Coverage with Evidence Development (CED) is a form of conditional reimbursement for pharmaceutical products, medical/surgical procedures, and medical devices. CED is characterized by restricted coverage occurring in parallel with targeted data collection and when the stated goal of the research is reducing material uncertainty. Material uncertainty exists when the clinical or cost-effectiveness impact of a new technology is not robustly characterized, and the effect of more accurately defining the clinical or cost-effectiveness impact may provide definitive information. Payers and other healthcare decision makers may interpret the additional evidence obtained from a CED program either positively or negatively, requiring a range of possible changes to the reimbursement status.
CED may be appropriate when the benefit profile of an innovation is uncertain, and data collection required by CED can be structured to minimize uncertainty around a specific aspect of the evidence base. CED has a potential impact on payers (e.g., private insurance companies, reimbursement agencies, and government bodies) and producers (e.g., pharmaceutical companies and device companies), but CED may also impact patients and healthcare providers who may want access to new treatments in the face of uncertainty. The key issue is striking an appropriate balance between the demands for prompt access to promising technologies and acknowledging that some degree of uncertainty about the clinical and cost-effectiveness of a technology is almost always present around the time of launch.
A manuscript developed by Hutton et al. describes the work of the Health Technology Assessment International (HTAi) Policy Forum in 2007 in consideration of CED (Reference Hutton, Trueman and Henshall10). The study concluded that when coverage decisions are made close to launch, the data required for regulatory approval may be not be sufficient for coverage decisions/reimbursement. CED provides an option for collecting evidence to meet the needs of payers without delaying access to new treatments for all patients.
In this study, we describe how CED differs from other conditional coverage agreements, provide a brief history of CED, describe several real-world examples of CED from different countries, describe the areas of consensus between the stakeholders, discuss the areas of contention between stakeholders, and propose criteria to assist payers in determining when CED could be appropriate.
For this study, stakeholders include patients, producers, payers, and healthcare providers. Producers are generally the developers, marketers, or owners of the new technology. Payers include private and public sector bodies that fund health services and the decision-making bodies that influence funding decision on a national or regional level.
The primary sources for this study were the CED presentations that took place at the Health Technology Assessment International (HTAi) meeting in Montreal, Canada, in July 2008. The information from the presentations was primarily from the perspective of producers and payers. Most of the examples of experience with CED presented at the meeting were those associated with large, government-run, healthcare delivery systems. The information from the presentations is supplemented with information from other publications and Internet sources identified by a search of MEDLINE (http://www.ncbi.nlm.nih.gov/pubmed/) and Google Scholar (http://scholar.google.com/) and referenced in this study.
COVERAGE WITH EVIDENCE DEVELOPMENT AS A FORM OF CONDITIONAL COVERAGE
Conditional coverage can be defined as a recommendation for reimbursement or coverage for a new product with associated limitations that may relate to the eligibility of specified patient groups or to specified dose or duration requirements. Conditional coverage agreements may be designed to limit the budget impact of a new innovation and to focus the promotion and utilization of the product to situations where cost-effectiveness is well defined. Table 1 describes different types of conditional coverage agreements including risk-sharing agreements, dose capping, price-volume agreements, and outcomes-based reimbursement schemes.
Table 1. Description of Types of Conditional Coverage for New Technologies in Health Care

CED, Coverage with Evidence Development.
CED is distinct from other forms of conditional coverage because the stated goal of CED is to generate evidence to validate current decisions and inform future decisions. CED differs from other conditional coverage agreements (e.g., risk sharing) in that it acknowledges that the coverage decision is characterized by uncertainty about the effectiveness of a technology but then goes on to put in place steps to address the uncertainty. One could argue that the other forms of conditional coverage are more limited in that they use uncertainty as a mechanism to adjust financial flows (e.g., by discounting prices or providing rebates under certain conditions) but make no attempt to reduce the uncertainty.
A BRIEF HISTORY OF COVERAGE WITH EVIDENCE DEVELOPMENT
The term CED was coined by the Center for Medicare and Medicaid Services (CMS) in the United States (US) when the organization developed a program to evaluate a specific surgical procedure for the treatment of emphysema. Other terms for CED include “Special Arrangement for Clinical Government Research and Audit” for interventional procedures and “Only in Research” (OIR) for pharmaceuticals in the United Kingdom (UK), “Conditionally Funded Field Evaluation” (CFFE) in Canada. Australia and France use the term CED. France also uses the term “Still in Research,” and Spain considers the practice “Monitored Use.”
