INTRODUCTION
Invertebrates are inhabitants of phytal substrates such as algae and seagrass (Dunstone et al., Reference Dunstone, O'Connor and Seed1997; Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003; Luizzi & Gappa, Reference Luizzi and Gappa2011). Algae hide animals from predators and the influence of physical characteristics of the environment—wave exposure, currents and ice abrasion (Connolly, Reference Connolly1997; Lippert et al., Reference Lippert, Iken, Rachor and Wiencke2001). In addition, algae increase habitat complexity in shallow waters providing the shelter for a great variety of organisms. In the Arctic seas a variety of habitats exists and a significant number of them is provided by different red algae species. Most studies of host algae have focused on fauna associated with a single red algae species (Valério-Berardo & Flynn, Reference Valério-Berardo and Flynn2002; Bussell et al., Reference Bussell, Lucas and Seed2007; Izquierdo & Guerra-García, Reference Izquierdo and Guerra-García2010) while reviews on epibionts of several species of red algae are fewer in number (e.g. Norderhaug, Reference Norderhaug2004).
Amphipods are among crustaceans inhabiting different phytal substrates. Species number in this group in the Arctic and subarctic seas appear to be significantly greater than other Malacostraca taxa dominating the marine benthic macrofauna, i.e. Cumacea, Isopoda and Decapoda (Sirenko, Reference Sirenko2001). Amphipods inhabit phytal substrates (Makkaveeva, Reference Makkaveeva1959, Reference Makkaveeva1963, Reference Makkaveeva and Vodyanitskiy1967; Denton & Chapman, Reference Denton and Chapman1991; Myers, Reference Myers1993; Conlan, Reference Conlan1994; Scipione, Reference Scipione, Schram and Vaupel Klein1999; Poore et al., Reference Poore, Watson, de Nys, Lowry and Steinberg2000) as well as such animal hosts as sponges (Amsler et al., Reference Amsler, McClintock, Amsler, Angus and Baker2009), ascidians (Sepúlveda et al., Reference Sepúlveda, Cancino and Thiel2003), cnidarians (Vader & Lönning, Reference Vader and Lönning1973), echinoderms (Vader, Reference Vader1978), molluscs (Vader & Beehler, Reference Vader and Beehler1983), brachyuran crustaceans (Dvoretsky, Reference Dvoretsky and Matishov2008) and vertebrates (Rowntree, Reference Rowntree1996). In fact, there is no commonly used procedure to study such association. In early studies the dredging in the algal beds used to be a common technique (Makkaveeva, Reference Makkaveeva1959, Reference Makkaveeva1963, Reference Makkaveeva and Vodyanitskiy1967; Rybnikov, Reference Rybnikov and Turpaeva1993; Raffaelli, Reference Raffaelli2000); few studies involved field and laboratory experiments (Raffaelli, Reference Raffaelli2000; Kley et al., Reference Kley, Kinzler, Schank, Mayer, Waloszek and Maier2009). In the last decades, underwater sampling and observations were applied to reveal patterns of the occurrence of amphipods on marine plants (Denton & Chapman, Reference Denton and Chapman1991; Rybnikov, Reference Rybnikov and Turpaeva1993). Association of amphipods with red algae was extensively studied in various marine regions (Makkaveeva, Reference Makkaveeva1959, Reference Makkaveeva1963, Reference Makkaveeva and Vodyanitskiy1967; Grese, Reference Grese1977; Rybnikov, Reference Rybnikov and Turpaeva1993; Norderhaug, Reference Norderhaug2004; Espinosa & Guerra-García, Reference Espinosa and Guerra-García2005; Izquierdo & Guerra-García, Reference Izquierdo and Guerra-García2010). At the same time Arctic and subarctic amphipods (in contrast to many malacostracan taxa that reach a remarkably high diversity in the polar seas of the northern hemisphere (Sirenko, Reference Sirenko2001; Piepenburg et al., Reference Piepenburg, Archambault, Ambrose, Blanchard, Bluhm, Carroll, Conlan, Cusson, Feder, Grebmeier, Jewett, Lévesque, Petryashev, Sejr, Sirenko and Włodarska-Kowalczuk2011)) are still poorly studied in that respect (Lippert et al., Reference Lippert, Iken, Rachor and Wiencke2001; Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003).
