INTRODUCTION
Outcomes of ant–plant mutualisms span a dynamic spectrum of possibilities, determined by spatiotemporal variation in biotic and abiotic factors (Bronstein Reference BRONSTEIN1994, Díaz-Castelazo et al. Reference DÍAZ-CASTELAZO, SÁNCHEZ-GALVÁN, GUIMARÃES, RAIMUNDO and RICO-GRAY2013, Kersch & Fonseca Reference KERSCH and FONSECA2005, Rico-Gray et al. Reference RICO-GRAY, DÍAZ-CASTELAZO, RAMÍREZ-HERNÁNDEZ, GUIMARÃES and HOLLAND2012). Thus, protection benefits received by ant-plants could vary with herbivory pressure, and availability of protective ants (Barton Reference BARTON1986, Rudgers & Strauss Reference RUDGERS and STRAUSS2004, Shenoy & Borges Reference SHENOY and BORGES2010); availability of protective ant partners may also be determined by quality and quantity of rewards, viz. extrafloral nectar (EFN) and nesting shelters (domatia) (González-Teuber et al. Reference GONZÁLEZ-TEUBER, SILVA BUENO, HEIL and BOLAND2012, Shenoy et al. Reference SHENOY, RADHIKA, SATISH and BORGES2012).
Few studies have investigated the actual spectrum of outcomes in ant–plant interactions resulting from geographical variation in plant traits, viz. domatia (Fonseca Reference FONSECA1999) and extrafloral nectaries (Rico-Gray et al. Reference RICO-GRAY, GARCIA-FRANCO, PALACIOS-RIOS, DÍAZ-CASTELAZO, PARRA-TABLA and NAVARRO1998, Rios et al. Reference RIOS, MARQUIS and FLUNKER2008). Furthermore, with few exceptions (Rios et al. Reference RIOS, MARQUIS and FLUNKER2008, Rudgers & Strauss Reference RUDGERS and STRAUSS2004), most research on geographical variation in ant–plant interactions has focused on multi-species communities (Rico-Gray & Oliveira Reference RICO-GRAY and OLIVEIRA2007), possibly because, being usually spread over similar habitats, most ant-plants show little intraspecific variation in characters. Fewer studies have examined temporal variation in such systems (Díaz-Castelazo et al. Reference DÍAZ-CASTELAZO, SÁNCHEZ-GALVÁN, GUIMARÃES, RAIMUNDO and RICO-GRAY2013). However, since costs and benefits in mutualistic interactions are finely balanced, with context-dependent dynamics (Baker-Méio & Marquis Reference BAKER-MÉIO and MARQUIS2012, Chamberlain & Holland Reference CHAMBERLAIN and HOLLAND2008), investigating spatiotemporal intraspecific variation in ant-related host-plant traits in the light of contexts (e.g. herbivory pressure, presence of protective ants at the sites) is important to our understanding of such mutualisms.
We investigate spatial and temporal variation in protection mutualism in a unique myrmecophytic plant Humboldtia brunonis Wall. (Fabaceae), in which every individual produces EFN on young leaves and bracts of floral buds, but only some individuals produce domatia. The domatia are occupied by 16 species of ants and myriad other invertebrates, most notably an arboreal earthworm Perionyx pullus Stephenson (Megascolecidae). However, only one ant species, Technomyrmex albipes (Smith) (Dolichoderinae), provides significant anti-herbivore protection (Gaume et al. Reference GAUME, ZACHARIAS, GROSBOIS and BORGES2005, Shenoy & Borges Reference SHENOY and BORGES2010). In an earlier study, done at three sites approximately 5 y before the present one, the geographical variation in protection indicated stronger ant–plant interaction towards the southern portion of the plant's distribution range in the Indian Western Ghats which exhibit a north–south gradient of seasonality in rainfall (Shenoy & Borges Reference SHENOY and BORGES2010). We hypothesize that the dynamics of the interaction between a host plant species and its associated ants varies over space and time, such that stronger mutualism, characterized by increased rewards to ants by the host plant and greater protection by the partner ant, would occur under conditions of greater herbivory pressure and at sites where protective ants are present. In the present study, we recorded five ant-related traits of H. brunonis, viz. (1) abundance of domatia-bearing trees, (2) number of extrafloral nectaries per leaf, (3) size of foliar nectaries, (4) volume of EFN per leaf and (5) composition of foliar EFN. We sampled foliar EFN since T. albipes prefers foliar EFN rich in essential amino acids and low sugar:amino acid ratios (Shenoy et al. Reference SHENOY, RADHIKA, SATISH and BORGES2012). We also recorded herbivory pressure and ant protection to leaves and floral buds at the five sites. Of these, data on volume and composition of EFN were also available from the earlier study at three sites, as were data on herbivory pressure and ant protection. Our study therefore re-sampled these site-specific factors to examine temporal stability in them, and additionally investigated spatial variation in three new ant-related plant traits, viz. abundance of domatia-bearing trees, number of nectaries per leaf, and nectary size, to confirm an earlier suggested north–south gradient in myrmecophytism in this species.
