INTRODUCTION
Tropospheric ozone (O3) is one of the most important phytotoxic pollutants in the atmosphere and has now reached a global mean of 35–50 nL L−1 (Cooper et al., Reference Cooper, Parrish, Stohl, Trainer, Nédélec, Thouret, Cammas, Oltmans, Johnson, Tarasick, Leblanc, McDermid, Jaffe, Gao, Stith, Ryerson, Aikin, Campos, Weinheimer and Avery2010; IPCC, 2013). Elevated tropospheric O3 has been reported to reduce plant photosynthesis, growth and production (Pang et al., Reference Pang, Kobayashi and Zhu2009; Wang and Mauzerall, Reference Wang and Mauzerall2004). The projected rise in O3 in Asia (IPCC, 2013) suggests that significant crop yield loss may likely happen in the near future.
Rice (Oryza sativa L.) is a major crop that feeds about half of the world's population (Witcombe et al., Reference Witcombe, Khadka, Puri, Khanal, Sapkota and Joshi2017) and is mainly grown in Asia. In Southeast China, a major rice growing area, the daily mean [O3] has exceeded 150 nL L−1 in some regions of Yangtze Delta and Pearl River Delta (Wang et al., Reference Wang, Manning, Feng and Zhu2007), which is a sufficiently high O3 level to adversely affect rice production. Many studies have shown that elevated O3 (EO3) negatively affects the growth and yield of rice crop (Kou et al., Reference Kou, Xu and Zhu2017a; Pang et al., Reference Pang, Kobayashi and Zhu2009; Wang et al., Reference Wang, Manning, Feng and Zhu2007). Grain yield is dependent on the growth of above and belowground parts, root activity and nutrient uptake (Kou et al., Reference Kou, Xu and Zhu2017a; Nouch et al., Reference Nouch, Ito, Harazon and Kobayashi1991; Zhao et al., Reference Zhao, Shao, Wang, Song, Wang and Yang2015) through the energy generated from root respiration (RR).
Studies on grass (Yoshida et al., Reference Yoshida, Gamon and Andersen2001) and tree (Cooley and Manning, Reference Cooley and Manning1987; Wittig et al., Reference Wittig, Ainsworth, Naidu, Karnosky and Long2009) indicated that EO3 had larger influence on roots than shoots. RR dissimilates 10–50% of carbohydrates assimilated per year and accounts for 12–50% of soil carbon emission (Hanson et al., Reference Hanson, Edwards, Garten and Andrews2000). However, the effects of EO3 on plant belowground processes of dryland crops, trees and grasses are inconsistent. For example, EO3 decreased the root to shoot ratio (RRS) (Andersen, Reference Andersen2003) but had no effect on that of loblolly pine (Edwards, Reference Edwards1991) or trembling aspen (Coleman et al., Reference Coleman, Dickson, Isebrands and Karnosky1996). In addition, EO3 increased the root activities and respiration of ponderosa pine (Scagel and Andersen, Reference Scagel and Andersen1997) but decreased those of bean (Hofstra et al., Reference Hofstra, Ali and Wukasch1981) and loblolly pine (Edwards, Reference Edwards1991). While EO3 reduced the root length of soybean (Wang et al., Reference Wang, Bai, Wen and Huang2004) and inhibited root growth of aspen (Coleman et al., Reference Coleman, Dickson, Isebrands and Karnosky1996), little is known about the effect of EO3 on root surface, diameter, volume or length density. Such inconsistent and scanty findings limit the understanding of the responses of belowground processes (root morphology and respiration) to EO3 in rice cropping systems (Nouch et al., Reference Nouch, Ito, Harazon and Kobayashi1991). This information is critical for advancing knowledge on rice production and soil carbon cycling in paddy systems under global climate change.
In the present study, a major rice cultivar (cv. Shanyou 63) commonly grown in East China was studied under free-air O3 fumigation system. Our aims were to determine the effects of free-air O3 enrichment on (i) the rice production and belowground biomass partitioning, (ii) the root morphology (root length, surface area, diameter, volume and length density) and (iii) the root activity and respiration at key development stages of rice.
MATERIALS AND METHODS
Experimental site
The experiment was conducted using the free-air O3 enrichment system located in Jiangdu county, Jiangsu province, China (119o42′ E, 32o35′ N). This site has been cultivated for over 100 years with rice–wheat rotations. The region has a subtropical marine climate with mean annual precipitation of 1100–1200 mm, mean annual air temperature of 16 °C, and total annual sunshine hours of >2000 h and a frost-free period of >230 days. The soil had a pH of 7.2 and bulk density of 1.16 g cm−3, and contained 13.6% clay, 28.5% silt, 1.5% soil organic carbon, 1.45 g kg−1 total N, 0.63 g kg−1 total P, 106 mg kg−1 available N, 33.8 mg kg−1 Olsen P and 96.4 mg kg−1 available K.
