Introduction
Type I programmed cell death (PCD) is characterized by DNA condensation and fragmentation and caspase-mediated cell death, whereas Type II PCD is characterized by autophagy, vacuolation and necrosis (Suzanne & Steller, Reference Suzanne and Steller2013). It is well known that caspase mediates apoptosis, nevertheless regulation of autophagy is under scrutiny in different model systems. Though Types I and II PCD showed typical symptoms, both are inter-connected, atleast in some models. Biological functions of autophagy are defined to be protective in early stages of stress/diseased conditions, whereas it favours cellular collapse on extreme degradation. Under autophagy, after fusion of lysosomes with autophagosome membrane, engulfed cellular cargo is degraded and recycled back into cytoplasm (Mizushima et al., Reference Mizushima, Levine, Cuervo and Klionsky2008). Autophagy is reported to activate the caspase-regulated apoptosis in different systems including Drosophila (Nezis et al., Reference Nezis, Shravage, Sagona, Johansen, Baehrecke and Stenmark2010), but a threshold level of caspase only could activate the PCD (Florentin & Arama, Reference Florentin and Arama2012). These processes are under regulation of genes such as autophagy-related genes (Atg) and apoptosis-associated genes (Jehn & Osborne, Reference Jehn and Osborne1997; Lorenzo et al., Reference Lorenzo, Susin, Penninger and Kroemer1999; He & Klionsky, Reference He and Klionsky2009; Li et al., Reference Li, Deng, Yang, Huang, Tettamanti, Cao and Feng2010; Ravikumar et al., Reference Ravikumar, Sarkar, Davies, Futter, Garcia- Arencibia, Green-Thompson, Jimenez-Sanchez, Korolchuk, Lichtenberg, Luo, Massey, Menzies, Moreau, Narayanan, Renna, Siddiqi, Underwood, Winslow and Rubinsztein2010; Suganuma et al., Reference Suganuma, Ushiyama, Yamada, Iwamoto, Kobayashi and Ikeda2011). The Atg were reported from yeast and many eukaryotic organisms including Drosophila and Bombyx mori (He & Klionsky, Reference He and Klionsky2009; Zhang et al., Reference Zhang, Hu, Li, Li, Deng, Yang, Cao and Zhou2009; Inoue & Klionsky, Reference Inoue and Klionsky2010), of which Atg5 is associated with membrane genesis, inactivation of which suppressed autophagy, led to diseased conditions (Simonsen et al., Reference Simonsen, Cumming and Finley2007). However, Atg5 performs not only the autophagic role but provokes apoptotic cellular death also (Yousefi et al., Reference Yousefi, Perozzo, Schmid, Ziemiecki, Schaffner, Scapozza, Brunner and Simon2006). Overexpression of another proapoptotic gene hid activated autophagy under starvation in different tissues of Drosophila; however, Bruce protein promoted degradation of hid and negatively regulate autophagy (Hou et al., Reference Hou, Hannigan and Gorski2009) exhibiting the inter-relation between autophagy and apoptosis.
Apoptosis-inducing factor (AIF) is a flavoprotein originally locates in mitochondria and relocates to cytosol and nucleus under diseased or stress conditions (Susin et al., Reference Susin, Lorenzo, Zamzami, Marzo, Brenner, Larochette, Prévost, Alzari and Kroemer1999). In the nucleus, AIF induces chromatin condensation and DNA fragmentation leading to apoptosis, in the absence of caspase (Daugas et al., Reference Daugas, Susin, Zamzami, Ferri, Irinopoulou, Larochette, Prévost, Leber, Andrews, Penninger and Kroemer2000). However, in β-cells of islets of Langerhans, AIF gene is expressed and protects it from oxidation stress (Schulthess et al., Reference Schulthess, Katz, Ardestani, Kawahira, Georgia, Bosco, Bhushan and Maedler2009). But caspase triggers permeability of the mitochondrial membrane and release of cytochrome C and/or AIF (Guo et al., Reference Guo, Srinivasula, Druilhe, Fernandes-Alnemri and Alnemri2002; Van Loo et al., Reference Van Loo, Demol, van Gurp, Hoorelbeke, Schotte, Beyaert, Zhivotovsky, Gevaert, Declercq, Vandekerckhove and Vandenabeele2002). Recent studies showed a cross talk between apoptosis and autophagy, which ensures the balance between survival and death of cell (Gordy & He, Reference Gordy and He2012).
