INTRODUCTION
Alveolar echinococcosis (AE) caused by the metacestode of Echinococcus multilocularis is a serious zoonotic disease. The life cycle of E. multilocularis involves various Microtus and other arvicolid rodent intermediate hosts and wild [especially red fox (Vulpes vulpes)] or domestic canid definitive hosts. Humans can act as accidental intermediate hosts (Davidson et al. Reference Davidson, Romig, Jenkins, Tryland and Robertson2012). The intermediate hosts of E. multilocularis become infected by ingesting parasite eggs, and then the metacestodes colonize at internal organs, generally the liver, where lesions develop. If these lesions are not diagnosed and treated early, they can lead to severe health problems and death due to their tumour-like growth (Eckert and Deplazes, Reference Eckert and Deplazes2004).
The parasite's distribution spans the northern hemisphere, including Europe, Asia and parts of North America (Ito et al. Reference Ito, Romig and Takahashi2003; Torgerson et al. Reference Torgerson, Keller, Magnotta and Ragland2010; Deplazes et al. Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). Many rodent species are defined as suitable intermediate hosts for E. multilocularis with varying significance in the maintenance of the life cycle in different regions of the world (Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003; Romig et al. Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). In central Europe, the most common intermediate hosts of E. multilocularis are the common vole (Microtus arvalis), the water vole (Arvicola terrestris), and the bank vole (Myodes glareolus) (Romig et al. Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). In the North America, intermediate host species listed to date include deer mouse (Peromyscus maniculatus), meadow vole (Microtus pennsylvanicus), southern red-backed vole (Myodes gapperi), house mouse (Mus musculus) and bushy-tailed woodrat (Neotoma cinerea) (Hnatiuk, Reference Hnatiuk1966; Leiby et al. Reference Leiby, Carney and Woods1970; Holmes et al. Reference Holmes, Mahrt and Samuel1971; Kritsky et al. Reference Kritsky, Leiby and Miller1977; Liccioli et al. Reference Liccioli, Duignan, Lejeune, Deunk, Majid and Massolo2013). In contrast to Europe and North America, a large variety of small mammal species act as intermediate host in this large region. Including more than 274 species of small mammals, many of which are candidate intermediate hosts for E. multilocularis, this diversity and excess in intermediate hosts leads increase in the transmission of the parasite (Giraudoux et al. Reference Giraudoux, Raoul, Afonso, Ziadinov, Yang, Li, Li, Quéré, Feng, Wang, Wen, Ito and Craig2013). Among small rodent and lagomorph intermediate hosts in Asia, Microtus spp., Ochotona spp., Cricetus spp. and Meriones spp. are leading (Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003).
Torgerson et al. (Reference Torgerson, Keller, Magnotta and Ragland2010) suggested that human AE is common in Turkey, and the estimated annual incidence is 100 cases. Deplazes et al. (Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017) summarized that approximately 500 human cases of AE have been documented in more than 60 studies in Turkey since 1939. The eastern Anatolian region of the country is defined with the most common AE cases (Deplazes et al. Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). Investigation of definitive and intermediate hosts of E. multilocularis has been neglected in Turkey. The first report of E. multilocularis in Turkey was recorded by Merdivenci (Reference Merdivenci1963) from a fox in the northwest of Turkey based on microscopy. The second report was informed by Avcioglu et al. (Reference Avcioglu, Guven, Balkaya, Kirman, Bia and Gulbeyen2016) from a fox in Erzurum Province of Turkey, identified using microscopy and PCR. Turkey is an endemic region for human AE, but the situation of the disease in intermediate hosts is unknown. Until now, there have been no reports of E. multilocularis in intermediate hosts in Turkey. The purpose of this paper is to describe the first findings of E. multilocularis in rodent intermediate hosts in Turkey and to discuss their importance in the transmission of the parasite in Turkey ecosystem.