METHODS
Study system and sites
Humboldtia brunonis is an understorey tree, endemic to the low-elevation, tropical wet evergreen forests of the Western Ghats of India (Pascal Reference PASCAL1988). It is polymorphic for the presence of domatia – modified swollen hollow internodes – which are inhabited by ants and various other invertebrates, including an arboreal earthworm. Domatia in southern sites are occupied mostly by ants; those in the north are dominated by earthworms (Shenoy & Borges Reference SHENOY and BORGES2010). Foliar nectaries are present on each of the two pairs of leaflets per leaf. Humboldtia brunonis is distributed between 11°10′N and 13°45′N in the Western Ghats within a narrow north–south strip. To sample the entire distribution, we divided it into five sections at intervals of 0.5° latitude, from which we chose sites within the altitudinal range 200–600 m asl: Agumbe (13°31′N, 75°04′E), Kudremukh (13°16′N, 75°07′E), Uppangala (12°33′N, 75°39′E), Sampaje (12°27′N, 75°37′E) and Solaikolli (12°05′N, 75°47′E) (Figure 1). These sites span a rainfall seasonality gradient in the Ghats with longer dry seasons (8 mo) towards the north compared with the south (6 mo) since the southern Western Ghats receive rain from both the south-west monsoon (June–September) as well as the north-east or retreating monsoon (October–November) (Davidar et al. Reference DAVIDAR, PUYRAVAUD and LEIGH2005, Gadgil & Joshi Reference GADGIL and JOSHI1983, Pascal Reference PASCAL1988, Shenoy & Borges Reference SHENOY and BORGES2010) and also suggested a natural geographical grouping between northern sites: Agumbe and Kudremukh, and southern sites: Uppangala, Sampaje and Solaikolli. Humboldtia brunonis occurs in clonal clumps (Dev et al. Reference DEV, SHENOY and BORGES2010) and is so abundant that it features within two forest type associations: (1) Dipterocarpus indicus–Humboldtia brunonis–Poeciloneuron indicum type, and (2) Dipterocarpus indicus–Kingiodendron pinnatum–Humboldtia brunonis type (Pascal Reference PASCAL1988, Rai Reference RAI2000). Agumbe and Kudremukh occur within the first, and the other three sites within the second forest type.
Figure 1. Map of Peninsular India indicating the Humboldtia brunonis study sites within the Western Ghats. The sites span the extant distribution of the species.
Our research was conducted during the dry season (December–April), during which flowers and young leaves are available (Shenoy & Borges Reference SHENOY and BORGES2010). The abundance of domatia-bearing trees and number of nectaries per leaflet were recorded from December 2007–March 2008. Leaf herbivory was measured from December 2009–March 2010. EFN collection and additional sampling of nectaries occurred between December 2010–March 2011.
Abundance of domatia-bearing Humboldtia brunonis trees
Within each site, three transects each of 300 m length were sampled. Each transect was divided into five equal sections, on which five points were randomly chosen. From each such point, one sampling point at randomly determined distances (between 10–60 m) from left and right sides alternately was selected (discounting 10 m on either side of foot-trails to avoid edge effects). Presence or absence of domatia was noted on the H. brunonis plant at each of the sampling points and five of its nearest conspecific neighbours. Thus we sampled 30 trees in each transect, and with three transects in each of the five sites, we sampled a total of 450 trees. Plants less than 1 m tall were avoided, since domatia in H. brunonis are formed only at heights ≥70 cm (Brouat & McKey Reference BROUAT and MCKEY2000).
Number of extrafloral nectaries per leaf
From each tree sampled for domatia abundance, five intact leaflets, independent of age, were haphazardly collected (n = 90 trees per site), and the number of nectaries per leaflet was counted. Average values of nectary numbers per leaflet per tree were obtained which were then used in a multivariate analysis to examine the effect of domatia presence on nectary numbers per leaflet (n = 450 tree-average nectary values from five sites). Subsequent sampling revealed that the leaflets of the distal pair had greater numbers of nectaries than the proximal pair, and this was true for all sites. Therefore, whole intact leaves (with four leaflets) were collected haphazardly from the transects, independent of domatia presence/absence on the trees (Agumbe: n = 34 whole leaves, Kudremukh: n = 30, Uppangala: n = 25, Sampaje: n = 40, Solaikolli: n = 30), and their nectaries counted.
Size of foliar nectaries
Young leaves were collected in 75% alcohol. Nectary size was estimated as the area of the circular nectary disc (Rudgers Reference RUDGERS2004). The diameter (and radius r) of each nectary was measured with a stereomicroscope using a micrometer on hand-cut, safranin-stained, transverse sections; nectary area was calculated as πr2 (Agumbe: n = 39 samples, Kudremukh: n = 39, Uppangala: n = 40, Sampaje: n = 39, Solaikolli: n = 40). Since there was no significant difference between the nectary sizes of very young red and expanding young green leaves (Chanam & Borges, unpubl. data), nectaries of such leaves were pooled for each site.