The White Sea is of particular interest for such studies because this basin holds a combination of Arctic and northern temperate conditions (Berger & Naumov, Reference Berger, Naumov, Berger and Dahle2001). The aim of the present study was to identify the composition of an amphipod community occurring in association with the red algae in the upper subtidal environment of Kandalaksha Bay of the White Sea.
MATERIALS AND METHODS
Sampling sites, substrates and techniques
Specimens were collected near Pertzov White Sea Biological Station of Moscow State University (WSBS) in Kandalaksha Bay (Figure 1) in August 2003. The sampling area (66°34′N 33°08′E) occupies the most narrow part of Velikaya Salma Strait and it is well protected from wave action by the shore. A 120 m long transect was set using a marked line. The transect was extended from 0 to 13.6 m depth and the line marks were used to define a position of each sampling station (Table 1). Sampling was undertaken by SCUBA divers. Sampling sites along the transect and in the additional locations were selected according to presence of substantial (>30%) coverage of rhodophytes. At each sampling site a visual description of the bottom landscape was made and the depth was identified using a hand dive-planner. A modified method developed by Grese (Reference Grese1977) was applied to collect red algae together with associated amphipods. Each sample was collected from the seabed area of approximately 0.07 m2. In total 21 samples were taken (Table 3).
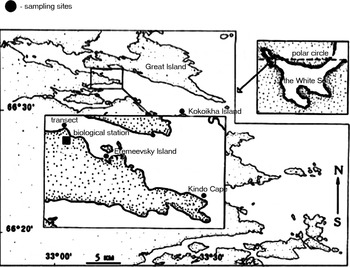
Fig. 1. Map of the study site.
Table 1. Description of the transect.

Six common macroalgal species represented in various proportions were studied as substrates for amphipod crustaceans: Ahnfeltia plicata (Hudson) Fries, 1836, Euthora cristata (C. Agardh) J. Agardh, 1847, Odonthalia dentata (L.) Lyngybe, 1819, Phycodrys sp., Phyllophora interrupta (Greville) J. Agardh, 1862 (now considered as a junior synonym of Coccotylus truncatus (Pallas) M.J. Wynne & J.H. Heine, 1992 but traditionally recognized in the White Sea under the first name), Ptilota gunneri P.C. Silva, Maggs & L.M. Irvine 1993 (=Ptilota plumosa (L.) Agardh, 1817; under this name the species is traditionally known in the White Sea). Red algae beds were most common at depths up to 10 m, so most amphipods were collected between 80′ and 100′ transect marks (Table 1). Additional samples were collected near Eremeevskiy Island (site description—muddy sand and small stones; abundant fields of brown/red algae), Cape Kindo (muddy-sand; red and brown algae are mostly at 8–9 depth and deeper) and Kokoikha Island (muddy-sand and small stones; red algae are on the surface of stones and on rhizoids of brown algae). Each algal thallus with associated epifauna was gently dislodged from the attached substrate and quickly but carefully collected in a plastic bag. Algae were examined and large amphipods were picked out; then algae were washed. Washouts were strained (using a 70-µm sieve) and examined under a stereo dissection microscope. Live amphipod specimens were preserved in 70% alcohol, others in 8% formalin. Identification of amphipods was to species level wherever possible using Gurjanova (Reference Gurjanova1951), Barnard & Karaman (Reference Barnard and Karaman1991), Martin & Davis (Reference Martin and Davis2001) and Bousfield & Hoover (Reference Bousfield and Hoover1997). Total length (from the tip of antenna to the apical part of uropod) of specimens was measured to the accuracy of 0.1 mm using an ocular-micrometer. Identified material has been deposited at the Zoological Museum of Moscow State University (registration nos. Mc 1131–1142). Colour photographs of Ampithoe rubricata, Acanthonotozoma inflatum, Crassicorophium bonellii, Pleustes panopla and Socarnes vahlii taken in natural conditions in the WSBS area are presented in Spiridonov et al. (Reference Spiridonov, Kosobokova, Malyutin, Petryashov, Pertsova, Bek, Uryupova, Neretin, Sinelnikov, Kuz'min, Tzetlin, Zhadan and Marfenin2010). Red algae were dried and identified using Zinova (Reference Zinova1955) while the nomenclature was updated using the ALGAEBASE (www.algaebase.org).