In 2006, CMS published a revised guidance document that clarified descriptions of two key elements of CED (2). The document describes “coverage with appropriateness determination,” which refers to the collection of data to confirm that a new technology is being used as described by the coverage decision documentation, and “coverage with study participation,” which refers to situations in which the new technology could be deemed reasonable and necessary if patients were enrolled in a clinical trial but adequate evidence for all necessary standards was lacking (Reference Tunis and Pearson17).
In Canada, CFFE is encouraged for products and innovations with a large potential investment, potentially disruptive effects, and a need for quality control. The application of field evaluations in health technology assessments in Ontario, Canada, is described in a recent publication (Reference Goeree and Levin9).
In the United Kingdom, the National Institute for Health and Clinical Excellence (NICE) does not negotiate the prices for pharmaceuticals or devices. The prices are set by the producer, and NICE conducts assessments and provides guidance for local health authorities. NICE is under remit to avoid rejection of a product because of estimates of total expenditure, but budget impact must be considered part of the evaluation. However, rejection of a product based on cost-effectiveness is implicitly a rejection on the grounds of cost. In 2010, NICE is expected to release guidelines with new suggestions for handling uncertainty including issues surrounding subgroup analyses, optimal stopping rules for therapy, real-world analyses, and scheme evaluations. NICE considers input from patients and the public when developing recommendations through the Citizens’ Council, and the Citizens’ Council has provided feedback on the conditions for applying OIR status (Reference Chalkidou3).
In Australia, the use of CED for pharmaceutical products has been limited. Difficulties with implementation and the limited remit of the Pharmaceutical Benefits Advisory Committee (PBAC), which evaluates submissions received with limited solutions to access for treatments, have contributed to slow uptake of CED. The role of the PBAC is to identify the presence and extent of uncertainty and to decide how that should impact recommendations for reimbursement.
SELECT EXAMPLES OF CED IN PRACTICE
Surgical Procedures and Medical Devices
In the United States, the CMS was concerned about evidence of a rapid uptake in lung volume reduction surgery (LVRS) that appeared to be based on positive results from a small case series. Coverage of LVRS for Medicare patients was limited coverage to patients enrolled in the National Emphysema Treatment Trial (NETT), which began in 1995 and collected 7 years of data (Reference Tunis and Pearson17). Patients (n = 1,218) were randomized to LVRS or nonsurgical treatment. The study cost US$60 million. No survival benefits were observed in the study population or high-risk patient subgroups. The surgery was associated with an increase in mortality. Due to deficiencies in coding designations, accurate tracking of the number of procedures was not possible. However, the code that included LVRS indicated that approximately 5,000 surgeries per year were being conducted before the results of the trial were reported, and that number has decreased to only a few hundred per year in the years since. This is an example of CED for a procedure without a designated producer.
In Ontario, Canada, an observational study was conducted to compare drug-eluting stents (DES) and bare metal stents (BMS) in an observational study using propensity score matched patients (Reference Tu, Bowen and Chiu15). DES were more effective, but greater benefits were observed in subgroups including patients with diabetes, and small and large diameter lesions. The recommendation was that DES should be used in patients who meet the criteria for one or more of these subgroups. While the uptake of DES in the absence of these data is not possible to calculate, researchers believe that the CAN$23 million per year spent on DES is less than it would have been without the program based on uptake rates from the United States.
In Australia, surgical intervention for abdominal aortic aneurysm was diffusing rapidly. In the late 1990s, the Medicare Services Advisory Committee (MSAC) granted limited funding under the condition of evidence development. The Department of Health funded the data collection for 5 years. Results from randomized controlled trials and reviews of data from other countries led the MSAC to recommend unrestricted funding of open aortic repair (11).
Pharmaceuticals
In the United Kingdom, the NHS and four manufacturers have agreed to a CED program that allows 5,000 patients access to beta interferon and glatiramer for the treatment of multiple sclerosis. Access was contingent upon the results of the data collected in patients enrolled in the program. The uncertainty here was around the claimed incremental cost-effectiveness ratio. However, because of the high unmet medical need and the basic plausibility of the claim, coverage was provided but contingent on the collection of data to close the evidence gap. Specifically, the study was designed to identify the cost-effectiveness ratio for the new treatment and compare it with the target threshold of £36,000 per quality-adjusted life-year (8). The expectation is that the prices may need to be adjusted to meet this target, depending on the effectiveness results of the ongoing studies. The study was scheduled to be completed in Fall of 2007; however, currently only limited results and guidance are available (13).