Gene activation associated with apoptosis in epithelial cells induced by macroparasitic infection is least examined in any invertebrate model systems. Though manipulations of host apoptosis by microbes were reported in mammalian model systems (Ubol et al., Reference Ubol, Tucker, Griffin and Hardwick1994; Sinai et al., Reference Sinai, Payne, Carmen, Hardi, Watson and Molestina2004), reports from the invertebrate systems are scanty (Minguez et al., Reference Minguez, Brule´, Sohm, Devin and Giambe´rini2013). Recently, we have shown immunocompetence of integument in the B. mori larvae exhibited after infection by the eukaryotic parasitoid, Exorista bombycis. Under the parasitic influence, integumental epithelium showed cellular responses such as signs of autophagy and apoptosis and activation of humoral immune components (Pradeep et al., Reference Pradeep, Anitha, Awasthi, Babu, Geetha, Arun, Chandrashekhar, Rao and Vijayaprakash2012). However, it is not known how parasitism regulates apoptosis in most model systems, particularly in invertebrates. In order to unravel the mechanism involved, we examined the activation of apoptosis-associated genes and its expression pattern in the larval integument of the lepidopteran model B. mori after infection by the tachnid parasitoid, E. bombycis.
Materials and methods
Materials
Larvae of mulberry silkworm, B. mori were reared under a photoregime of 13L: 11D, at 26±1 °C and humidity of 85% and fed with mulberry leaves. Day 1 fifth (final) instar larvae were exposed to mated gravid females of E. bombycis for an hour, for oviposition and is considered as the 0 h of infection.
Methods
The integumental tissue from control and infected larva of B. mori was dissected out and processed for transmission electron microscopy (TEM) as described earlier (Pradeep et al., Reference Pradeep, Anitha, Awasthi, Babu, Geetha, Arun, Chandrashekhar, Rao and Vijayaprakash2012). From integument, protein was extracted using total protein extraction reagent (T-PER; Thermo-Pierce) and quantified (Lowry et al., Reference Lowry, Rosebrough, Farr and Randall1951). The integumental protein was resolved by 10% SDS-PAGE and two-dimensional (2D) electrophoresis. Exclusive bands observed from parasitized tissue in the PAGE and 2D matrix were cut and processed for mass spectrometry and peptide mass fingerprinting (MASCOT). From another batch, integument was dissected out and kept in RNA stabilization reagent, RNA Later (Qiagen). Total RNA was extracted using a phenolic solution, RiboZol (Amresco). cDNA was synthesized from 1 μg total RNA using oligo d(T) primer (Protoscript; New England Biolabs) as per manufacture's protocol and amplified using gene-specific primers designed (Primer 3) from the mRNA sequences obtained from the NCBI database (table 1). For real time analysis, MESA GREEN qPCR Master Mix Plus for SYBR® Assay I Low ROX was used (Eurogentec, Belgium) on Agilent Stratagene Mx3005P qPCR system.
Table 1. Key to the apoptosis-associated genes showing differential expression in integumental epithelium of B. mori larvae after infection with the parasitoid E. bombycis.

1 Calculated molecular weight as per NCBI website.
2 Primer from Pradeep et al. (Reference Pradeep, Anitha, Awasthi, Babu, Geetha, Arun, Chandrashekhar, Rao and Vijayaprakash2012).
At least two replications of quantitative expression analysis (qPCR) experiments using independent templates from control and parasitized samples were performed at five different time points at 24 h intervals after infection. After performing the qPCR, the reaction mixture was resolved on 1.5% agarose gel and confirmed the single amplicon of predicted molecular weight and primer specificity.
Statistical analyses
Quantitation of gene expression in the parasitized tissue relative to the calibrator (defined as 1.0) was calculated using Mx3500P qPCR software (Agilent). Average threshold cycle (C t) value of transcript expression was calculated by ΔΔ C t method (Livak & Schmittagen, Reference Livak and Schmittagen2001) from the duplicates and normalized with the house-keeping gene, β-actin. Comparative C t values of the gene of interest were standardized by C t values for the control gene β-actin. C t values were standardized relative to the average value for the control, yielding the delta C t value, and these values were standardized to make the average control value ‘1’ (the ΔΔ C t values) (Gerardo et al., Reference Gerardo, Altincicek, Anselme, Atamian, Barribeau, de Vos, Duncan, Evans, Gabaldón, Ghanim, Heddi, Kaloshian, Latorre, Moya, Nakabachi, Parker, Pérez-Brocal, Pignatelli, Rahbé, Ramsey, Spragg, Tamames, Tamarit, Tamborindeguy, Vincent-Monegat and Vilcinskas2010). Fold change in gene expression relative to the calibrator was calculated, which allowed to display the down regulated relative quantities as negative values. The data (mean±SE) represented is the gene expression induced after parasitism, excluding the changes in control introduced by injection or developmental processes. Moreover, this calculation allowed displaying the downregulated relative quantities as negative values when fold change in gene expression was calculated.