MATERIALS AND METHODS
Study area
Erzurum is located in the Eastern Anatolia Region (39°52′N, 41°17′E) of Turkey and has the fourth biggest surface area (25 066 km2) in the country (Fig. 1). Erzurum has 20 counties and its total population is 762 000. The province's central districts (Yakutiye, Palandöken and Aziziye) constitute the majority of the total population (411 000). The total population of the other 17 provinces which are in rural areas is 351 000. The majority of the province is elevated and it is situated 1853 m above sea level. Continental climate rules in the province with cold and snowy winters, and warm and dry summers. The average low temperature is −15 °C, while the average high temperature is 27 °C. Average annual precipitation is 453 mm. The majority of region's vegetation cover meadows, and in some parts forests are encountered.
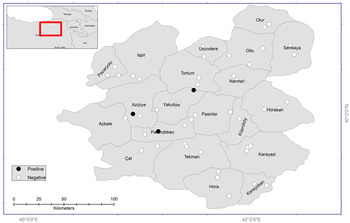
Fig. 1. Study areas, trap locations (circles) and positive findings of E. multilocularis (black circles) in Erzurum Province.
Sample collection
Ethical approval was obtained from the Ataturk University Animal Research Local Ethics Committee (Approval no: 2015/27) for the study. Fieldwork was performed with ethical permits from the Ministry of Forestry and Water Affairs, General Directorate of Nature Protection and National Parks (Approval no: 2015/43002).
Trapping was conducted between February and December 2016, in 20 counties of Erzurum Province. Intensive and active mouse nests in the rural areas of counties were determined and traps were established in these areas. In this way, 49 regions were selected in the rural areas of 20 counties (Fig. 1). An average of 70 traps was set for each of the 49 regions. Consequently, a total number of 3430 traps were placed during the investigation period. The traps [small Sherman live trap (25 × 10 × 10 cm3)] were set with different baits, including cheese, walnuts, potato chips and cucumber. All traps were checked and trapped animals were collected daily, and then euthanized by cervical dislocation. Sampled animals were labelled with date and place of sampling, and stored at –20 °C until further process.
Laboratory methods
Parasitological and histopathological examinations
Rodents were identified at the genus level using the standard morphological criteria (Corbet, Reference Corbet1978). After dissection, the thoracic and peritoneal cavity, brain and visceral organs, were examined macroscopically for metacestodes of E. multilocularis. Suspicious lesions (e.g. white spots) were excised and preserved in 70% ethanol and 10% formalin for molecular and histopathological examinations.
Fluid, drawn from suspicious lesions >6 mm, was examined under the light microscope for the presence of protoscoleces. In parasite lesions smaller than 6 mm in diameter, histologic examination of tissue sections was performed. For histopathology, pieces of suspicious lesions were fixed in 10% formalin, routinely processed and embedded in paraffin. Five μm thick sections were cut and stained with haematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) (Presnell and Schreibman, Reference Presnell and Schreibman1997).
DNA isolation and PCR amplification
Isolation of DNA from suspicious lesions was executed by using Intron DNA extraction kit (G-spin™ Total DNA Extraction Kit; Intron, Korea) according to the manufacturer's instructions. The obtained DNA was stored at −20 °C until the next step. The mitochondrial 12S ribosomal RNA (rRNA) gene was subjected to analysis using classic PCR with species-specific primers Em.nest/for and Em.nest/rev (Dyachenko et al. Reference Dyachenko, Beck, Pantchev and Bauer2008).
Bidirectional sequencing was performed commercially with an ABI PRISM 310 genetic analyser (Applied Biosystems, Foster City, CA) using the ABI PRISM® BigDye terminator cycle sequencing kit. The sequence data were then subjected to BLASTN (RefSeq mRNA) searches of the E. multilocularis genome database of the National Center for Biotechnology Information (www.ncbinlm.nih.gov/). All the sequenced data were edited and aligned using Bioedit 7·0 (www.mbio.ncsu.edu/BioEdit/bioedit.html) following naked eye checking. Nucleotide sequences of E. multilocularis isolates obtained from rodents were deposited in GenBank under accession numbers KY863531 to KY863535.
Tissue samples from infected rodents were extracted using Intron DNA extraction kit (G-spin™ Total DNA Extraction Kit; Intron, Korea) following the manufacturer's instructions. The mitochondrial cytochrome b gene was subjected to analysis using classic PCR with primers L14727-SP and H16498 (Meyer et al. Reference Meyer, Kocher, Basasibwaski and Wilson1990; Jaarola and Searle, Reference Jaarola and Searle2002).