Ozone fumigation treatment
Three plots (240 m2) were maintained at ambient [O3] (AO3) and three others were subjected to EO3 (1.5 times as much as AO3). Adjacent plots were separated by at least 70 m to avoid O3 cross contamination. A free-air O3 enrichment system was used for the O3 fumigation from 9:00 a.m. to sunset except in rainy days, and from July 1 (10 days after rice transplanting) to October 16 (harvest) in 2012. The daytime mean [O3] during experimental period was 40 and 60 nL L−1, and AOT40 was 5.2 and 13.4 ppmh for AO3 and EO3, respectively. More details of the free-air O3 fumigation system were described in Kou et al. (Reference Kou, Xu and Zhu2017a).
Plant cultivation and sampling
One major cultivar of rice in Yangtze River delta, Shanyou 63 (SY63), was grown in all experimental plots. To facilitate the measurements of morphological and physiological parameters of rice roots, a polyvinyl chloride growth chamber (PGC, 20 × 20 × 15 cm) with open top and bottom and three equal-interval rows of holes on the chamber wall was used in the study. The walls and bottom of PGC were wrapped with a nylon cloth of 0.1 mm aperture to keep the roots within the chamber but allow water uptake by roots. Five PGCs were buried into the field at 15 cm depth in each plot. Topsoil was collected from corresponding plot, mixed through a 2-cm sieve, and packed in each PGC to a bulk density of approximately 1.16 g cm−3. Seedlings were sown on May 10, transplanted on June 11 at each PGC with two seedlings per hill (equivalent to 24 hills m−2), and harvested on October 5. Urea was applied at 200 kg N ha−1, superphosphate at 33 kg P ha−1 and potassium chloride at 62 kg K ha−1 to the rice field. The P and K fertilizers were applied before transplanting, while urea at the beginning, tillering and flowering stages at a ratio 3:3:4. Fertilizer application, irrigation and drainage managements followed the standard cultivation practices in the region.
Root activity and respiration, morphology and biomass measurements
Fresh RR rates, specific root respiration rate (SRR) and root morphology were measured at the tillering (July 22), flowering (August 17), grain filling (September 1) and milky mature stages (September 24). During the RR measurement, the PGC was dug out (three for each treatment). Subsequently, the roots in PGC were washed with water to remove the adhered soil and separated from shoot. All roots were put into the distilled water for 1 h cultivation to relieve the potential priming effect due to injury (Zogg et al., Reference Zogg, Zak, Burton and Pregitzer1996). Then the roots of each PGC were transferred to a 5-mL plastic beaker filled with distilled water with CO2 saturation. The beaker was immediately put into a 2-L gas collection equipment (Supplementary Figure S1, available online at http://dx.doi.org/10.1017/S0014479718000170) as described by Kou and Zhu (Reference Kou and Zhu2013). Three 2-mL gas samples of RR in each beaker were collected using the static opaque chamber technique at 0, 20 and 40 min after chamber closure. Gas samples were analysed by a gas chromatograph (Shimadzu, GC-14B) with a thermal conductivity detector. The RR and SRR were calculated according to Kou et al. (Reference Kou, Zhu, Xie, Hasegawa and Heiduk2007). After gas sample collection, the roots in the beaker were used for measuring root morphology (length, surface area, average diameter, volume and length density) with a root scanner (EPSON Perfection V700 Photo). The above and belowground biomass of rice grown in the PGC was oven-dried at 80 °C for 72 h and weighed. Whole plants were divided into root, straw and grain.
Statistical analysis
All data were subjected to ANOVA using the SPSS 11.5 (Windows version 11.5; SPSS, Inc., Chicago, IL). The difference between O3 treatments at each stage was determined using t-test. The effects of O3, growth stage and their interactions were analysed by two-way ANOVA. Correlations of yield with biomass and its partitioning, root activity and morphology were analysed through one-way ANOVA. Differences were considered marginally significant at P = 0.05–0.1 and significant at P < 0.05.
RESULTS
Crop production and dry matter partitioning
EO3 significantly decreased grain production by 19.3% (Table 1). EO3 also significantly decreased total biomass at the grain milky mature stage (−11.7%), and the RRS by 35.7% and 13.3% at the flowering and grain filling stages, respectively. EO3 decreased root biomass by 9.3–16.0%, but the decrease was only significant at the tillering stage (Tables 1 and 2). Significant interaction between O3 and growth stage was noted only for RRS.
