INTRODUCTION
Trypanosoma rangeli is a digenetic haemoflagellate parasite from the Herpetosoma subgenus that infects humans as well as domestic and wild animals in Central and South America. Triatomine bugs, especially those of the Rhodnius genus, are vectors of T. rangeli among the vertebrate hosts (D'Alessandro and Saravia, 1992). During the last few years, biological, biochemical and molecular characterization of several T. rangeli isolates has revealed the genomic and antigenic plasticity of this parasite, for which the life-cycle in the mammalian hosts is still unknown (Cuba, 1998; Grisard et al. 1999; Guhl and Vallejo, 2003).
This parasite has an overlapping distribution with Trypanosoma cruzi, the causative agent of Chagas disease, allowing the occurrence of single and/or mixed infections in both vertebrate and invertebrate hosts in the same geographical region. About 2600 cases of T. rangeli infection have been described in Latin-American countries (Vallejo et al. 2002). In Brazil, the first case was detected in 1996 in the Amazon region (Coura et al. 1996) and today T. rangeli has already been described in 7 states, showing a wide geographical distribution of the parasite (Cuba, 1998; Ramirez et al. 1998; Grisard et al. 1999). Despite its non-pathogenic characteristic in vertebrate hosts, T. rangeli infection presents a crossed antigenic reaction with T. cruzi due to homology of at least 60% of their antigens (Guhl and Vallejo, 2003). So, the differential diagnostic between the two species is very difficult and a serious problem for the diagnosis of Chagas disease, since infection with T. rangeli will give false-positive results.
While in the insect stage trypanosomatids can use some amino acids as a source of energy (Bursell, 1981; Ter Kuile, 1997), all parasite bloodstream forms are entirely dependent upon rapid D-glucose metabolism through glycolysis for survival. Since D-glucose transport across the plasma membrane is generally the rate-limiting step for metabolism (Ter Kuile and Opperdoes, 1991; Bakker et al. 1999), the study of D-glucose transport in trypanosomatids has received considerable attention as the plasma membrane D-glucose transporters can be potential drug targets for chemotherapy (Barrett et al. 1998; Tetaud et al. 1997; Szablewski, 2000; Bayele, 2001; Azema et al. 2004).
In several trypanosome species (T. brucei, T. congolense, T. cruzi, T. equiperdum, T. rhodesiense; T. b. gambiense and T. vivax) D-glucose transporters have been already cloned and described (Bringaud and Baltz, 1994; Barrett et al. 1998; Tetaud et al. 1997; Szablewski, 2000), and they all belong to the Major Facilitator Superfamily of transporters with high amino acid sequence homology among them (52–80% identity). While in some species 2 isoforms with different affinities can be found, in other parasites only 1 form has been described, which may reflect an adaptation to the different environments to which these parasites are exposed. For example, T. brucei and T. congolense have 2 different glucose transporter isoforms (Gruenberg et al. 1978; Munoz-Antonia et al. 1991; Vendrenne et al. 2000) that allow these parasites to develop in both the mammalian bloodstream (with D-glucose concentrations in the 5 mM range) and also the insect vector (with a far lower abundance of D-glucose). A low affinity transporter (Km 10−3–10−4M), denominated THT1 (or TcoHT1 for T. congolense), is highly expressed in bloodstream forms, while a high affinity isoform (Km 10−5–10−6M), denominated THT2, is predominantly expressed in T. brucei procyclic forms. The high affinity TcoHT2 transporter is expressed in all the developmental stages of the parasite (Bringaud and Baltz, 1993, 1994; Barrett et al. 1998; Vendrenne et al. 2000). The T. brucei THT transporters are 80% identical, with the genes arranged in tandem repeats on 2 homologue chromosomes with 6 copies of THT1 and 5 copies of THT2. Both T. congolense TcoHT isoforms are 92·4% identical, and encoded by a single cluster of genes containing 2 copies of TcoHT1 and 3 copies of TcoHT2 arranged alternately.
A different situation is found in T. cruzi where a single D-glucose transporter isoform of moderated affinity (Km 10−4–10−5M) is expressed at similar levels in epimastigote and trypomastigote forms, indicating that provably the molecular properties of this transporter are sufficient to allow the survival of the parasite in D-glucose-poor environments (insect vector and intracellularly in the mammalian host). This parasite has 8 tandem copies of the TcrTH1 gene in 1 chromosome, but Northern blot analysis revealed 2 transcripts that differ in size, a finding with still unknown function (Tetaud et al. 1994). T. vivax, which only transiently stays in the insect vector, has only a single low affinity isoform and the gene (TvHT1) occurs as a cluster of at least 6 tandemly repeated identical copies (Waitumbi et al. 1996).