Significance of variation between control and infected samples was tested by Student's t-test. Correlation in expression levels between the genes, Atg 5, AIF and caspase was analysed by linear regression analysis.
Results and discussion
Infection of B. mori larvae by E. bombycis maggot induced signs of apoptosis in the integumental epithelial cells. Formation of autophagic vesicles, accumulation of protein in vacuoles, dilation and degranulation of cisterne and emptying of rough endoplasmic reticulum in to vacuoles were noticed in a temporal manner after the infection. During infection, the epithelial nuclear morphology showed gradual variation from branched morphology in the early stages to an unorganized one in the later stages (fig. 1). Moreover genomic DNA showed condensation and fragmentation in the later stages of infection. Similar signs of autophagy, apoptosis and cellular death were noticed during PCD occurred during metamorphosis of insects (Misch, Reference Misch1965; Li et al., Reference Li, Deng, Yang, Huang, Tettamanti, Cao and Feng2010). A composite TEM profile of the integument of the parasitized B. mori larva demarcated the infection-induced autophagy in the early stage, its progress in the middle stage followed by apoptosis in the later stage of infection.

Fig. 1. Induction of apoptosis in the epithelium followed by lysis of integument of final instar larvae of B. mori after infection through the parasitoid E. bombycis: (a) Saline injected 72 h control larvae showed a spot of melanization at the point of needle injection; no lysis was observed in the surrounding area of cuticle. However at 72, 96 and 120 h post infection (hpi) (b–d), progressive increase in lysis area was observed (arrows) under stereo zoom microscope (magnification: 400× each). TEM observation on the changes in nucleus of integumental epithelium: Nucleus of 24 h control larvae (e) and 24 h post infected (hpi) larvae (f) showed branched nuclear membrane and uniformly distributed chromatin material however did not show variations in nuclear morphology. In 48 hpi larvae of B. mori (g), the cytoplasm was free of organelles, but with network of cytoplasmic channels. At 96 hpi (h), the nuclei (arrows) become deformed, pyknotic, lost ramifications and with condensed chromatin. BL, basal lamina; CT, cuticle; N, nucleus.
Infection of B. mori larva by E. bombycis has induced an enhancement in production of immune- and host-response proteins in the integument as revealed earlier by 2D electrophoresis and SDS-PAGE coupled with mass spectrometry (Pradeep et al., Reference Pradeep, Anitha, Awasthi, Babu, Geetha, Arun, Chandrashekhar, Rao and Vijayaprakash2012). The analysis showed activation of signalling, melanization, proteolysis and humoral immunity components. Genes encoding these immune-associated proteins showed expression in a co-regulatory way in the control larvae whereas the co-regulation was lost in different sets of genes after the infection (Anitha et al., Reference Anitha, Pradeep, Awasthi, Murthy, Ponnuvel, Sasibhushan and Rao2013).
Advanced analysis showed activation of proteins associated with autophagy and apoptosis in the integumental epithelium. Three key proteins associated with Types I and II apoptosis viz. autophagy 5-like (Atg5L), AIF and Nedd2-like caspase were identified by mass spectrometry from the infected larval integument. Activation of genes encoding these apoptosis-associated proteins in the integumental epithelium after the infection was examined by qPCR. In early stages of infection, Atg5 gene showed upregulation till 48 h after infection, followed by significant (P<0.045) decrease (fig. 2a). On the other hand, AIF gene showed lower level of relative expression in the early stages of infection followed by gradual increase to significantly (P<0.001) greater expression at 96 h and 120 h after infection and correlation (R 2=0.75) with increase in age (fig. 2b). Consequently in the infected larvae, relative expression of AIF showed negative correlation (R 2=0.4698) with that of Atg5 (table 2). However, Atg5 showed larger level of quantitative expression than AIF gene.