Table 1. Dry matter and ratio of root to shoot of Shanyou 63 rice as affected by elevated O3 at different growth stages.

Data are expressed as means ± 1 SE (n = 3). AO3 refers to ambient O3 concentration. EO3 refers elevated O3 concentration with 150% daily average AO3. ‘–’ indicates no data. Different letters in the same column mean significant difference at the 0.05 level.
Table 2. Analysis of variance for biomass and its partitioning, root morphology and activity in response to elevated O3.

F is the F-value, and P is the P-value for each effect in ANOVA. ms and ns indicate marginally significant (P = 0.05–0.10) and no significant (P > 0.05), respectively.
Root morphology
Significant effect of O3 and interaction of its and growth stage on root morphology indices were observed (Table 2). Root length, length density, surface area and volume of rice plants increased rapidly from the tillering stage, followed by a gradual decrease at the grain filling (AO3) and milky mature (EO3) stages (Table 3). The first three parameters reached their maximum at the grain filling stage under EO3 but at the flowering stage under AO3, whereas root volumes peaked at the grain filling stage regardless of O3 treatment. Average root diameter increased with growth development under EO3 but peaked at the grain filling stage under AO3. EO3 significantly decreased root length by 42.3% and 24.4% at the flowering and grain milky mature stages, respectively (Table 3). EO3 significantly decreased root surface area at all key growth stages and also the average root diameter by 3.6–11.6% and root volume by 23.3–42.8% from the tillering to grain filling stages. Root length density was also reduced under EO3 at the tillering (−31.0%), flowering (−42.3%) and grain milky mature (−24.4%) stages.
Table 3. Root morphology index of Shanyou 63 rice as affected by elevated O3 at different growth stages.

Data are expressed as means ± 1 SE (n = 3). AO3 refers to ambient O3 concentration. EO3 refers elevated O3 concentration with 150% daily average AO3. Different letters in the same column mean significant difference at the 0.05 level.
Root activity and respiration
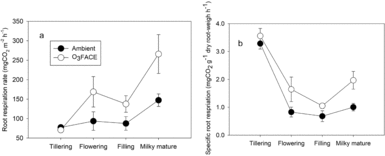
The RR under EO3 and AO3 increased from the tillering to the flowering stage, and decreased slightly at the grain filling stage followed by a sharp increase (Table 2, Figure 1a). EO3 significantly increased RR by 57.3–80.4% at the flowering, grain filling and milky mature stages. The SRR under EO3 and AO3 declined from the tillering stage to a minimum at the grain filling stage, but increased thereafter (Figure 1b). EO3 increased the SRR by 8.3–98.5% across growth stages.

Figure 1. Root respiration rate (a) and specific root respiration rate (root respiration rate per unit root biomass) (b) of Shanyou 63 rice at tillering, flowering, grain filling and milky mature stages under ambient (AO3) and elevated O3 (EO3). Values are means ± 1SE (n = 3).
Correlations of yield with biomass and its partitioning, root activity and morphology
The yield was positively correlated with total biomass at the tillering and flowering stages when averaged across [O3] treatments (Table 4). However, no significant correlation between yield and root biomass, or between yield and RR at the four key stages was found. Significant positive correlations between yield and root morphology (e.g. length, surface area, length density and volume) were found at the flowering stage (r ranging from 0.69 to 0.80, P < 0.05, n = 8).
Table 4. Correlations of yield with biomass and its partitioning, root activity and morphology when averaged across [O3] treatments (n = 8).

r is the correlation coefficient, and P is the P-value for each linear model. *is significant at the 0.05 level.
DISCUSSION
We found that rice yield was reduced by EO3, which was attributed to the adverse effect of EO3 on biomass during the vegetative growth and root morphological index at the flowering stage (Tables 1–). This was in agreement with previous findings (Kou et al., Reference Kou, Xu and Zhu2017a; Reference Kou, Yu, Lam, Chen, Hou and Li2017b; Wang et al., Reference Wang, Manning, Feng and Zhu2007) as photosynthesis (Pang et al., Reference Pang, Kobayashi and Zhu2009) and C assimilation decrease under EO3 (Cooley and Manning, Reference Cooley and Manning1987; Fiscus et al., Reference Fiscus, Booker and Burkey2005). The decrease in RRS under EO3 in our study (Table 1) further indicates that dry matter accumulation and belowground growth (root biomass, root morphology) would be lower under projected [O3] increase. A negative response in RRS to EO3 was also reported for rice (cv. Koshihikari) (Nouch et al., Reference Nouch, Ito, Harazon and Kobayashi1991) and wheat (McCrady and Andersen, Reference McCrady and Andersen2000). Significant decrease in RRS under EO3 might be attributed to an increase in allocation of assimilated C for repairing damaged leaf tissues under EO3 (Andersen, Reference Andersen2003).
