Introduction
An outbreak of peritonitis in Finnish reindeer occurred in 2003. Severe peritonitis with large numbers of nematodes in the peritoneal cavity was a consistent finding at post-mortem examination (fig. 1). The location of the nematodes in reindeer, as well as their large size, was highly suggestive of Setaria sp. (Laaksonen et al., Reference Laaksonen, Kuusela, Nikander, Nylund and Oksanen2006).
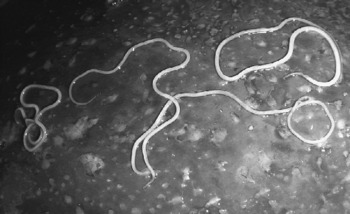
Fig. 1 Four adult female worms of Setaria tundra on the surface of the liver of a reindeer with severe fibrinous perihepatitis.
The genus Setaria Viborg consists of nematodes commonly found in the peritoneal cavity of ungulates. They are long, slender and milk-white in colour. The posterior end of the male is spirally coiled. The mouth is surrounded by a cuticular ring and a peribuccal crown. The tail of the male usually bears four pairs of precloacal and four pairs of postcloacal papillae. Spicules are unequal and dissimilar with the longer (left) spicule being slender with or without a long sclerotized membrane. On the tail, near the tip, there is a pair of small ventrolateral appendages. Larvae, known as microfilariae, are transmitted by arthropods (Anderson, Reference Anderson and Anderson2000).
Setaria spp. are usually well-adapted nematodes and thus considered harmless inhabitants of the abdominal cavity of ungulates (Urquhart et al., Reference Urquhart, Armour, Duncan, Dunn and Jennings1996). However, earlier papers describe outbreaks of peritonitis and poor body condition associated with heavy Setaria sp. infection in reindeer in Scandinavia in 1973–1974 (Rehbinder et al., Reference Rehbinder, Christensson and Glatthard1975; Kummeneje, Reference Kummeneje1980). The presumed causative agent in these outbreaks was S. tundra. Nevertheless, the identification of these nematodes was based on the host and location in the host rather than on detailed morphological studies. Setaria yehi has been associated with chronic peritonitis in reindeer in Alaska (Dieterich & Luick, Reference Dieterich and Luick1971). The aim of the present study is therefore to accurately identify the causative agent of peritonitis. A description of Setaria tundra associated with peritonitis in the reindeer Rangifer tarandus is provided.
Materials and methods
In January 2004, 10 female and 10 male specimens of Setaria sp. were collected from the abdomen of 8-month-old reindeer calves slaughtered in the Kuusamo slaughterhouse in the south-eastern part of the Finnish reindeer herding area (Laaksonen et al., Reference Laaksonen, Kuusela, Nikander, Nylund and Oksanen2006). Samples were preserved in 70% alcohol, dehydrated in a series of alcohol and cleared in lactophenol. To enable comparison with previous descriptions and morphometrics of various Setaria spp., several measurements were taken, including lengths of the body, oesophagus and tail, distance from the nerve ring to the vulva, widths at the vulva and anus, and morphology of the spicules.
In the same slaughterhouse, in December 2005, specimens (n = 95) of Setaria sp. were collected manually from the abdominal cavity of 40 reindeer calves. In addition, the viscera were rinsed with tap water into a steel container, and the worms were collected and counted. Worms were first washed in tepid physiological saline, and then fixed in a hot mixture of 40% formaldehyde (10 parts), acetic acid (1 part) and water (89 parts). The lengths of 30 females were measured, and 13 males were dehydrated and cleared in lactophenol for morphometry. For scanning electron microscopy (SEM), five females and five males were dehydrated in increasing concentrations of ethanol and critical point dried (Bal-Tec, CPD 030). Dried samples were mounted on aluminium stubs, coated with platinum (Agar Sputter Coater), and examined under a Zeiss DSM 926 scanning electron microscope.