Volume and composition of EFN per leaf
Young leaves were enclosed in cloth bags to prevent nectar consumption. EFN volume (pooled across four leaflets) was measured the next morning (after 24 h) between 06h00 and 09h00 using micropipettes (Agumbe: n = 21 leaves; Kudremukh: n = 43; Uppangala: n = 23; Sampaje: n = 21; Solaikolli: n = 46). EFN was collected in sterile gas chromatography vials, using micropipettes with sterile tips (Agumbe: n = 9 EFN samples; Kudremukh: n = 10; Uppangala: n = 9; Sampaje: n = 7; Solaikolli: n = 5) in the same way. Up to 50 μL of HPLC-grade methanol was added to each vial to prevent microbial growth; vials were stored on ice, and their contents later lyophilized.
The composition of sugars and amino acids in EFN was determined using gas chromatography-mass spectrometry (GC-MS). A mixture of 1 mg each of several sugars (sucrose, glucose, fructose, inositol, galactose, mannose, arabinose) and each of the 20 naturally occurring amino acids (Sigma-Aldrich, Germany) was used as reference. Each component was converted to its trimethyl silylated derivative by treating the mixture with 1 ml of N-methyl-N-(trimethyl silyl) trifluoro acetamide and 2 ml pyridine (adapted from Kost & Heil Reference KOST and HEIL2005). The standard solution was diluted 1:10 using dichloromethane, and 1μL of it was injected into the GC-MS instrument (Agilent-HP GC model 6890N, MS model 5973N) fitted with an HP-5 MS column (30 m × 0.25 mm × 0.25 mm; J and W Scientific, USA). The GC was operated with a split-ratio of 10:1 with a temperature program adapted from Shenoy et al. (Reference SHENOY, RADHIKA, SATISH and BORGES2012). EFN samples were analysed as above. The concentration of each component was estimated and compared across sites as were the sugar:amino acid mass ratios. Amino acids were grouped into essential or non-essential categories using standard human criteria since standard insect criteria are not available.
Herbivory and protection to young leaves and floral buds
At each site, one pair of young leaves per tree was tagged (Agumbe: n = 18 pairs; Kudremukh: n = 26; Uppangala: n = 27; Sampaje: n = 21; Solaikolli: n = 30). Ants, irrespective of the species present on the trees at each site, were allowed access to one leaf (control) of each pair, while they were excluded from the other using Tanglefoot® glue. After 10 d the leaves were collected, photocopied and later scanned. Herbivory, measured as percentage leaf area consumed using the software ImageJ 1.36b (http://rsb.info.nih.gov/ij), was compared between ant-excluded and control leaves. Pairs of control and ant-excluded inflorescence primordia were tagged (Agumbe: n = 36 pairs; Kudremukh: n = 29; Uppangala: n = 30; Sampaje: n = 31; Solaikolli: n = 27) and the numbers of floral buds on each inflorescence counted. After 10 d, the proportion of floral buds damaged or removed by herbivory was determined, and compared between ant-excluded and control inflorescences. Herbivory pressure was defined as herbivory on ant-excluded leaves or floral buds. Ant protection was estimated as reduction in herbivory by the presence of ants, by comparing the herbivory on ant-excluded and control samples of each pair.
Statistical analysis
Non-parametric statistics were used for all inter-site comparisons. Kruskal–Wallis ANOVAs (denoted by the χ2 test statistic) were employed, followed by post hoc Wilcoxon rank sum tests (denoted by the W test statistic) after appropriate Bonferroni corrections. Wilcoxon rank sum tests were also used for comparison between northern and southern groups of sites. To determine the effect of domatia and site, and their interaction on the number of nectaries per leaflet, we used a linear modelling framework, and simplified the model by sequentially dropping terms (Crawley Reference CRAWLEY2012) to find the most parsimonious model. Wilcoxon matched-pairs signed ranks tests were used for comparing herbivory on ant-excluded versus control pairs of leaves or floral buds. All statistical analyses were performed using R software (version 2.14.1). All values mentioned in the results are mean ± SD.
RESULTS
Geographical variation in morphological ant-plant traits
The relative abundance of domatia-bearing trees varied across the five sites (Kruskal–Wallis test: χ2 = 22.5, P = 0.0002, n = 450; Agumbe: 8.9%; Kudremukh: 22.2%; Uppangala: 33.3%; Sampaje: 48.9%; Solaikolli: 31.1%; Figure 2). The southern group of sites had a significantly greater relative abundance (an average of 2.4 times higher abundance) of domatia-bearing trees than the northern group (Wilcoxon rank sum test: W = 308, P < 0.001) (Figure 2).