The reductions in root length, length density, surface area, volume and average diameter of rice plants demonstrated the inhibitory effect of EO3 on root growth under higher [O3] exposure. Similar responses in wheat to EO3 were observed in our previous study (Kou et al., Reference Kou, Yu, Lam, Chen, Hou and Li2017b) and other researches have also found that EO3 decreased root volume (Zhao et al., Reference Zhao, Cao, Wang, Dai, Liu and Liu2012) and root length of soybean (Wang et al., Reference Wang, Bai, Wen and Huang2004) and alfalfa (Renaud et al., Reference Renaud, Allard and Mauffette1997). The impaired root morphology weakened the ability of roots to absorb nutrients and moisture in soil, reducing rice growth and production. Kou et al. (Reference Kou, Xu and Zhu2017a) found that EO3 decreased the N, P and K uptakes of SY63 under the same free-air O3 enrichment facility, whereas Zhao et al. (Reference Zhao, Shao, Wang, Song, Wang and Yang2015) observed a decrease in N uptake of SY63 rice grown under EO3 in open-top chambers. The delayed occurrence of the maximum root development (except for root volume) may reflect a maintained rice growth under oxidative stress conditions (Coleman et al., Reference Coleman, Dickson, Isebrands and Karnosky1996; Witcombe et al., Reference Witcombe, Khadka, Puri, Khanal, Sapkota and Joshi2017). However, this would also imply that less assimilated C could be used for reproductive growth and grain formation.
While (RR is affected by plant development and environmental conditions (Kou and Zhu, Reference Kou and Zhu2013), and the SRR can represent root activity (Kou et al., Reference Kou, Zhu, Xie, Hasegawa and Heiduk2007). We observed a significant increase in RR under EO3 from the flowering to grain milky mature stages but a slight decrease at the tillering stage (Figure 1a). Nouch et al. (Reference Nouch, Ito, Harazon and Kobayashi1991) also observed that EO3 slightly decreased RR at the tillering stage but increased RR from the jointing to heading stages. Likewise, our previous study reported that EO3 increased RR of rice at the jointing stage (Kou and Zhu, Reference Kou and Zhu2013). In the present study, RR and root biomass were significantly correlated (r = 0.88, P < 0.05) across [O3] and growth stage. The negative effect of EO3 at the tillering stage was likely due to the reduction in carbohydrate allocation to belowground in view of the negative response of root biomass and RRS to EO3 (Tables 1 and 2). The increase in RR under EO3 from the flowering to grain milky mature stages (Figure 1a) suggests that the proportion of total photosynthate used for RR was very high. Kou et al. (Reference Kou, Cheng, Zhu and Xie2015) observed that EO3 increased rhizospheric respiration but decreased total biomass in SY63. According to Nouch et al. (Reference Nouch, Ito, Harazon and Kobayashi1991), the energy produced from the increased root respiration might be used for repairing the tissue damage caused by EO3. The increase in SRR under EO3 in our study (Figure 1b) indicates that the C assimilation and allocation to the belowground parts satisfied the increased respiratory demands of root tissues under O3 stressed conditions. In other words, rice grown under O3 stressed conditions would adjust carbohydrate allocation to achieve balance between plant root and shoot and their activity for maintaining plant growth. This was achieved through changes in RRS, root morphology, RR and activity. Although the negative correlations (r ranging from −0.072 to −0.333) between yield and RR at all key stages were not significant (Table 4), the increase in RR under EO3 for repairing tissue damage would mean less assimilated C for root growth (e.g. inhibitory root morphology), decreasing crop production.
CONCLUSIONS
Inhibition caused by EO3 on total biomass at the vegetative growth and root morphology at the flowering stage decreased rice yield. While EO3 decreased biomass partitioning belowground, it enhanced root activity from the flowering to milky mature stages. Rice RR was increased to satisfy the energy demands for repairing the damage of plant tissues caused by O3 stress, decreasing the allocation of assimilated carbon for root growth (as reflected by the impaired root morphology). Understanding the responses of belowground processes to EO3 is critical for breeding O3-tolerant cultivars and adjusting field management in a paddy field in a high O3 world.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 41003030), the Innovation Team Foundation of Henan University of Science and Technology (grant no. 2015TTD002), Open Research Fund Program of State Key Laboratory of Soil and Sustainable Agriculture (grant no. Y052010030).
SUPPLEMENTARY MATERIAL
For supplementary material for this article, please visit https://doi.org/10.1017/S0014479718000170