After bidirectional sequencing, the sequences of cytochrome b were edited and visually optimized using SEQUENCHER, v. 4.1.4 (Gene Codes Corporation). The sequences were cut an 1140 bp fragment and aligned in BioEdit, v. 7.2.5 (Hall, Reference Hall1999) with all previously obtained from GENBANK sequences. Finally, the sequences containing 1140 bp was blasted using Bioedit 7·0. (www.mbio.ncsu.edu/BioEdit/bioedit.html).
Statistical analyses
Data were subjected to 2 × 2 cross tabulation to evaluate association of breed, gender, location with positivity using χ 2 test (Fisher Exact Test if necessary). Relative risks were reported at 95% confidence interval. Statistical significance was declared at the P value of 0·05.
RESULTS
A total of 498 rodents [females 205 (41·2%) and males 293 (58·8%)] were trapped on 49 trapping sites (catch rate: 14·5%, 498/3430). Among counties, Aziziye (n = 31, 6·22%), Palandoken (n = 43, 8·63%) and Yakutiye (n = 8·63, 8·63%) municipalities are within Erzurum Metropolitan, consisting of 23·49% of the samples. Other municipalities were 50–200 km away from city centre, and consisted of 76·51% of the samples (n = 381).
Based on morphological criteria, rodents were represented with 391 (78·5%) Microtus spp., 93 (18·7%) Apodemus spp., 12 (2·4%) Mesocricetus spp. and 2 (0·4%) Crocidura spp. (Table 1). Microtus genus was determined from the most prevalent rodent in the study area. Counties neighbouring the Black Sea region was high in Apodemus spp. and those within city centre and plato were rich in Microtus spp. (χ 2 = 229·02, P < 0·0001). Microtus spp. was the predominant both in rural and urban areas. However, Apodemus spp. was more predominant in rural than urban areas (χ 2 = 19·30, P < 0·0002).
Table 1. E chinococcus multilocularis infection in captured rodents in 20 counties from Erzurum Province

NP, number of E. multilocularis positive rodents; NC, number of captured rodents.
Macroscopic examination of visceral organs showed liver lesions in 48 of 489 (9·6%) animals. No lesion was found in the brains of any of the rodents. Five rodents were molecularly confirmed to be infected with E. multilocularis, leading to a prevalence of 1·3% in Microtus genus. All infected rodents were confirmed to be Microtus irani by the mitochondrial cytochrome b gene sequence analysis.
The positivity was not specific to counties (χ 2 = 16·91, P < 0·60) and gender (χ 2 = 3·14, P < 0·0762). There was no species by positivity association (χ 2 = 1·38, P < 0·71), the positivity rate of being 0 for Apodemus spp. (n = 93), Crocidura spp. (n = 2) and Mesocricetus spp. (n = 12), and 5 for Microtus spp. (n = 391). All infected rodents were seen in urban [χ 2 = 16·45, P < 0·0001, logit Odd ratio value of 37·30 (2·04–679·76, 95% CI) and relative risk ratio of 1·05 (1·01–1·09, 95% CI)].
One of the five positive rodents contained liver with two metacestode lesions (15–13, 10–9 mm in diameter) (Fig. 2A and B). Protoscoleces were found after examining the cysts vesicle fluid under a light microscope (Fig. 2C and D). The other four positive rodent livers contained metacestode lesions were smaller than 5 mm in diameter.

Fig. 2. (A) Metacestodes (arrows) of E. multilocularis in the liver. (B) The liver containing multiple metacestodes. (C) Protoscoleces in the cysts vesicle fluid. (D) Rostreller hooks (in the black circle). (E) Histological sections of the liver (H&E stained). Section through cysts containing protoscoleces (yellow arrows) bordered by the homogenous laminated layer (blue arrows). (F) The cellular inner germinal layer (red arrow), liver parenchyma (asterisks).