Since little is known concerning the basic biochemistry of carbohydrate transport and metabolism by T. rangeli, in the present study we describe the characterization of D-glucose transport in different life-stage forms of the parasite, and isolate a partial DNA sequence of a putative D-glucose transporter gene from T. rangeli.
MATERIALS AND METHODS
Parasites
The T. rangeli Choachi strain, isolated from naturally infected Rhodnius prolixus in Colombia (Schottelius, 1987), was used in the present study. The epimastigote forms were cultivated at 27 °C in liver-infusion tryptose (LIT) medium supplemented with 10% of fetal bovine serum, 100 μg/ml of streptomycin and 100 units/ml of penicillin. The trypomastigotes forms were obtained by cultivating epimastigotes in DMEM medium, pH 8·0 at 28 °C, according to Koerich et al. (2002).
Transport assays
The transport assay was adapted from a method used to characterize L-proline transport in T. cruzi (Silber et al. 2002). T. rangeli epimastigote and trypomastigote forms were centrifuged at 2000 g for 10 min at 25 °C. The pellet cells were washed 3 times in phosphate-buffered saline (PBS) pH 7·4, re-suspended in the same buffer, and conserved in an ice bath until use. Approximately 50 μl of epimastigotes (5×106 cells) or trypomastigotes (1×107 cells) were placed in a microcentrifuge tube, incubated for 5 min at room temperature, and the reaction started by addition of 10 μl of different glucose concentrations in the presence of 0·2–0·5 μCi of D-[U-14C]glucose (Amersham Bioscience Corp.). After 5 sec at room temperature, the uptake of the sugar was interrupted by the addition of 1 ml of cold 100 mMD-glucose in PBS containing 100 μM HgCl2, followed by a short (>1 min) centrifugation and 1 additional wash in the same buffer. The radioactivity incorporated into the cells was determined by liquid scintillation, and negative controls with previously boiled parasites were used.
Competition assays were performed with 0·3 mMD-glucose containing 1 μCi of D-[U-14C]glucose in the presence of the several analogues (D-manose, 2-deoxy-D-glucose, D-glucosamine, D-galactose, D-fructose) at a final concentration of 3 mM. Inhibition assays were performed by pre-incubating the parasites for 5 min at 25 °C with 150 μM cytochalasin B or phloretin, or with 5 mM phloridzin. After pre-incubation, transport assays were performed using 0·3 mMD-glucose containing 1 μCi of D-[U-14C]glucose. For the calculation of Ki a concentration of 0·01 mMD-glucose containing 1 μCi of D-[U-14C]-glucose was used with sugar analogues at a concentration of 0·05, 0·1, and 0·5 mM, and transport inhibitors at a concentration of 75, 150, and 200 μM for cytochalasin B and phloretin, or 1, 5, and 10 mM of phloridzin. Apparent Ki values were determined using the equation v0/v=1+[I]/Ki, where v0 and v are uninhibited and inhibited transport rates, respectively, and [I] is the inhibitor concentration (Eisenthal et al. 1989).
Statistical analysis
All experiments were performed in triplicate. The mean and standard error of at least 3 distinct experiments were determined.
Isolation of a T. rangeli partial DNA sequence encoding a D-glucose transporter
In order to clone a T. rangeliD-glucose transporter gene, we used degenerate oligonucleotides corresponding to conserved amino acid sequences found in other trypanosomatid hexose transporters (Barrett et al. 1998). Initially, a 1·1 kb DNA fragment was obtained with primer THXT1-F (5′ ATGATYGTTGGYTCRATGATTGCSTCG 3′), corresponding to amino acids MIVGS/AMV/IGSI present in the middle of the predicted second transmembrane (TM-2) helix, and primer THXT2-R (5′ CTGCAGMACGAAGCARAYMARRCC 3′) that corresponds to amino acids GL/IV/ICFVLQ present in the middle of the last transmembrane helix (TM-12). The PCR reaction was performed in a 25 μl volume containing 0·5 μl dNTP 10 mM, 1 μl of 0·5 mM of each primer, 40 ng of phenol-buffer purified T. rangeli epimastigote total genomic DNA, and 1 unit of Taq DNA polymerase (Invitrogen-USA). The reaction tube was subjected to pre-denaturation for 2 min at 95 °C and for 25 cycles: denaturation for 1 min at 95 °C; annealing for 1 min and 10 sec at 45 °C, and elongation for 2 min at 72 °C. A final 10 min extension at 72 °C was included. The 1·1 kb fragment was purified following agarose gel electrophoresis with a Concert® Rapid gel extraction kit (Invitrogen-USA), cloned into pGEM-T easy® vector (Promega-USA), and sequenced by DYEnamic™ ET Dye Terminator (Amersham Bioscience Corp) using T7 sequencing primers and THXT3-F (5′ ACGTTTGGCATTATGTTTGCGGCGGCG 3′) and THXT4-R (5′ CTGCTGGCTCAGCACAAAAAAGCACGG 3′). Nucleotide sequence obtained from 5 independent clones were identical, and the high homology to TcrTH1 (see Results section above) allowed the extension of the sequence into TM-1 by further cloning a 315 bp DNA fragment using primers THXT5-R (5′ CACCAGAAAGCTCACCTTGTGGCCAAA 3′) and THXT6-F (5′ TTTTGCAGCCTGCAGAACCTGAAGGTG 3′, corresponding to amino acids 22 to 30 of the TcrTH1 permease).