A related example in the United Kingdom is an agreement where manufacturers and payers agreed that bortezomib (Velcade®, Millennium) could be reimbursed for multiple myeloma if data were collected and reported to NICE. The results will inform a NICE recommendation in 2011 (Reference Chalkidou3). However, this program is operating as an outcomes-based risk share, rather than true CED, as the effective price of the product is modulated by the individual patient outcomes being experienced, and the data are not designed to fill an evidence gap.
In Australia, the most comprehensive example of CED as part of a risk-sharing program is that of bosentan monohydrate (Tracleer®) for the treatment of pulmonary artery hypertension (PAH). A study measuring survival outcomes to inform drug-pricing decisions is under way as part of a risk-sharing agreement between the Pharmaceutical Benefits Scheme (PBS) and Actelion Pharmaceuticals. Bosentan is a dual endothelin receptor agonist that has been approved by the Australia's Therapeutic Goods Administration (TGA) and the PBS for the treatment of primary pulmonary hypertension and PAH associated with scleroderma, and PAH associated with a congenital systemic-to-pulmonary shunt (12;14). A recent cost-effectiveness analysis estimated that bosentan costs $A39,300 per patient per year using 2004 PBS costing (Reference Wlodarczyk, Cleland and Keogh19). The Bosentan Patient Register, funded by Actelion, evaluates the survival outcomes of bosenan-treated patients over a 3-year period (1). Enrollment is optional for patients and independent of access to bosentan. Patients who disenroll may retain access to bosentan. However, all bosentan-treated patients receive treatment based on their response to the drug over the previous 6-month period. Nonresponders are denied access to treatment regardless of their registry enrollment status, but nonresponders remain enrolled in the registry. The PBS intends to use the survival data to evaluate the cost-effectiveness results predicted by economic modeling and will use the information to adjust the price of bosentan with the goal of maintaining the incremental cost-effectiveness ratio predicted by the model (Reference Wlodarczyk, Cleland and Keogh19).
AREAS OF CONSENSUS AND CONTENTION SURROUNDING CED
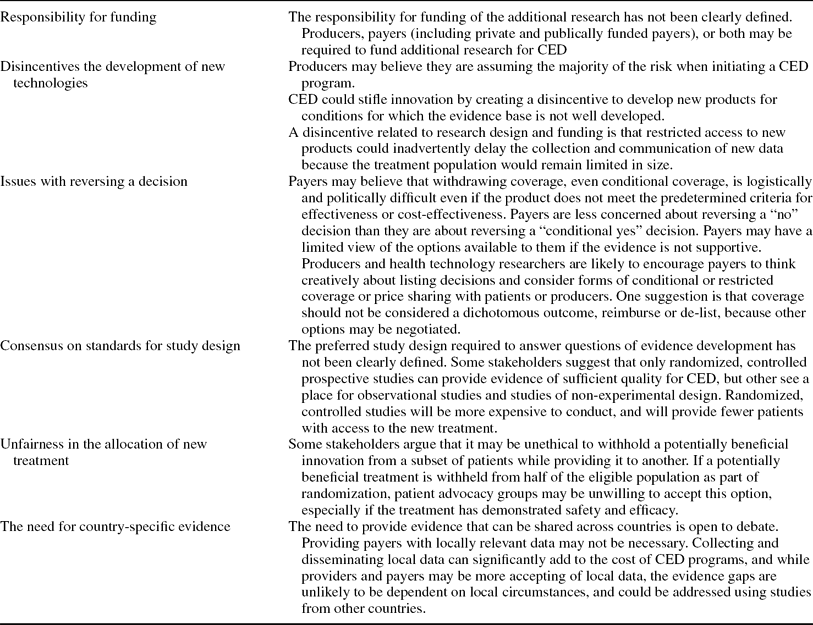
Based on the presentations at the HTAi meeting, the authors identified six areas of agreement between payers and producers (Table 2), and six areas that the authors believe require further consideration and discussion by stakeholders (Table 3). The authors do not consider these issues to be barriers to the uptake of CED, but rather issues to consider when developing programs and studies.
Table 2. Areas of Consensus Among CED Stakeholders

CED, Coverage with Evidence Development.
Table 3. Areas Requiring Future Consideration by CED Stakeholders
CED, Coverage with Evidence Development.
When considering the issues for future consideration, the first two issues listed in Table 3 are potentially the most pressing issues to be addressed by stakeholders. The first area for consideration focuses on the responsibility for funding the additional research required for CED—the payer or the producer. While evidence suggests that public sector coverage of research costs could be offset by price discounts or other pricing agreements (Reference Claxton6), payers are concerned that publically funded, evidence development could inadvertently incentivize producers to provide incomplete submission packages. Other experts believe that payer-funded research is necessary to provide control over research design and data collection (Reference Tunis16). For technologies without producers, insurers could share the costs of research based on a percentage of collected premiums (Reference Steinberg, Tunis and Shapiro18).