Data analysis
The structure of amphipod associations was evaluated by the total number of individuals (N), diversity (Margalef/Menchinik, D(Mg)/D(Mn); Shannon, H′) and dominance (Berger–Parker, d). Indices were calculated for individual samples (Magurran, Reference Magurran2004). The formulae used were:

where S = number of species in a sample, N = total number of individuals, N (max) = number of the most abundant species and ni = number of individuals of the i th species. Kendall's rank-correlation coefficient was used to investigate the association between the abundances of pairs of amphipod species. To visualize the similarity of each sample a non-metric multidimensional scaling (MDS) ordination based on the Bray–Curtis similarity index was plotted giving a position of each red algae sample in two-dimensional space based on its epibiont composition (Bray & Curtis, Reference Bray and Curtis1957). Indices were calculated using SYSTAT 7.0 (SYSTAT Software, Richmond, CA) and PAST (Hammer et al., Reference Hammer, Harper and Ryan2001).
RESULTS
In total 12 species of amphipods were found in association with red algae (Tables 2 & 3). Ampithoe rubricata (synonym based on another spelling Amphithoe rubricata, fide Gurjanova (Reference Gurjanova1951)) was the most abundant species (Table 2). It was found in 16 samples in almost all sampling sites (76% of a total number of samples). Crassicorophium bonellii was found in 17 samples (81% of samples). Gammaropsis melanops had a similar frequency of occurrence; this species was found in 12 samples (57% of samples). In particular, this species was the most abundant at the site near Eremeevskiy Island. Pleusymtes glaber was found in 14 samples (67% of samples) in all observed habitats. Pleustes panopla occurred at ten stations of the transect as well as near Cape Kindo and Kokoikha Island (48% of samples). The other species were neither frequently occurring nor were they numerous (Table 3).
Table 2. Species composition of amphipods collected on red algae in Kandalaksha Bay, the White Sea.

Table 3. Station data, red algae species, composition, diversity and dominance indices of amphipods that occurred on the algae.
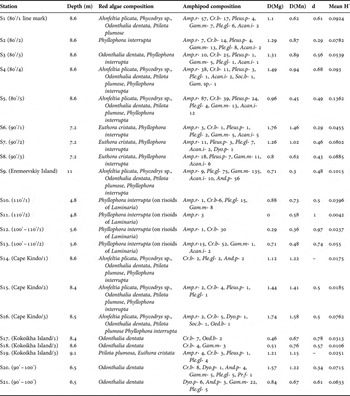
Abbreviations: Amp.r, Ampithoe rubricata; Cr.b, Crassicorophium bonellii; Dyo.p, Dyopedos porrectus; Soc.b, Socarnes bidenticulatus; And.p, Andaniella pectinata; Gam. sp., Gammarus sp.; Pleus.p, Pleustes panopla; Gam.m, Gammaropsis melanops; Ple.gl, Pleusymtes glaber; Acan.i, Acanthonotozoma inflatum; Pr.f, Protomedeia fasciata; Oed.b, Oediceros borealis. D(Mg), Margalef diversity index; D(Mn), Menhinik diversity index; d, Berger–Parker dominance index; H′, Shannon diversity index.