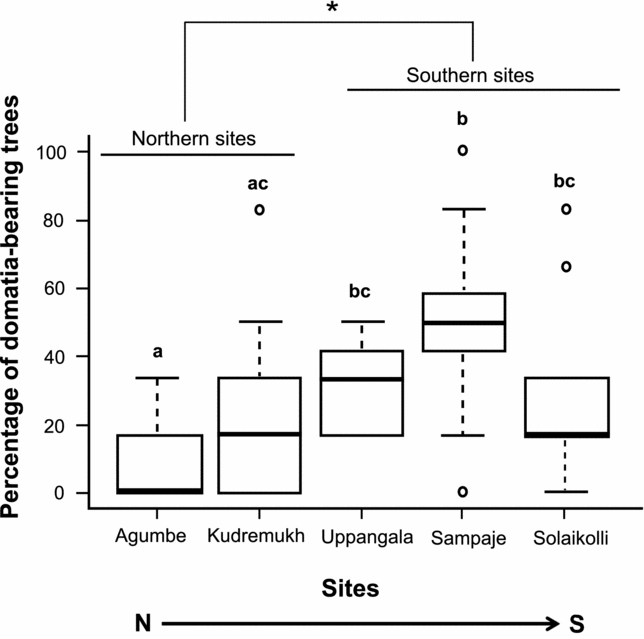
Figure 2. Abundance of domatia-bearing trees of Humboldtia brunonis across sites spanning its distribution in the Indian Western Ghats (n = 90 trees per site). The lower and upper boundaries of each box indicate lower and upper quartile values; the bar in the box indicates the median. Error bars indicate minimum and maximum values, excluding outliers which are indicated by open circles. Different letters above the boxes indicate significant differences (Kruskal–Wallis test followed by post hoc Wilcoxon rank sum tests after Bonferroni correction). * P < 0.05, Wilcoxon rank sum test between northern and southern sites.
Site identity was the only factor to affect the nectary number per leaflet significantly (F4,445 = 31.9, P ≪ 0.001), while domatia presence (F1,444 = 0.001, P = 0.97) and the interaction term (F4,440 = 0.95, P = 0.43) had no effect. The average number (± SD) of nectaries per leaflet was lowest in the northern sites, viz. Agumbe (5.8 ± 2.2) and Kudremukh (5.7 ± 2.4), and higher in the southern sites, being highest in Sampaje (8.3 ± 2.7), while there was no significant difference between Uppangala (6.7 ± 2.7) and Solaikolli (6.7 ± 2.5) (Figure 3a). At each site, the number of nectaries per leaflet varied amongst the four leaflets of a leaf (Kruskal–Wallis tests: Agumbe: χ2 = 18.2, df = 3, P < 0.01; Kudremukh: χ2 = 29.2, df = 3, P < 0.01; Uppangala: χ2 = 15.3, df = 3, P < 0.01; Sampaje: χ2 = 29.8, df = 3, P < 0.01; Solaikolli: χ2 = 42.6, df = 3, P < 0.01), with the two distal leaflets bearing more nectaries that the two proximal leaflets (Figure 3b). The number of nectaries per whole leaf varied significantly across the five sites (χ2 = 50.0, df = 4, P < 0.0001), being higher in southern (Uppangala: 32.2 ± 9.6, Sampaje: 30.7 ± 11.1 and Solaikolli: 29.2 ± 9.8) than northern sites (Agumbe: 17.3 ± 5.4, Kudremukh: 21.8 ± 6.7) (W = 972, P < 0.001) (Figure 3b) with the average nectary number per leaf in the southern sites being 1.6 times higher than that of the northern sites.

Figure 3. Abundance of foliar extrafloral nectaries in Humboldtia brunonis across sites spanning its distribution in the Indian Western Ghats. Number of extrafloral nectaries per leaflet (n = 90 trees per site and 450 leaflets in total) in domatia- and non-domatia bearing trees (a). Numbers of extrafloral nectaries per leaf (summed across four leaflets of each leaf) across sites (b). Bar plots depict mean ± SD. Letter notation and symbols as in Figure 2. Agumbe: n = 34 whole leaves, Kudremukh: n = 30, Uppangala: n = 25, Sampaje: n = 40, Solaikolli: n = 30.
The area of foliar nectaries varied across the five sites (χ2 = 26.7, df = 4, P < 0.05), with a trend of increases in area from north to south (Agumbe: 0.14 ± 0.08 mm2; Kudremukh: 0.18 ± 0.06 mm2; Uppangala: 0.21 ± 0.06 mm2; Sampaje: 0.20 ± 0.06 mm2; Solaikolli: 0.19 ± 0.06 mm2) (Figure 4a). The southern group of sites had significantly larger nectaries (1.2 times larger on average) than the northern group (W = 3022, P < 0.001) (Figure 4a).