Five metacestode lesions were histopathologically examined after H&E and PAS staining, and multicystic lesions were detected as shown in Fig. 2E and F. Histopathologically, metacestode lesions were consisted of numerous small vesicles with well-developed germinal and PAS-positive thin laminated layers. Most of the vesicles contained protoscoleces, presenting the features of larval E. multilocularis (Fig. 2C and D). All cysts sampled from the infected rodents had protoscoleces.
DISCUSSION
Echinococcosis is a serious veterinary public health concern in most areas of the world. Cystic echinococcosis (CE) occurs throughout Turkey, whereas AE predominantly occurs in the Eastern Anatolian Region of the country (Deplazes et al. Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). Occurrence in definitive hosts is used to define endemicity for areas of Europe and North America (Romig et al. Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). Because of investigation of definitive and intermediate hosts of E. multilocularis has been neglected in Turkey, human AE data were considered the most reliable source of information in Turkey. Large parts of Turkey are considered as endemic for E. multilocularis. In definitive hosts, there is one record of an infected red fox (Merdivenci, Reference Merdivenci1963) from the European part of the country. Despite the large number of human cases particularly in the eastern part of Turkey, there was an absence in data of animal infection from the Asian part of the country (Romig et al. Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017). Recently, Avcioglu et al. (Reference Avcioglu, Guven, Balkaya, Kirman, Bia and Gulbeyen2016) recorded E. multilocularis infection from one out of ten red foxes, which confirmed microscopically and molecularly, in Erzurum Province from the Asian part of the country. To date, there are no data available on E. multilocularis in intermediate hosts in Turkey. In the present study, E. multilocularis metacestode was found in the liver of five out of the 498 rodents. This is the first report describing the presence of E. multilocularis in rodent intermediate hosts in Turkey.
Microtus spp. was the most frequently captured species (78·5%) and the dominant rodent in the study area. The study area, at an elevation of 1400–2400 m, have open grasslands such as meadows, and pastures is a suitable habitat for the voles of genus Microtus. In general, the subdivision of the genus Microtus into subgenera and species groups is very complicated and controversial (Zagorodnyuk, Reference Zagorodnyuk1990; Nowak, Reference Nowak1999). Because of the morphological similarities between species, rodents were identified at the genus level in this study. Only five positive rodents were molecularly analysed and were confirmed to be M. irani. The main intermediate hosts of E. multilocularis are M. arvalis, A. terrestris and M. glareolus in Europe (Romig et al. Reference Romig, Deplazes, Jenkins, Giraudoux, Massolo, Craig, Wassermann, Takahashi and de la Rue2017) and Microtus spp., Ochotona spp., Cricetus spp. and Meriones spp. (Vuitton et al. Reference Vuitton, Zhou, Bresson-Hadni, Wang, Piarroux, Raoul and Giraudoux2003) in Asia. In this study, M. irani is determined as an intermediate host in E. multilocularis life cycle in Turkey.
Reports of the worldwide prevalence of E. multilocularis infection have varied between >1% and more than 83% in various rodent species (Eckert et al. Reference Eckert, Schantz, Gasser, Torgerson, Bessonov, Movsessian, Thakur, Grimm, Nikogossian, Eckert, Gemmell, Meslin and Pawlowski2001; Gottstein et al. Reference Gottstein, Saucy, Deplazes, Reichen, Demierre, Busato, Zuercher and Pugin2001; Eckert and Deplazes, Reference Eckert and Deplazes2004). The infection rate (1·3%) reported in this study is in accordance with the previous records throughout the world. Differences can be based on a wide spectrum of sensitive intermediate hosts, the number of examined hosts and the diagnostic methods used (Eckert et al. Reference Eckert, Schantz, Gasser, Torgerson, Bessonov, Movsessian, Thakur, Grimm, Nikogossian, Eckert, Gemmell, Meslin and Pawlowski2001). For Turkey, which is an endemic region for E. multilocularis, this rate seems to be low, but other endemic regions also have the similar situation. As quoted by Oksanen et al. (Reference Oksanen, Siles-Lucas, Karamon, Possenti, Conraths, Romig, Wysocki, Mannoci, Mipatrini, La Torre, Boufana and Casulli2016) pooled that the prevalence of E. multilocularis in intermediate hosts on 371 studies in Europe was estimated to be 1·1–6%. Eckert (Reference Eckert, Palmer, Lord Soulsby and Simpson1998) reported that the average prevalence of E. multilocularis in rodents is usually low in central Europe, but higher prevalences have been determined in certain foci. Similarly, in our study the prevalence was found to be 1·3% for the whole study area, whereas the infection was more common in certain regions such as Palandöken 5·3%, Yakutiye 5·1% and Aziziye 3·4% (Table 1). Deplazes et al. (Reference Deplazes, Hegglin, Gloor and Romig2004) pointed out the crucial significance of anthropogenic food resources for the urbanization of foxes. They have adapted to synanthropic life and increasingly use anthropogenic food sources within urban settings. In this study, all positive rodents captured from Erzurum's central districts (Yakutiye, Palandöken and Aziziye), which could be explained by the synanthropic life of foxes.