The diversity of amphipod assemblages was analysed using Margalef (D(Mg)) and Menchinik (D(Mn)) indices (Table 3). Maximum D(Mg) = 1.76, 1.74, 1.57 and D(Mn) = 1.46, 1.58, 1.22 respectively, were calculated for Stations 6, 16 and 20. This means the number of species in these samples was maximal for the survey while the species abundances in these assemblages were more or less evenly distributed. Noteworthy, there was no relation to the diversity of red algae: five species were present in sample 16 collected near Cape Kindo (Ahnfeltia plicata, Phycodrys sp., Odonthalia dentata, Ptilota plumosa and Phyllophora interrupta), two species (Euthora cristata and P. interrupta) occurred at Station 6, only O. dentata was found at Station 20. Minimum D(Mg) = 0, 0.29 and D(Mn) = 0.58, 0.36 values were at Stations 11 and 12. These samples (11 and 12) were characterized by the minimum number of species, e.g. only Ampithoe rubricata occurred at Station 11. Mean Shannon diversity indices (H′) ranged from 0.0042 (sample 11) to 0.1362 (sample 5) (Figure 2). No correlation between diversity indices of amphipods and the number of algae species was found. The Berger–Parker index (d) was used to calculate species dominance in the assemblages (Table 3). The results ranged from 0 (minimum value in samples 14, 19 and low value in samples 2, 6) to 1 (single species assemblage at Station 11). The dominant species were as follows: A. rubricata in 7 samples (d ranged from 0.43 to 1); C. bonellii in 8 samples (0.29–0.97); G. melanops in 3 samples (0.29–0.61); and Pleusymtes glaber in 1 sample (d = 0.25).

Fig. 2. Mean Shannon diversity index (H′) values for each sample.
The Kendall's rank-correlation coefficient (Table 4) showed weak negative correlation between abundances of Andaniella pectinata/Ampithoe rubricata and A. pectinata/Pleustes panopla. The dominant species A. rubricata was found together with P. panopla and Acanthonotozoma inflatum. The group of species: Andaniella pectinata, G. melanops and Pleusymtes glaber was characterized by statistically significant positive cross-correlation. Acanthonotozoma inflatum was less abundant but found in the samples with Ampithoe rubricata, G. melanops and P. glaber. Crassicorophium bonellii did not show correlation with any other species. In general, the values of Kendall's rank-correlation coefficient were not high and ranged from 0.34 to 0.66 (Table 4) which meant a lack of clear trend in amphipod co-occurrence on red algae.
Table 4. Values of Kendall's rank-correlation coefficient calculated between the abundances of particular amphipod species; species occurring at a single station only are excluded.

Abbreviations: Amp.r, Ampithoe rubricata; Cr.b, Crassicorophium bonellii; Dyo.p, Dyopedos porrectus; Soc.b, Socarnes bidenticulatus; And.p, Andaniella pectinata; Pleus.p, Pleustes panopla; Gam.m, Gammaropsis melanops; Ple.gl, Pleusymtes glaber; Acan.i, Acanthonotozoma inflatum; Pr.f, Protomedeia fasciata; Oed.b, Oediceros borealis. Statistical significance: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
According to the MDS plot there is weak correlation between the amphipod assemblages and the location, the depth, and composition of algal substrate (Figure 3). The most abundant and rich in species group (with dominance of A. rubricata) occurred on multi-species red algae assemblages at depth about 8.6 m of the transect (area protected from the waves). The diverse and abundant amphipod assemblages are on the multispecies algal substrate while less diverse and abundant amphipod associations are on Phyllophora interrupta and kelp rhizoids, and on the substrate including Odonthalia dentata (Figure 3, upper part of the plot). The abundance of A. rubricata decreased along this gradient up to the complete absence of this species on O. dentata. The abundance of C. bonellii remained relatively constant within the described line while the contribution of Pleusymtes glaber and/or G. melanops considerably varied (Table 3). The diverse amphipod assemblage in the transect (8.6 m depth) differed considerably from the quantitatively poor assemblage inhabiting multispecies algal substrate at similar depths near Cape Kindo that it was sure to be caused by wind and wave action. However, the amphipod assemblage from Cape Kindo preferred such algal assemblages as Euthora cristata + Ptilota plumosa and E. cristata + Phyllophora interrupta at depths from 7.2 to 9.1 m along the transect. An assemblage associated with the red algae/sponge biotope near Eremeevskiy Island differed from other amphipod groups by a strong co-dominance between Pleusymtes glaber, G. melanops and Andaniella pectinata (Figure 3; Table 3).