Fig. 2. Relative expression of genes encoding apoptosis-associated proteins in the integumental epithelium of final instar larvae of B. mori after infection through the parasitoid E. bombycis. (a) Atg5 showed upregulation in the early stages whereas (b) AIF showed upregulation in the later stages of infection. Allometric line represents the linear relation between AIF expression and larval age. (c) Caspase showed consistent expression throughout infection with a dip at 72 h post infection. (d) Fold change in expression of Atg5, AIF and caspase at various time points after infection. Caspase was upregulated before the AIF increase and also in the later stages of infection, indicating genetic regulation of organized cell death events induced by the parasitic infection. The transcript level was normalized with that of the house keeping gene, β-actin. Each point represents mean of two independent analysis (mean±SE) performed at five time points (age in hours) after infection. Quantitation of gene expression in the parasitized tissue relative to the calibrator (defined as 1.0) was calculated using Mx3500P qPCR software (Agilent). Average C t value of transcript expression was normalized with the house-keeping gene, β-actin. The C t values were standardized relative to the average value for the control treatment, yielding the delta C t value, and these values were standardized to make the average control treatment value ‘1’ (the ΔΔ C t values) (Gerardo et al., Reference Gerardo, Altincicek, Anselme, Atamian, Barribeau, de Vos, Duncan, Evans, Gabaldón, Ghanim, Heddi, Kaloshian, Latorre, Moya, Nakabachi, Parker, Pérez-Brocal, Pignatelli, Rahbé, Ramsey, Spragg, Tamames, Tamarit, Tamborindeguy, Vincent-Monegat and Vilcinskas2010). Thus the gene expression induced after parasitism is presented here, excluding the changes in control introduced by injection or developmental processes. Moreover, this calculation allowed displaying the down regulated relative quantities as negative values when fold change (d) in gene expression was calculated.
Table 2. Correlation between relative expression of apoptosis-associated genes in the integumental epithelium of B. mori final instar larvae infected by the parasitoid, E. bombycis.
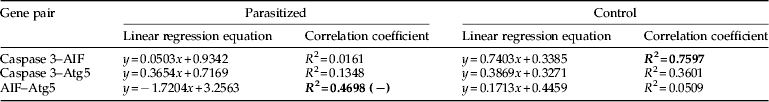
AIF, apoptosis-inducing factor; Atg5, autophagy-related gene 5; (−) indicates negative correlation.
Bolded values indicate higher correlation coefficients.
In the earlier stages of infection, upregulation of Atg5 gene coincides with appearance of autophagic vacuoles at 24 h after infection, unswerving with its role in initiation of autophagy (Sakoh-Nakatogawa et al., Reference Sakoh-Nakatogawa, Matoba, Asai, Kirisako, Ishii, Noda, Inagaki, Nakatogawa and Ohsumi2013; Tian et al., Reference Tian, Ma, Guo, Deng, Ma, Xia, Cao and Li2013), which supports the viability of cell (Palmer et al., Reference Palmer, Wittrock and Christensen1986; Schaub & Sehnitker, Reference Schaub and Sehnitker1988; Pinheiro et al., Reference Pinheiro, Silva and Gregorio2010). However, when the parasitic influence is intensified on the epithelial cells, relative expression of Atg5 was decreased in the B. mori larvae (fig. 2a). It is reported that Atg5 enhances cellular susceptibility towards apoptosis showing that Atg5 is required not only for inducing autophagosome formation (Scott et al., Reference Scott, Schuldiner and Neufeld2004; Berry & Baehrecke, Reference Berry and Baehrecke2007), but also acts as a molecular link between autophagy and apoptosis (Yousefi et al., Reference Yousefi, Perozzo, Schmid, Ziemiecki, Schaffner, Scapozza, Brunner and Simon2006). However, in the lepidopteran Galleria mellonella, Atg8 expression was noticed in different tissues during larval-pupal transformation and before the PCD (Khoa & Takeda, Reference Khoa and Takeda2012).