Figure 4. Size of foliar extrafloral nectaries of Humboldtia brunonis across sites spanning its distribution in the Indian Western Ghats. Size of nectaries of young leaves (a), and volume of extrafloral nectar (EFN) per leaf (b). For nectaries: Agumbe: n = 39 nectaries, Kudremukh: n = 39, Uppangala: n = 40, Sampaje: n = 39, Solaikolli: n = 40. For EFN volume per leaf: Agumbe: 21 leaves; Kudremukh: 43; Uppangala: 23; Sampaje: 21; Solaikolli: 46. Box-and-whisker plots, letter notation and symbols as in Figure 2.
Geographical variation in foliar nectar traits
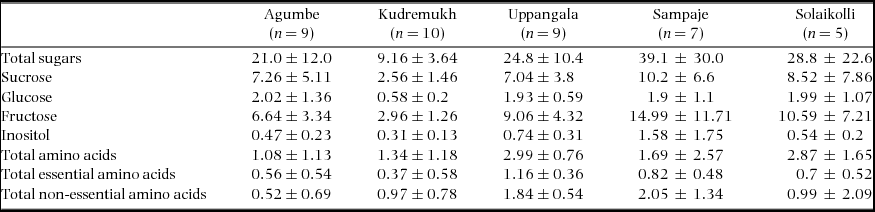
The volume of EFN per leaf varied across the five sites (χ2 = 24.0, df = 4, P < 0.0001; Agumbe: 5.6 ± 4.3 μl; Kudremukh: 3.4 ± 2.7 μl; Uppangala: 11.7 ± 9.3 μl; Sampaje: 5.9 ± 7.6 μl; Solaikolli: 9.6 ± 11.4 μl). EFN volume was significantly greater in the southern group (2.24 times greater on average) compared with the northern group (W = 1870, P < 0.001) (Figure 4b). Sugars had higher EFN concentrations than amino acids (Figure 5); however, the sugar:amino acid ratios (per cent weight/volume) varied across the five sites, ranging from 17.2 (Uppangala) to 48.6 (Agumbe); ratios for the other sites were 47.8 (Kudremukh), 32.0 (Sampaje) and 35.5 (Solaikolli). Overall, seven sugars (sucrose, glucose, fructose, inositol, galactose, mannose and altrose) were detected (Figure 4a). The total concentration of sugars (g per 100 ml volume) in EFN varied across the five sites (χ2 = 14.7, df = 4, P = 0.005), being significantly higher in the southern group (2.1 times higher on average) than the northern group (W = 88.0, P = 0.002) (Figure 5a, inset).
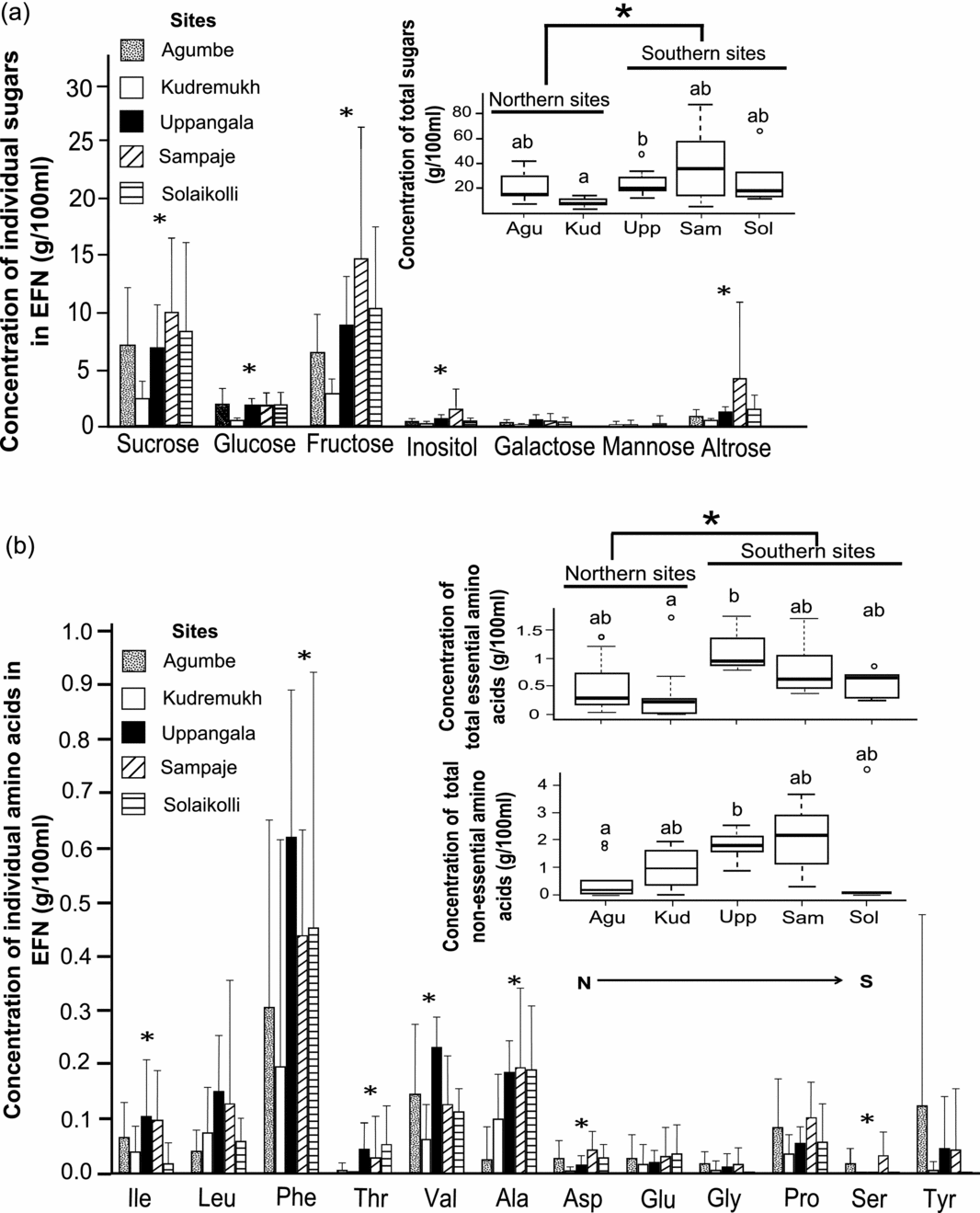
Figure 5. Geographical variation in concentrations of sugars and amino acids in foliar extrafloral nectar (EFN) of Humboldtia brunonis at study sites in the Indian Western Ghats. Concentrations of individual sugars (a) and amino acids (b) are compared across sites. Concentrations of total sugars (inset of a), and total essential and non-essential amino acids (inset of b) are compared across sites as well as between northern and southern groups of sites. Bar plots depict mean ± SD. Box-and-whisker plots, letter notation and symbols as in Figure 2. Agu (Agumbe): n = 9 EFN samples; Kud (Kudremukh): n = 10; Upp (Uppangala): n = 9; Sam (Sampaje): n = 7; Sol (Solaikolli): n = 5. Ile: Isoleucine, Leu: Leucine, Phe: Phenylalanine, Thr: Threonine,Val: Valine, Ala: Alanine, Asp: Aspartic acid, Glu: Glutamic acid, Gly: Glycine, Pro: Proline, Ser: Serine, Tyr: Tyrosine.