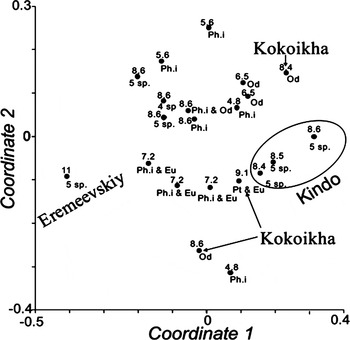
Fig. 3. Multidimensional scaling (MDS) plot based on a presence/absence Bray–Curtis similarity matrix of amphipod species collected from each red algae assemblage. Arabic numerals (i.e. 5.6 etc.) show the depth of sampling in metres, abbreviations indicate the red algae species: Eu, Euthora cristata; Od, Odonthalia dentata; Ph.i., Phyllophora interrupta; Pt, Ptilota plumosa; 5 sp., mixed assemblage of five species; 4 sp., mixed assemblage of four species.
The main trends are: multispecies red algae substrates provide a habitat more abundant and rich in species than amphipod assemblages; there is no clear evidence of a relationship between amphipods and location, depth or algal composition.
DISCUSSION
Ecology of the amphipod species collected in red algae biotopes
Data on biotopes are available only for some species collected in our study. According to our results Ampithoe rubricata, one of the common species of the red algae biotope, occurs mostly on the multi-species algae substrate but appears to avoid Odonthalia dentata at the transect. Dredging in the inner Kandalakasha Bay revealed A. rubricata to occur on various types of sediments from mud to stones but mostly within the growing zone of algae (Grishankov et al., Reference Grishankov, Ninburg and Shkliarevich2000). In recent studies it was reported to be abundant in shallow areas of Onega Bay in the community dominated by polychaetes as well as in the kelps with red algae (Golikov, Reference Golikov1985). In other areas A. rubricata building tubes using plant material (tube construction is probably facilitated by secretion of glands present on pereopods 1 and 2) was reported to live under stones and on algae (Skutch, Reference Skutch1926). Skutch's study showed that A. rubricata occurred on various algae, including six species of rhodophytes, three species of chlorophytes and two species of brown algae, also living near rhizomes of seagrass Zostera marina L. Experimental studies (Norderhaug, Reference Norderhaug2004) emphasized the importance of red algae as a food source for this species. The grazing habit of A. rubricata is also indicated by the morphology of its mouthparts—mandibles with well-developed cutting edge and rigid setae on maxillae (Uryupova, Reference Uryupova2005). Different species of red algae may also have a different nutritional value for this amphipod (Norderhaug, Reference Norderhaug2004). In general, the species of the genus Ampithoe were reported to live on various algal substrates (Gurjanova, Reference Gurjanova1951) and feed on this material (McDonald & Bingham, Reference McDonald and Bingham2010). In particular, the amphipod assemblage living in the community of Phyllophora nervosa (A. De Candolle) Greville, 1830 (current accepted name P. crispa (Hudson) P.S. Dixon, 1964) in the Black Sea had similar number of species (11–19) to the White Sea and was dominated by Ampithoe vaillanti Lucas, 1846 (Makkaveeva, Reference Makkaveeva1963; Grese, Reference Grese1977; Rybnikov, Reference Rybnikov and Turpaeva1993). It was concluded that similarly to several congeners in temperate waters A. rubricata may be regarded as a dominant species in the association of amphipod taxa living in the red algae biotopes in the White Sea. This species uses red algae as both the source of food and the shelter (Norderhaug, Reference Norderhaug2004) but apparently it is not an obligate inhabitant of the red algae communities and occurs in other biotopes mostly associated with macrophyte habitats.