To estimate worm fecundity, two fresh female nematodes collected in February 2004 were dissected under a stereomicroscope. Uteri were removed, opened, rinsed in 10 ml of physiological saline and larvae were counted using a modified Knott's method (Bowman, Reference Bowman and Bowman2003).
The results were then compared with previous descriptions by Rajewsky (Reference Rajewsky1929), Yeh (Reference Yeh1959), Desset (Reference Desset1966) and Shoho & Uni (Reference Shoho and Uni1977), and certain morphological features of the Setaria nematodes were highlighted. The morphological terms used are those adopted from Willmott (Reference Willmott, Anderson, Chabaud and Willmott1977).
Results
Of 95 nematode specimens collected from 40 reindeer calves, 77 were females and 18 males (sex ratio = 4.3). Uteri of females were filled with microfilariae, which confirmed that the nematodes belonged to the superfamily Filarioidea. The location of worms in the abdominal cavity, the anterior end of the adult worm with a peribuccal crown and elevations, and the morphology of the unequal spicules were typical of the genus Setaria.
Female worms
The morphometrics of 10 female worms are summarized in table 1, with the morphological details shown in fig. 2. The mean worm length is 67 mm, compared to a mean length of 56 mm for 30 ‘younger’ female worms (data not shown). Using SEM, the cuticle of the worm is smooth with scattered bosses, which are more abundant near the posterior end. Subcuticular longitudinal ridges and fine striations are visible only in the lactophenol-cleared specimen under light microscopy (LM).
Table 1 Morphometrics of female worms (n=10) of Setaria tundra from the reindeer in Finland.


Fig. 2 Morphology of female Setaria tundra from SEM and light microscopy to show: A, cephalic region with peribuccal crown (pc), round oral opening, dorsal and ventral bifid projections (be), four cephalic papillae (cp), four externolabial papillae (elp) and an amphid (a), scale bar = 20 μm; B, lateral view of the cephalic region with the peribuccal crown (pc) and marked buccal thickening (arrowhead), scale bar = 50 μm; C, an amphid with a round slightly elevated porous area and narrow slit-like opening with serrated edges, scale bar = 2 μm; D, posterior end with a long caudolateral appendage (cla), see G for an enlargement of the area enclosed within the rectangle, scale bar = 50 μm; E, side view of an anterior deirid, scale bar = 5 μm; F, tip of the tail with a small and roundish caudolateral appendix (cla), scale bar = 10 μm; G, bud-like knob at the tip of the tail, longitudinal grooves and pores are present on the surface and the neck is striated and clearly demarcated from the tail by the row of bosses, scale bar = 10 μm; H, opening of vulva covered by a flap (vf), scale bar = 10 μm.
The peribuccal crown is oval with a dorsoventral longer axis. The crown possesses two slightly bifid elevations without lateral lips. The mouth is round and surrounded by a slightly elevated smooth circumoral ring. The wall of the buccal cavity is thick, with a rough texture. There are four externolabial and four prominent cephalic papillae and a pair of lateral amphids on the head (fig. 2A,B). The amphids consist of a round, slightly elevated, porous area with 40–50 pores, behind which is a narrow slit-like opening with serrated edges situated crosswise (fig. 2C). The anterior deiride is a dome-shaped knob with a thorn-like structure protruding from the middle (fig. 2E). The tail is long, slender and slightly bent with many bosses on the surface (fig. 2D). At the tip of the tail, a row of bosses forms a collar, resembling a turtleneck ending with a bud-like knob, which possesses longitudinal grooves and pores (fig. 2F,G). The caudolateral appendages appear triangular under LM, but under SEM appear as roundish cones (fig. 2D,F). The shape and size of appendages are varied, with a phasmidial pore situated near the base of each appendage. A phasmidial pore can be observed a short distance from the appendage. The vulva is located anteriorly, and its mean distance from the anterior end is 271 μm. The vulvar opening is partially blocked by a flap (fig. 2H).