The differing findings in the studies might be related to the identification method based on gross and histopathologic examinations (Barabasi et al. Reference Barabasi, Marosfi, Barabasi and Cozma2011) or PCR (Hofer et al. Reference Hofer, Gloor, Müller, Mathis, Hegglin and Deplazes2000; Burlet et al. Reference Burlet, Deplazes and Hegglin2011). In this study, cysts were found in one of the 498 examined liver samples by gross examination. Positivity increased to five after PCR analysis conducted on 48 suspicious liver lesions. This finding indicated the complexity of distinguishing small immature cysts, especially in lesions smaller than 5 mm in diameter (Torgerson and Deplazes, Reference Torgerson and Deplazes2009). On the other hand, parasite DNA can be easily detected by species-specific PCR from these small suspicious lesions. These data showed that early infection in intermediate hosts can be reliably diagnosed by PCR (Deplazes et al. Reference Deplazes, Dinkel and Mathis2003).
The presence of protoscoleces in infected rodent species indicates that they can serve as competent intermediate hosts of E. multilocularis. Burlet et al. (Reference Burlet, Deplazes and Hegglin2011) reported high E. multilocularis prevalence in Arvicole spp. in Switzerland, and also indicated that the potential infectivity of these voles may be much lower (12 with protoscoleces from 129 infected). In a study by Hofer et al. (Reference Hofer, Gloor, Müller, Mathis, Hegglin and Deplazes2000), only 2/19 Arvicola spp. positive for E. multilocularis had protoscoleces. Similar figures were reported by Stieger et al. (Reference Stieger, Hegglin, Schwarzenbach, Mathis and Deplazes2002) where 26/81 positive Arvicola spp. contained protoscoleces, and Reperant et al. (Reference Reperant, Hegglin, Tanner, Fischer and Deplazes2009) where 2/31 positive Arvicola spp. contained protoscoleces. In addition, seven with protoscolex from 13 infected voles in Romania (Barabasi et al. Reference Barabasi, Marosfi, Barabasi and Cozma2011), one with protoscoleces from five infected M. gapperi in North America (Liccioli et al. Reference Liccioli, Duignan, Lejeune, Deunk, Majid and Massolo2013), three with protoscoleces from eight infected A. amphibius in Sweden (Miller et al. Reference Miller, Olsson, Walburg, Sollenberg, Skarin, Ley, Wahlstrom and Hoglund2016) were reported. Our histological observations showed that all infected M. irani (5/5) had fertile metacestodes, suggesting that this rodent species could act as a competent intermediate host and thus contribute to the transmission of E. multilocularis in Turkey.
Our findings of all infected M. irani with protoscoleces show that this rodent can act as suitable intermediate hosts for E. multilocularis in Turkey. It is recommended that molecular analysis should be conducted in addition to gross and histological examinations to determine the presence of E. multilocularis in rodents. An extensive survey should be conducted to investigate the prevalence of E. multilocularis in definitive hosts (especially fox and dog) in Erzurum Province.
ACKNOWLEDGEMENTS
We thank to the Ministry of Forestry and Water Affairs, General Directorate of Nature Protection and National Parks for fieldwork permission.
FINANCIAL SUPPORT
This work was supported financially by a grant (no. 115S420) from the Scientific and Technical Research Council of Turkey (TUBITAK).
CONFLICTS OF INTEREST
None.