Another common species Crassicorophium bonellii was reported as highly abundant in green filamentous and brown algae beds in Kandalaksha Bay (Ninburg et al., Reference Ninburg, Ivanuyshina and Aleksandrov1986). High density aggregations of this species occupied muddy-sands in the Velikaya Salma Strait (authors, personal observations). Contrary to A. rubricata, C. bonellii is considered to be a deposit seston feeder according to the literature. This conclusion is supported by the morphology of its mouthparts, i.e. dense setation of mandibles and both pairs of maxillae (Uryupova, Reference Uryupova2005). This species prefers mixed assemblages of red algae with deposition and accumulation of seston. Crassicorophium bonellii apparently uses red algae substrate in a different way compared with the species (similarly to A. rubricata) feeding on plant material. The red algae habitats may also just be characterized by local hydrodynamic and sedimentation conditions preferred by C. bonellii. This explains why there is no correlation between its abundance and the abundance of any other common amphipod species (Table 4) which do not feed on seston and organic deposits. However, it is also probable that rhodophytes are a shelter for C. bonellii.
In Velikaya Salma Strait Gammaropsis melanops, Pleusymtes glaber and Andaniella pectinata apparently preferred single species in red algae patches (along the main transect) and multi-species algal assemblages associated with sponges. As their abundances were mutually positively correlated it may be concluded that they have some similarity in biotope preferences. The mouthparts morphology of G. melanops and P. glaber shows their ability to feed on plant material (Uryupova, Reference Uryupova2005). Wildish & Peer (Reference Wildish and Peer1983) characterized P. glaber both as a deposit feeder and an algal scraper. Gammaropsis melanops is supposed to tackle encrusted algal surfaces: apical parts of its mouthparts are broadly separated and this can help to reach the algae surface more effectively (Uryupova, Reference Uryupova2005). Gammaropsis sp. from the Antarctic Peninsula is also known as a sponge-associated species (Amsler et al., Reference Amsler, McClintock, Amsler, Angus and Baker2009). However, details of the trophic specialization of these three species, the role of algae and sponges in nutrition and their association with red algae are not known.
Pleustes panopla found on all six species of rhodophytes is considered to be a common inhabitant of algal beds in the North Atlantic and the Barents Sea (Gurjanova, Reference Gurjanova1951). It was also reported to occur on Ptilota serrata in Newfoundland waters (Fenwick & Steele, Reference Fenwick and Steele1983). In inner Kandalaksha Bay this species was reported to occur among hydroids growing on Laminaria spp. (Grishankov et al., Reference Grishankov, Ninburg and Shkliarevich2000). Dyopedos porrecta, a relatively rare species in our samples, is known to be associated with hydroids. This species is able to build tubes of small diameter composed of detritus (the crustacean glues particles using secretions of glands located on pereopods 3 and 4). Usually these tubes are on other organisms, such as hydroids (Laubitz, Reference Laubitz1979). These species are apparently not closely associated with red algae but amphipods commonly inhabit rhodophyte biotopes.
Although all of the recorded species are previously known from the White Sea (Gurjanova, Reference Gurjanova1951; Bulycheva, Reference Bulycheva1957; Tschesunov et al., Reference Tchesunov, Kalyakina and Bubnova2008), some of them are not commonly reported for Kandalaksha Bay. For example, Dyopedos porrectus, Gammaropsis melanops and Socarnes bidenticulatus have not been recorded from the northern part of Kandalaksha Bay (Grishankov et al., Reference Grishankov, Ninburg and Shkliarevich2000). Andaniella pectinata was found once in 648 dredge and 610 grab samples in the Northern Archipelago of Kandalaksha Bay and 304 stations in Por'ya Inlet (Grishankov et al., Reference Grishankov, Ninburg and Shkliarevich2000). However, it was not found in Kovda Inlet, somewhat north of the Pertsov Biological Station of MSU (Vinogradov & Kobuzeva, Reference Vinogradov and Kobuzeva2006). Probably, sampling techniques were not adequate in previous studies for the habitats in which these species usually occurred.