RESULTS
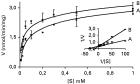
In order to characterize the D-glucose uptake systems present in T. rangeli, D-glucose transport was analysed in epimastigote and trypomastigote forms of the Choachi strain using zero-trans kinetic assays. For kinetic analysis, D-glucose transport assays were performed at a fixed incubation time of 5 sec, during which the rate of uptake was linear over a concentration range of 0·01–1 mM. As shown in Fig. 1 the two parasite forms exhibit a saturable process typical for a carrier-mediated transport system. A Lineweaver-Burk plot of the data suggests a single transport system with similar Vmax (~2·6 nmol/min/mg) and a high affinity for D-glucose with Km values of 30 μM for epimastigotes and of 80 μM for trypomastigotes, respectively.

Fig. 1. Kinetics of initial D-glucose uptake by Trypanosoma rangeli epimastigote (A) and trypomastigote (B) forms. In both forms the rate of D-glucose uptake was measured over a concentration range of 0·01–1 mM at 25 °C. Assays were conducted by determining the initial rate of transport (fixed 5-sec time-point) using zero-trans uptake. Values are expressed as mean±S.D. (n=3). Insert, Lineaweaver-Burk plot.
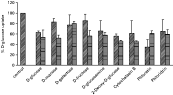
Several D-glucose analogues were used in competition experiments in order to study the substrate specificity of the transporter present in both forms of T. rangeli. Fig. 2 shows the effects of a 10-fold excess of some monosaccharides on the transport of 0·3 mM D-glucose, and Table 1 gives a summary of the Ki values for each sugar. No significant inhibition by D-galactose was observed in both parasite forms, indicating that modification in C-4 allows D-glucose binding to the transporter. The position of less importance seems to be C-2, because D-glucosamine and 2-deoxy-D-glucose inhibited the transport with values close to D-glucose. In the case of D-fructose and D-manose significant differences were observed between epimastigotes and trypomastigotes, and while these two sugars did not inhibit D-glucose uptake by epimastigotes, in trypomastigotes both sugars inhibited significantly the transporter present in this parasite stage. Significant inhibition of D-glucose uptake in T. rangeli forms was also observed with cytochalasin B, phloretin, and to a lower extent, also with phloridzin (Fig. 2 and Table 1).

Fig. 2. Specificity and inhibition of D-glucose uptake by Trypanosoma rangeli epimastigotes () and trypomastigotes () forms. The inhibition of 0·3 mM D-[U-14C]glucose uptake by unlabelled analogues (3 mM), or by the inhibitors of eukaryotic D-glucose-transport systems cytochalasin B and phloretin (both at 150 μM) or phloridzin (5 mM), was determined with the final concentrations indicated for each compound as described in the Materials and Methods section. D-Glucose transport in the absence of analogues and inhibitors was set to 100%. Values are expressed as mean±S.D. (n=3).
Table 1. Inhibition constants (Ki) for analogues and inhibitors (The values of Ki for T. rangeli forms were determined by the inhibition of D-glucose incorporation as described in the Materials and Methods section. Other values are from references: (a) (Tetaud et al. 1994); (b) (Tetaud et al. 1997) and (c) (Vendrene et al. 2000).)

Using PCR and degenerated oligonucleotides corresponding to conserved sequences present in other trypanosomatids high-affinity D-glucose transporters (see Fig. 3 and Materials and Methods section), we next isolated a 1·1 kb DNA fragment that encoded a protein with significant similarity to these transporters, especially the T. cruzi TcrHT1 high-affinity permease. Indeed, by use of a sequence corresponding to amino acids close to the first TM from the TcrHT1 gene we could extend the cloned sequence further, totalizing 457 amino acids (approximately 82–85% of a full-length permease). While the DNA sequence showed a high homology with T. vivax (87%) and T. cruzi (83%) high-affinity hexose transporter genes (and ~35% homology with the other THT2 transporters), the amino acid identity was higher (64%) with T. cruzi TcrHT1, while with the permeases of other trypanosomatids values of 37–43% identity were obtained (Fig. 4). We have also used the cloned sequence to search the ongoing EST's database from T. rangeli (Snoeijer et al. 2004), but unfortunately no sequence corresponding to the cloned DNA was found.