Second, the incentive for producers to agree to a CED program is open for debate. The speculation is that producers and HTA agencies may disagree on the adequacy of available data or whether a CED approach is justified. Producers may agree to CED for products that would otherwise receive a negative coverage decision based on the data available at launch. Producers may also be concerned that the likelihood of a price decrease in a scenario where the technology does not perform as well as predicted is more likely than a price increase in situations where the performance of the new technology is better than expected.
Producers are concerned that CED could negatively impact their return on investment by slowing the uptake of their product thus delaying peak sales until near the end of their period of exclusivity. In some cases, this may unintentionally provide an incentive for “fast followers” in a new drug class or for new type of technology by placing a greater evidence burden on the first product to market. This concern illustrates the need to regard evidence development as fit for purpose and plan accordingly
Suggested Criteria for CED
While stakeholders appear to be in agreement that CED is not appropriate for all types of interventions, the criteria for determining the situation for which CED should be applied remains unclear. Some stakeholders believe that CED should only be used in situations where material uncertainty exists, but what may be deemed as “material” to a payer may not seem so to the producer and vice versa. A second suggestion is that CED is more likely to be applied when treatments may be highly effective for a subset of patients and less likely to be effective for a large group of patients. Studies designed to measure the safety and efficacy of a new technology may not be appropriately designed or powered to determine efficacy or safety in a subpopulation. Payers may be interested in restricting coverage to a subpopulation and a CED program may be appropriate for defining the characteristics of the target subpopulation. A third observation is that CED could provide a new coverage option for technologies with incomplete evidence and the potential to diffuse quickly. A final observation is that CED is more likely to be applied in conditions with high unmet need.
This study proposes the following six criteria to evaluate new technologies to ascertain if CED could be appropriate: (i) High unmet clinical need or significant improvement in outcomes is still required. (ii) The value proposition for the product in question is logical and theoretically valid, but one or more pieces of evidence are still lacking. In some cases, the evidence that is lacking may relate to the impact of the treatment on long-term outcomes, and stakeholders may need to identify one or more suitable interim markers of effectiveness that will help resolve the uncertainty. (iii) Data collection is the best solution to resolve the uncertainty (and can be done in a “fit for purpose” manner, such as a RCT, observational study, registry, or other well-designed study). (iv) More traditional coverage tools are not appropriate (e.g., price-volume agreements) to resolve the clinical or cost-effectiveness uncertainty. (v) The primary concern is uncertainty surrounding clinical or cost-effectiveness outcomes and not purely financial/budget impact. (vi) Stakeholders agree that the evidence development can be achieved in a timely manner to ensure that the context of the findings is still relevant within the current operating environment.
When used selectively for innovative procedures, pharmaceuticals, or devices in the appropriate disease areas, CED may help provide access to promising medicines or technologies while data that will minimize the uncertainty that surrounds key issues are collected. However, steps should be taken to ensure that the benefits of additional evidence are greater than the cost of a delayed decision. Claxton and others have described approaches to Value of Information analysis (Reference Claxton5;Reference Claxton and Sculpher7). Whereas this is an important perspective, there will be circumstances where the most pragmatic approach is to undertake CED. Whether or not the Value of Information justifies the conduct of the CED will relate to the unmet clinical need, the type of data required, and the ease of agreeing the action to be taken when the further evidence is available (whether that is supportive or not).
CED has been used successfully in several countries, albeit mainly for devices and nonpharmaceutical interventions. This study illustrates the areas of consensus and the areas of outstanding issues to assist stakeholders in determining the appropriateness of the applicability of CED for a particular innovation. Further experience, especially with pharmaceuticals, will demonstrate the benefits and shortfalls of CED. As more examples of CED become available, the authors are confident that consensus around many of the elements is likely to develop.
CONTACT INFORMATION
Paul Trueman, MA (Paul.Trueman@brunel.ac.uk), Professor of Health Economics, Health Economics Research Group, Brunel University, Uxbridge, Middlesex UB8 3PPH, UK
David L. Grainger, BSc (grainger_david@lilly.com), Global Public Policy Director, Department of Corporate Affairs, Eli Lilly and Company, Lilly Corporate Center, McCartey Street, Indianapolis, Indiana 46285, Kristen E. Downs, MSPH (ked@relevanthealthoutcomes.com), President, Relevant Health Outcomes, Inc., 514 Riverside Drive, Morganton, North Carolina 28655