Uteri of female worms contain eggs, developing larvae as well as microfilariae varying in number between 180,000 and 220,000.
Male worms
The morphometrics of 23 male worms are summarized in table 2 and morphological details are provided in figs 3 and 4. The bifid elevations, the round mouth surrounded by a circumoral ring, cephalic papillae and amphids are in accordance with the corresponding structures in female worms, although the bifid elevations and the mouth are generally smaller in males. The tail is 4 mm long, coiled into a corkscrew of four loops (fig. 3A). The ventral surface (area rugosa) of the tail consists of many distinct transverse ridges (about 145) with microstriation, bosses in rows and papillae (figs 3A–D, 4A–D). Three pairs of precloacal papillae, one medial precloacal papilla, a pair of adcloacal papillae, three pairs of ventral postcloacal papillae, two pairs of dorsolateral papillae, a pair of lateral appendages and a pair of very small papillae close to the caudal end of the tail are observed near the tightly curved end (diameter about 200 μm) of the tail (figs 3B,C, 4A). Each male possesses 23 papillae. The spicules are unequal and dissimilar in structure, the longer (left) spicule being a thin-walled sclerotized tube with a funnel-shaped head (manubrium). The shaft (calomus) consists of a straight tube which widens slightly before dividing. The blade (lamina) is bifurcated with narrow protrusions with one branch straight, ending in a rudder-like blade and the other shorter branch being bent against a longer protrusion (fig. 3D,F). The longer spicule also possesses a membraneous tube-like extension. The narrow distal ending of this tube can protrude from the cloaca. The shorter (right) spicule resembles a short spout with a stout bent cylinder (fig. 3E,G). The cuticle is smooth with scattered bosses, and a delicate cross-striated laminar structure is seen on the cuticular surface (fig. 4D).
Table 2 Morphometrics of male worms (n=23) of Setaria tundra from reindeer in Finland.


Fig. 3 Morphology of male Setaria tundra, from SEM and light microscopy to show: A, clockwise-coiled tail consisting of four loops, see D for an enlargement of the area enclosed within the rectangle, scale bar = 200 μm; B, papillae of the tail, with three pairs of precloacal papillae (pcp), one medial precloacal papilla (mpcp), a pair of adcloacal papillae (acp), three pairs of ventral postcloacal papillae (vpcp), and a cloaca (c); see C for an enlargement of the area within the rectangle, scale bar = 50 μm; C, papillae at the tip of the tail, with a posterior pair of ventral postcloacal papillae (vpcp), two pairs of lateral postcloacal papillae (lpcp) and a short caudolateral appendage (cla) with a phasmid pore (pp) at the base; one pair of very small papillae (arrowhead) situated close to the tip of the tail, scale bar = 20 μm; D, an extension of the longer spicule (mt) seen as a membranous thin-ended tube protruding from the cloaca; precloacal papillae (pcp) and lateral line (l) are clearly visible, scale bar = 50 μm; E, a shorter spicule (s) and a membranous extension of the longer spicule protruding from the cloaca; papillae located close to the cloaca consist of adcloacal papilla (acp) and a pair of ventral postcloacal papillae (vpcp), scale bar = 20 μm; F, the longer spicule (s), scale bar = 100 μm; G, the shorter spicule (s), scale bar = 100 μm.

Fig. 4 Morphology of male and female Setaria tundra, from SEM and light microscopy to show: A, posterior part of male with the longer spicule (sl) situated more anteriorly than the shorter spicule (ss); the extension of the longer spicule forms a membranous tube (mt), which partly protrudes from the cloaca (c), lateral line (lal), scale bar = 100 μm; B, female and male in mating position, vulva at the anterior part of female is in close proximity to the posteriorly locating cloaca (c) of the male; the coiled tail and transversal cuticular folds are likely to aid copulation; see C and D for SEM of the areas within the rectangles, scale bar = 400 μm; C, ventral transverse folds of the male with microstriations, which prevent rotation of the female around her longitudinal axis during copulation, scale bar = 10 μm; D, transverse bands of bosses between the anterior precloacal papillae and the tranverse microstriated folds, scale bar = 20 μm; E, the ventral surface of the tail anterolaterally to the anterior precloacal papillae with transverse striations and numerous bosses, scale bar = 3 μm.