Continuous presence of the parasite in vicinity of integument induced prolonged autophagy followed by integumental lysis (fig. 1a–d), which was accompanied by increase in AIF and caspase expression (fig. 2). Both the genes are encoding enzymes that stimulate apoptosis. Caspase showed increase in expression at 48 h followed by decrease at 72 h after infection. Subsequent increase was noticed in the later stage of infection, at 120 h after infection. The initial rise in caspase expression is associated with cellular protection while the later enhancement is related with cellular death (Martin & Baehrecke, Reference Martin and Baehrecke2004; Liu et al., Reference Liu, Tang, Fu, Peng, Yang, Li and Hong2007; LeBlanc & Saleh, Reference LeBlanc and Saleh2009; Suganuma et al., Reference Suganuma, Ushiyama, Yamada, Iwamoto, Kobayashi and Ikeda2011). In the control B. mori larvae, relative expression of caspase showed marginal positive correlation with that of Atg5 gene (R 2=0.36), whereas it showed larger positive correlation (R 2=0.76) with AIF expression (table 2). When the cells were under stress, AIF, located in the mitochondria is translocated to cytosol and then to nucleus, and induced DNA fragmentation leading to cell death (Lorenzo et al., Reference Lorenzo, Susin, Penninger and Kroemer1999; Joza et al., Reference Joza, Susin, Daugas, Stanford, Cho, Li, Sasaki, Elia, Cheng, Ravagnan, Ferri, Zamzami, Wakeham, Hakem, Yoshida, Kong, Mak, Zúñiga-Pflücker, Kroemer and Penninger2001; Cande´ et al., Reference Cande, Cecconi, Dessen and Kroemer2002a , Reference Cande, Cohen, Daugas, Ravagnan, Larochette, Zamzami and Kroemer b , Reference Cande´, Vahsen, Garrido and Kroemer2004). Moreover, the DNA fragmentation is accompanied by upregulation of caspase gene expression, in the later stages of parasitism (fig. 2c) confirming that apoptosis is mediated by a combined action of AIF and caspase. However, the relation and/or regulation between caspase expression and other genes become void after the infection (table 2) showing derailing of regulation on caspase activity under the parasitic influence (Castino et al., Reference Castino, Isidoro and Murphy2005; LeBlanc & Saleh, Reference LeBlanc and Saleh2009). Similar loss of co-regulation of other host-response genes with caspase expression was reported (Anitha et al., Reference Anitha, Pradeep, Awasthi, Murthy, Ponnuvel, Sasibhushan and Rao2013). Co-regulated caspase expression seems essential to prevent surge in caspase activity in the final larval instar, which is under the endocrine control during larval-pupal transformation (Liu et al., Reference Liu, Sheng, Liu, Wen, He, Wang, Shao, Jiang, An, Sun, Bendena, Wang, Gilbert, Wilson, Song and Li2009). The deregulatory gene activities induced by the parasitism in B. mori shows the requirement of an effector caspase to control both autophagy and apoptosis as noticed in Drosophila (Hou et al., Reference Hou, Hannigan and Gorski2009).
Fold increase in expression of cell death-associated genes at 24 h after infection showed upregulation of caspase gene and downregulation of AIF gene in the early stage of infection (fig. 2d). This revealed an early activation of caspase gene before the upregulation of AIF, confirming the observations in Drosophila (Susin et al., Reference Susin, Daugas, Ravagnan, Samejima, Zamzami, Loeffler, Costantini, Ferri, Irinopoulou, Prévost, Brothers, Mak, Penninger, Earnshaw and Kroemer2000; Gabriel et al., Reference Gabriel, Sureau, Casselyn, Teissié and Petit2003). Apoptosis preceded by autophagy was also reported during larval–pupal transformation of midgut in B. mori (Franzetti et al., Reference Franzetti, Huang, Shi, Xie, Deng, Li, Li, Yang, Zeng, Casartelli, Deng, Cappellozza, Grimaldi, Xia, Feng, Cao and Tettamanti2012). But no caspase activation was observed during PCD of larval fat body in Manduca sexta (Muller et al., Reference Muller, Adori and Sass2004). Present observations confirm the co-occurrence of both autophagy and apoptosis and an organized PCD (Zhang et al., Reference Zhang, Hu, Li, Li, Deng, Yang, Cao and Zhou2009, Reference Zhang, Pan, Sun, Huang, Yu, Liu, Zhao and Lu2010) induced by the parasitoid infection in the integument of B. mori.
In summary, we observed early upregulation of Atg5 gene in association with appearance of signs of autophagy and late upregulation of AIF gene in association with nuclear deformation, DNA condensation and fragmentation. Caspase gene was active throughout the infection period. The expression profile of Atg5 showed negative correlation with that of AIF in the infected larvae led to a novel observation of regulated expression of these apoptosis-associated genes in the parasitized epithelium. Enhanced expression of Atg5, AIF and caspase genes coupled with the appearance of cell death symptoms indicate parasitism-induced activation of genetic machinery to modulate cell death events in the epithelium, which was hither to unknown in invertebrate systems.
Acknowledgements
The authors express sincere thanks to the reviewers for critical comments. The authors are thankful to Central Silk Board for facilities. AJ was supported by DBT senior research fellowship from the project. The authors are thankful to Springer-Verlag GmbH, Tiergartenstr. 17, 69121 Heidelberg, Germany, for granting permission to reproduce data on caspase expression published by us in Cell and Tissue Research (2013, vol. 352, pp 371–385). The authors acknowledge the financial support received from Department of Biotechnology (DBT), Government of India, New Delhi, in the form of a research project to ARP (BT/PR11722/PBD/19/197/2008 dated 11/6/2009).