The total concentration of the major sugars (sucrose, glucose and fructose) varied across the five sites (χ2 = 9.45, df = 1, P = 0.002). The southern group had significantly greater concentration of total major sugars (W = 86.0, P = 0.002) than the northern group. Minor sugars included galactose, mannose and altrose (Appendix 1). Interestingly, there was no significant difference in total minor sugars between the northern and southern groups. Inositol was present in much lower proportion compared with major sugars in all sites (0.3 g per 100 ml in Kudremukh to 1.6 g per 100 ml in Sampaje), with variation across the five sites (χ2 = 10.2, df = 4, P = 0.037); post hoc pairwise tests revealed significant difference in inositol concentrations only between Kudremukh and Uppangala (W = 11, P < 0.001).
Twelve amino acids were detected, comprising five essential (isoleucine, leucine, phenylalanine, threonine and valine) and seven non-essential (alanine, aspartic acid, glutamine, glycine, proline, serine and tyrosine) amino acids (Figure 5b). The average total concentration of the amino acids ranged between 1.08 ± 1.13 g per 100 ml (Agumbe) and 2.99 ± 0.76 g per 100 ml (Uppangala) (Table 1), with significant variation across the five sites in concentrations of total amino acids (χ2 = 13.8, df = 4, P = 0.008), total essential amino acids (χ2 = 14.6, df = 4, P = 0.006), and total non-essential amino acids (χ2 = 13.0, df = 4, P = 0.01). The southern group of sites had higher concentration of essential (W = 79.0, P = 0.001), as well as non-essential amino acids (W = 109, P = 0.01) than the northern group (Figure 5b, inset) with average concentration of total amino acids in the southern sites being 2.2 times higher than the northern sites.
Table 1. Concentration (mean ± SD, g per 100 ml) of important sugars and total essential and non-essential amino acids in foliar EFN of Humboldtia brunonis at study sites in the Indian Western Ghats.
Geographical variation in herbivory pressure
Herbivory pressure varied significantly across the five sites for both young leaves (χ2 = 21.0, df = 4, P = 0.0003) and floral buds (χ2 = 12.7, df = 4, P = 0.01), with the highest herbivory occurring in the southern site Uppangala (approximately 60% and 50% herbivory for leaves and floral buds respectively). The control leaves had significantly lower herbivory than ant-excluded leaves only at Uppangala (Wilcoxon matched pairs test: V = 88, P = 0.045, n = 27 pairs). There was no significant reduction in herbivory in the control floral buds at any site. In Kudremukh, ant-excluded floral buds demonstrated lower herbivory levels (Wilcoxon matched pairs test: V = 74, P = 0.006, n = 29 pairs).