Fig. 3. (A) PCR amplification of a TrHT1 fragment. Lane 1, 1 kb Plus DNA Ladder (Invitrogen). Lane 2, PCR product. The gene was amplified from Trypanosoma rangeli epimastigote genomic DNA using the primers described in the Materials and Methods section. (B) Amino acid sequence alignment of the trypanosomatid D-glucose transporters. Identical amino acids are underscored with asterisk, and the 12 putative transmembrane segments are indicated with their corresponding number above.

Fig. 4. Relationship between TrHT1 and other trypanosomatid hexose transporters. Sequences were retrieved from GenBank, and the phylogram was obtained using the PHYLIP package. TbgHT2 – T. brucei gambiense hexose transporter.
DISCUSSION
Kinetic analysis of D-glucose transport by T. rangeli epimastigotes and trypomastigotes indicates that a high-affinity transporter is present in both life-cycle stages studied, a situation similar to that found in other insect-form kinetoplastids including Crithidia luciliae and some Leishmania species (Knodler et al. 1992; Langford et al. 1994; Burchmore and Hart, 1995; Tetaud et al. 1997), and with no evidence of a low-affinity transporter similar to that found in bloodstream forms of other trypanosomes (Tetaud et al. 1997; Barrett et al. 1998; Szablewski, 2000). Inhibitor analysis of the mode of D-glucose transport indicates that facilitated diffusion is probably the mechanism responsible for sugar uptake. Although inhibition by phloridzin could indicate an active transport system (e.g. a Na+/glucose symporter), this inhibitor has also been shown to interfere with the facilitated diffusion of sugars (Tetaud et al. 1997), and thus the use of other inhibitors (e.g. ionophores, uncoupling agents and/or H+-ATPase inhibitors) may be more informative on the exact mechanism of D-glucose uptake by T. rangeli. As is the case for other trypanosomatidal transporters, the T. rangeli permease seems not to recognize D-galactose, and probably transports D-fructose and other sugars modified at C-2, a characteristic shared with TcrHT1 and clearly different from other mammalian (e.g. GLUT1) permeases (Barrett et al. 1998). Indeed, the partial DNA sequence that we have obtained from T. rangeli shows significant identity with the moderated-affinity D-glucose transporter TcrHT1 from T. cruzi. Although the results are preliminary and await further functional characterization that this gene is indeed a D-glucose transporter, and that more than one permease isoform is present in T. rangeli (as suggested by the differential inhibition with D-fructose and D-manose observed in epimastigote and trypomastigote forms), the results presented here may explain some important aspects of the biology of this parasite.
For example, the lack of evidence for a low-affinity (and high capacity) D-glucose transporter in T. rangeli, like those found in bloodstream forms of other trypanosomes, indicates that this parasite is probably not adapted to this D-glucose rich environment, justifying its non-pathogenic status in the mammalian hosts. Another possibility is that the parasite may stay just transiently in the blood before invading some cell, as already described by some authors that found amastigote forms in cell tissues and in fibroblast cultures (Urdaneta-Morales and Tejero, 1986; Osorio et al. 1995), although this finding is still controversial.
In conclusion, our results on D-glucose transport by T. rangeli cells describe a new pattern of sugar uptake in trypanosomatids: a parasite transmitted by bite that lacks 2 transporter isoforms as observed for a typical salivarian species, but presenting just a high-affinity transporter typical of the stercoraria group. Other biochemical characterizations have produced further conflicting information on the T. rangeli phylogeny. In a study of β-tubulin gene sequences on a limited number of isolates, it was suggested that T. rangeli was more closely related to T. brucei than to T. cruzi (Amorin et al. 1993). However, a recent study (de Santa Izabel et al. 2004) detected in T. rangeli proteolytic enzymes from the cysteine-proteinase group only, enzymes that have close homology to the major cysteine-proteinase of T. cruzi, cruzipain. Thus, despite intensive discussions on taxonomic position and evolutionary relationships, T. rangeli is probably a phylogenetic link between the Stercoraria and the Salivaria trypasonomatids (Grisard, 2002).
This work was supported by the Brazilian research agencies CNPq, FAPESP (04/10067-6) and FAPESQ-SC. L.C.M. was recipient of a post-doctoral fellowship from CNPq (305173/02-2).