Discussion
Reindeer slaughtered in December 2005 and born during the preceding spring had contracted the nematode infection during the previous summer. This allowed us to determine that worms were collected about 6 months, or less, after infection, and it is likely that not all worms had reached their maximum measurements. On average, the females were 18% shorter than those measured from the first sampling (January 2004). In northern Finland, S. tundra appears to be transmitted by mosquitoes of the genera Anopheles and Aedes, possibly also other haematophagous insects (S. Laaksonen et al., unpublished). The potential vectors are active during the warm months of the year, mainly May to September.
Based on morphology and morphometrics, the nematodes recovered from the abdomen of reindeer with peritonitis were identified as Setaria tundra, described by Issaitschikow and Rajewsky in Rajewsky (Reference Rajewsky1929) in reindeer from Arkhangelsk. Current measurements of female and male S. tundra correlate relatively well with values given in Rajewsky (Reference Rajewsky1929) and the caudolateral appendages of the female, considered important for its identification (Yeh, Reference Yeh1959), are 28 μm from the tip of the tail, compared with 30–40 μm in specimens drawn by Rajewsky (Reference Rajewsky1929). The tip of the tail with a clearly demarcated knob correlates well with Rajewsky's observations.
Coiled tails in both sexes of S. tundra as well as the transversal folds with microstriations present in the ventro-posterior section of the male are probably morphological structures aiding copulation. The slightly coiled tail of the female may be useful in keeping the male parallel to the female during copulation. The coiled tail of the male forms a funnel-shaped trap with the diameter of the first anterior loop being widest, then decreasing gradually, and the diameter of the last loop near the cloaca being 200 μm. This trap is almost equal to the width of the female near the vulva. Hypothetically, when the anterior end is held, the female can move forward until the vulva is close to the cloaca of the male. The membranous tube with a slender distal part is an extension of the longer spicules, and this tube probably guides the transmission of spermatozoa to the female (fig. 4C). The area rugosa with its transverse rows of bosses and folds with longitudinal microstriations prevents rotation around the longitudinal axis of the worm (fig. 4B).
Rajewsky (Reference Rajewsky1929) observed a total of 13 pairs of papillae on male S. tundra including four pairs of precloacal and postcloacal genital papillae, three pairs of lateral papillae, a small pair in the dorsal direction from the mid-lateral papilla and a very small pair close to the tip of the tail. In addition, Rajewsky described a single medial precloacal papilla. In males observed in the present study, the dorsal pair of papillae was not seen. In Rajewsky's drawings, the buccal crown is oval but drawn without an oral opening. Yeh (Reference Yeh1959) redescribed S. tundra from white-tailed deer Odocoileus virginianus and mule deer O. hemionus from the USA and this description is almost identical to that of Rajewsky (Reference Rajewsky1929), except for the tail of the female and male. According to Yeh, the ‘female tail ends in a roughened knob which may be furnished with a number of spikes, and often looking very much like the tail of Artionema labiato-papillosa [currently Setaria labiato-papillosa, remark by the authors of the present study by Nikander et al.], and the pair of large cone-shaped caudolateral appendages are 50–70 μm from the extremity’. According to Yeh, there are only eight paired papillae and there is no mention of the three pairs of distinct lateral papillae reported by Rajewsky (Reference Rajewsky1929) and in the present study. This led Yeh to question the identification of S. tundra from roe deer Capreolus capreolus and European elk Alces alces by Böhm & Supperer (Reference Böhm and Supperer1955) and Yeh was convinced that the species referred to by the latter authors