ‘motivation spill-over’. No attentional bias to either of the incentive stimuli in any of the other groups was observed. Along with previous research, these findings further suggest that attentional bias to drug stimuli may reflect a pathological mechanism that only occurs in drug-dependent groups. It further corroborates anecdotal reports of ketamine dependence by documenting a population of such users who demonstrate similar attentional biases to other drug-dependent groups. While it may be confined only to a subgroup of individuals, amid reports of rising ketamine use it is important that both drug users and drug workers are informed that ketamine may be dependence forming.
Acknowledgements
This study was supported by an Economic and Social Research Council grant (RES-000-23-0945).
Declaration of Interest
None.