DISCUSSION
Context dependency in ant–plant mutualisms has usually been demonstrated in relation to the identity of protective ants, types of herbivore and abiotic factors (Barton Reference BARTON1986, Kersch & Fonseca Reference KERSCH and FONSECA2005, Pringle & Gordon Reference PRINGLE and GORDON2013). We found that conditionality in an ant–plant mutualism was linked to myrmecophytic traits which varied with geography and demonstrated a clear north–south pattern. Herbivory pressure in the absence of ants was high towards the south, especially at the Uppangala site, but its reduction in the presence of ants suggests that greater ant attraction by suitable myrmecophytic traits could have led to stronger protection services by ants at this site.
Context dependency of rewards to ants in relation to protection services
Nesting space availability can affect ant colony size and consequently protection benefits (Fonseca Reference FONSECA1999). In H. brunonis, greater availability of domatia could promote ant protection services since the sole protective ant, T. albipes, being polydomous, is likely attracted by abundant nesting spaces (domatia) (Shenoy & Borges Reference SHENOY and BORGES2010). Greater abundance of domatia-bearing trees and of the protective ant in the southern sites (Shenoy & Borges Reference SHENOY and BORGES2010) supports the facilitation of ant protection by increased availability of domatia as also suggested by Fiala & Maschwitz (Reference FIALA and MASCHWITZ1992). However, the southernmost site Solaikolli had relatively fewer domatia-bearing trees for reasons that cannot currently be explained.
In H. brunonis, there may be greater selective advantage to increased foliar nectary numbers in the southern sites due to site-specific effects, e.g. herbivory pressure and abundance of the protective ant. However, whether in H. brunonis, as in other ant-plants, nectary number is genetically determined (Rudgers Reference RUDGERS2004, Rudgers & Strauss Reference RUDGERS and STRAUSS2004) or modulated by herbivory (Mondor et al. Reference MONDOR, TREMBLAY and MESSING2006, Pulice & Packer Reference PULICE and PACKER2008) is still unknown. The greater EFN volume in the southern sites could result from the larger nectary size (Baker-Méio & Marquis Reference BAKER-MÉIO and MARQUIS2012) characteristic of the southern plants. These results differ from those of Shenoy et al. (Reference SHENOY, RADHIKA, SATISH and BORGES2012) who reported similar volumes at three sites (northern: Agumbe; southern: Sampaje and Solaikolli). This spatiotemporal variation in EFN volume, in relation to differing herbivory pressure and protection, suggests that EFN volume is a plastic trait as found in other ant-plants (Bixenmann et al. Reference BIXENMANN, COLEY and KURSAR2011, Escalante-Pérez et al. Reference ESCALANTE-PÉREZ, JABORSKY, LAUTNER, FROMM, MÜLLER, DITTRICH, KUNERT, BOLAND, HEDRICH and ACHE2012, Heil et al. Reference HEIL, FIALA, BAUMANN and LINSENMAIR2000, Reference HEIL, KOCH, HILPERT, FIALA, BOLAND and LINSENMAIR2001). We recorded only seven of the 12 sugars reported in Shenoy et al. (Reference SHENOY, RADHIKA, SATISH and BORGES2012), while the rest (maltose, arabinose, xylose, ribose and arabinoic acid) were either undetected or present in trace amounts. Of the amino acids, methionine and tryptophan, reported by Shenoy et al. (Reference SHENOY, RADHIKA, SATISH and BORGES2012), were undetected in the present study. The sugar:amino acid ratio of Uppangala (17.2) was lower than the lowest ratio value reported by Shenoy et al. (Reference SHENOY, RADHIKA, SATISH and BORGES2012) (Agumbe: 50, Sampaje: 323 and Solaikolli: 34); therefore Uppangala had the richest amino acid concentration recorded in any site at any time for this plant. In the two studies separated by 5 y, Agumbe and Solaikolli showed little variation in sugar:amino acid ratios, while the ratio in Sampaje changed considerably. The absence of a north–south gradient in minor sugars and in inositol, which may not be important to ants (Rudgers & Gardener Reference RUDGERS and GARDENER2004) or whose effect on ants is equivocal (Shenoy Reference SHENOY2008), alludes to the fact that the latitudinal trend in EFN composition is also likely under context-based selection such that EFN is rich in sugars important to ants at sites where protective ants need to be rewarded for their services. Our results also emphasize that repeated sampling of EFN volume and composition is necessary to uncover the range of variation expressed within an ant–plant mutualism, and that data from a single study at a single point in time may be inadequate.
Increased investment in EFN volume and composition can increase ant protective efficacy (González-Teuber et al. Reference GONZÁLEZ-TEUBER, SILVA BUENO, HEIL and BOLAND2012, Shenoy et al. Reference SHENOY, RADHIKA, SATISH and BORGES2012). Since T. albipes is a tramp ant and may shift between populations of H. brunonis (while remaining ‘faithful at the meta-population scale’, Gaume et al. Reference GAUME, ZACHARIAS, GROSBOIS and BORGES2005), and also prefers ‘costly’ EFN with higher essential amino acid content, it would be beneficial to the host plant to express context dependency in the production of attractive rewards, producing them only as and when it would most benefit the plant. Our findings of higher EFN volumes and attractive EFN compositions only at the site most threatened by herbivory in the south, demonstrates such context dependency in the relationship of this myrmecophyte with its protective ant in the southern sites. Therefore, plants invest in ant protection when herbivory is high and when protective ants are available. Furthermore, plants appear to be able to modulate some ant rewards to suit the physiological requirements of their protective ants.