differs from S. tundra in the female tail having very small subterminal appendages; these caudolateral appendages are large in S. tundra and further from the extremity. The female tail ends in a terminal knob, unlike S. tundra, in which it ends in a series of small knobs or spikes. In the present case there was clear variation in the size and shape of the caudolateral appendages in females (see fig. 2D,F), suggesting that the identification to species level cannot be based solely on the morphology of these structures. Yeh (Reference Yeh1959) described the S. tundra of Böhm & Supperer (Reference Böhm and Supperer1955) as a new species Artionema hartwichi. He also proposed a division of the genus Setaria into three genera: Setaria, Hyraconema and Artionema based on differences in morphology and hosts. The Artionema species were parasites of artiodactyls, characterized by having unequal spicules, the left one possessing a long sclerotized membrane. This new classification was not, however, widely accepted since the members of these three genera had a similar and distinctive morphology as well as the same niche (peritoneal cavity) within the host. The taxonomy suggested by Yeh was first criticized by Nelson (Reference Nelson1962) and later by Ansari (Reference Ansari1966), who proposed that Hyraconema and Artionema be rejected and the former status of the genus Setaria maintained.
Desset (Reference Desset1966) reported that S. tundra is a parasite of the reindeer Rangifer tarandus and describes the rounded caudolateral appendages and the oval peribuccal crown as typical morphological features of S. tundra. She redescribed S. kabargi, a parasite of musk deer Moschus moschiferus, and stated that S. kabargi is not a synonym of S. tundra as proposed by Yeh (Reference Yeh1959). Desset gave the new name Setaria yehi to Artionema tundra (syn. Setaria tundra) described by Yeh (Reference Yeh1959), a parasite of North American cervids. However, Desset based the differentiation between S. tundra and S. yehi on differences in the morphology of the caudolateral appendages of female worms. Such species as the white-tailed deer, mule deer, moose Alces alces, caribou Rangifer tarandus, fallow deer Cervus dama and bison Bison bison are described as hosts for S. yehi by Sonin (Reference Sonin1977), with the reindeer, elk and roe deer as hosts for S. tundra in Europe. Compared with the intraspecies morphological variation in the present study, the distinction between S. tundra and S. yehi is based on rather minor differences. Thus, the validity of S. yehi as a species should be verified.
In our previous study, we showed that S. tundra from reindeer (five specimens), elk (two specimens) and roe deer (two specimens) in Finland were identical along the 1389-bp mtDNA sequence (Laaksonen et al., Reference Laaksonen, Kuusela, Nikander, Nylund and Oksanen2006). Although there were six nucleotide substitutions compared with the S. tundra sequence originating from roe deer in Italy (648 bp) (GenBank AJ544874, Casiraghi et al., Reference Casiraghi, Bain, Guerro, Martin, Pocacqua, Gardner, Franceschi and Bandi2004) (Favia et al., Reference Favia, Cancrini, Ferroglio, Casiraghi, Ricci and Rossi2003), these differences were regarded as minor and the identification as S. tundra was still justified. Based on current morphological studies as well as the polymerase chain reaction, the outbreak of peritonitis in reindeer in Finland was caused by S. tundra. Laaksonen et al. (Reference Laaksonen, Kuusela, Nikander, Nylund and Oksanen2006) showed that a positive correlation existed between the intensity of S. tundra infection and the degree of peritonitis.
Acknowledgements
The authors are grateful to the staff of the Electron Microscopy Unit of the Institute of Biotechnology, University of Helsinki for preparing specimens for SEM and the use of the scanning electron microscope. In addition, the authors would like to thank Dr Odile Bain, Muséum National d'Histoire Naturelle et Ecole Pratiques des Hautes Etudes, Paris, for fruitful discussions about filaroid nematodes.