Biogeographical characteristics and composition of assemblages of amphipod species associated with red algae
Eleven species of amphipods associated with red algae in Kandalaksha Bay have been classified with regard to patterns of latitudinal distribution using biogeographical characterization provided by Gurjanova (Reference Gurjanova1951), Bulycheva (Reference Bulycheva1957) and Sirenko (Reference Sirenko2001). Seven species are characterized by the Arctic-boreal distribution: Acanthonotozoma inflatum, Andaniella pectinata, Dyopedos porrectus, Gammaropsis melanops, Oediceros borealis, Protomedeia fasciata and Socarnes bidenticulatus. The other four amphipods (Ampithoe rubricata, Crassicorophium bonellii, Pleusymtes glaber and Pleustes panopla) are known as amphiboreal species. The first three taxa of this group are the most abundant and common species in the red algae belt while P. panopla is not abundant but relatively common there.
The present study was restricted to a single summer season. It aimed to examine amphipod diversity associated with red algae with some details on the factors effecting a variation of these assemblages. The minimum number of species was found within the shallow inshore part of the transect and near Kokoikha Island. In some samples low diversity was associated with dominance of predominantly amphiboreal species, i.e. Ampithoe rubricata, Crassicorophium bonellii, Gammaropsis melanops and Pleusymtes glaber. In the upper subtidal zone (up to 4–5 m) of the White Sea, water temperature undergoes drastic daily and seasonal changes (from May to October) (Chernovskaya, Reference Chernovskaya1956). Amphiboreal species are presumably adapted to a broader temperature range and, in particular, to higher summer temperature than the Arctic-boreal species and they have an advantage when occupying red algae biotopes at the most shallow depth in the White Sea.
The highest abundance and diversity was found within the part of the main transect at depths between 7 and 9 m where a mixture of rhodophyte species occurred. These sites were located deeper; probably the temperature and salinity conditions were more stable there. So, not only the common species living in the uppermost subtidal zone but other species can inhabit the red algae biotope. The transect was located within a coastal zone effectively protected from northern and north-eastern winds by the Velikiy and Eremeevskiy Islands. Similar multi-species red algae biotopes near Cape Kindo and Kokoikha Island were more exposed. Probably waves resulted in less amphipod abundance (in particular, Ampithoe rubricata) but a relatively high diversity of them was found.
Environmental factors affect the assemblage composition of algal epibionts at various spatial and temporal scales (Christie et al., Reference Christie, Jorgensen, Norderhaug and Waage-Nielson2003; Huang et al., Reference Huang, Amsler, McClintock, Amsler and Baker2007; Reichert et al., Reference Reichert, Buchholz, Bartsch, Kersten and Giménez2008; Jacobucci et al., Reference Jacobucci, Tanaka and Leite2009). The form and function of the algal host produce a specific three-dimensional space for living and could play a significant role in the distribution of amphipods while nutritional value of particular algal species for mesograzers also matters (Norderhaug, Reference Norderhaug2004; Poore, Reference Poore2004; Huang et al., Reference Huang, Amsler, McClintock, Amsler and Baker2007). In the present study we did not examine individual host characteristics (i.e. a displacement volume, wet weight, other branching architectural characteristics of an alga and nutrition values of algal species) in a way that it was done in a series of recent studies (Norderhaug, Reference Norderhaug2004; Huang et al., Reference Huang, Amsler, McClintock, Amsler and Baker2007). In order to analyse in detail the factors influencing variation of the amphipod assemblages of algal biotopes, future studies should combine individual treatment of algal hosts with setting sampling sites (covering a standard area) along environmental gradients (depth, exposure and surrounding biotopes). Furthermore, seasonal variation may dramatically vary in different geographical regions. The White Sea with its drastic inter-seasonal changes of temperature and a relatively long ice cover in winter should be regarded as one of the most promising regions for these aspects of studies on macroalgae and associated fauna.
ACKNOWLEDGEMENTS
We thank the diving team of Pertzov White Sea Biological Station of Moscow State University (WSBS) as well as the master and crew of the RV ‘Nauchnyi’ for the sampling support. We would also like to acknowledge the constant support provided by the director of the WSBS Professor Alexander Tzetlin. We thank the anonymous referees who greatly helped to improve this manuscript. This work was supported by grants from the Russian Ministry of Education and Science (Grant Nos. 02.740.11.0280, P1291, NSh-4456.2010.4).