A gradient conducive to the evolution of myrmecophytism
Northern Western Ghat sites receive only the south-west monsoon (June–September) while southern sites also receive the north-east monsoon (October–December) (Gadgil & Joshi Reference GADGIL and JOSHI1983). Furthermore, the southern and northern sites occur within two distinct forest types which probably result from differences in rainfall and other geophysical factors (Pascal Reference PASCAL1988, Rai Reference RAI2000). The dry season is shorter towards the southern sites while the interval between dry spells is longer towards the north (Shenoy & Borges Reference SHENOY and BORGES2010). This long dry period in the north may explain the greater dominance of interlopers such as arboreal earthworms in the domatia of the northern sites (Shenoy & Borges Reference SHENOY and BORGES2010). These dominant interloping earthworms that cannot survive outside the domatia in the dry season, coupled with the lower relative abundance of trees bearing domatia in the northern sites, further reduces the availability of domatia for ants in the northern sites. The earthworms have a negative association with ants and may displace them from domatia (Gaume et al. Reference GAUME, SHENOY, ZACHARIAS and BORGES2006). Since caulinary woody-stem domatia are costly to produce (Blatrix et al. Reference BLATRIX, RENARD, DJIETO-LORDON and McKEY2012), the domatium trait may be favoured when the net benefits of domatia production outweigh their costs. The domatia inhabitants of H. brunonis also provide nitrogen to the plant that is incorporated into plant tissue (Chanam et al. Reference CHANAM, SHESHSHAYEE, KASINATHAN, JAGDEESH, JOSHI and BORGES2014). How the benefits of domatia accrued from protective ants nesting within domatia interact with the nutritional benefits obtained from miscellaneous and even interloping domatia inhabitants and affect selection on the domatium trait is still unknown.
Invoking the geographic mosaic model (Gomulkiewicz et al. Reference GOMULKIEWICZ, THOMPSON, HOLT, NUISMER and HOCHBERG2000, Thompson Reference THOMPSON1999), it appears that the south zone is a ‘hot spot’ of protection mutualism for the H. brunonis system, whereas the north zone is a ‘cold spot’. Unlike the semi-myrmecophyte H. brunonis, Humboldtia laurifolia Vahl is a true myrmecophyte, one in which all individuals bear stem domatia. The occurrence of this true myrmecophyte, H. laurifolia, in Sri Lanka (Krombein et al. Reference KROMBEIN, NORDEN, RICKSON and RICKSON1999) which lies to the south of the Western Ghats (where it is mostly associated with the protective dolichoderine T. albipes), further supports the north–south trend in protection mutualism in this region. Sri Lanka also receives two monsoons (Suppiah Reference SUPPIAH1996) as does the southern Western Ghats. Latitudinal effects on ant-plant distributions are known in Asia (Pemberton Reference PEMBERTON1988) and may be dictated by temperature, rainfall and rainfall seasonality gradients. Similar latitudinal effects in India resulting from the length and timing of the monsoons have been demonstrated for other plant–animal dependent interactions such as the dispersal phenology of plants (Aravind et al. Reference ARAVIND, GANESHAIAH and UMA SHAANKER2013). It is therefore entirely possible that only the less seasonal southern sites in the Western Ghats are most conducive to the development of ant–plant mutualisms. However, some southern sites such as Solaikolli did not follow the north–south trend in all ant-related traits; greater attention in future research must be paid to factors that could have caused such deviations. Our findings of an overall stable north–south gradient of myrmecophytism in the Western Ghats of India have implications for the existence of such gradients in several other plant–animal interactions that merit exploration in this region.
ACKNOWLEDGEMENTS
This research was funded by the Ministry of Environment and Forests (Government of India). We are grateful to the Karnataka Forest Department for research permits. We thank Jean-Marie Bessière, Megha Shenoy, Geetha Ramaswami, Soumya Prasad, Raman Kumar and Kavita Isvaran for important suggestions; Mary Sunitha, G. Yathiraj, Balaji, Ganesh Venkataraman and Soya Mary Jose for logistic support, S. P. Vijayakumar for the map illustration; Anusha Krishnan, Mahua Ghara, Martin Heil, Yuvaraj Ranganathan and an anonymous reviewer for critical comments on the manuscript.
APPENDIX 1. Concentrations (mean ± SD, g per 100 ml) of minor sugars, and individual amino acids in foliar EFN of Humboldtia brunonis at study sites in the Indian Western Ghats.