Introduction
The Antarctic dragonfishes (family Bathydraconidae) include small benthic species endemic to the Southern Ocean, occurring on the Antarctic continental shelf and slope, and around Antarctic and sub-Antarctic islands (Gon Reference Gon1990). Among them, the genus Bathydraco is characterized by a single lateral line of tubular scales extending from opercle to at least the rear third of the body (Gon Reference Gon1990). Based on a recent taxonomic revision (DeWitt Reference DeWitt1985), it consists of five species: B. antarcticus Günther, 1878, B. joannae DeWitt, Reference DeWitt1985, B. macrolepis Boulenger, 1907, B. marri Norman, 1938, and B. scotiae Dollo, 1906. Two other species of Bathydraco, B. nudiceps Waite, 1916 and B. wohlschlagi DeWitt and Tyler, 1960, were placed in synonymy and redescribed as Akarotaxis nudiceps (Waite, 1916), a monotypic genus with two lateral lines and other morphological characters different from Bathydraco (DeWitt & Hureau Reference DeWitt and Hureau1979). Cladistic analysis based on the skull morphology and skeletal characteristics of the pectoral and caudal fins suggested close relationships between B. marri and B. joannae and between B. macrolepis and B. antarcticus, respectively (Voskoboinikova Reference Voskoboinikova1999).
Compared to other notothenioids, Akarotaxis and Bathydraco are typically eurybathic and deep-living fishes, occurring between 230 and 2950 m depth (Eastman Reference Eastman2017). The geographical distributions of B. antarcticus and B. joannae are limited to sub-Antarctic waters, where they inhabit the slopes of the Scotia Sea Islands and the Kerguelen–Gaussberg Ridge. Akarotaxis nudiceps, B. macrolepis and B. marri are circum-Antarctic and occur on the Antarctic continental shelf and slope (Gon Reference Gon1990). Bathydraco scotiae probably also has a circum-Antarctic distribution, but it has been found exclusively in deeper waters (2100–2950 m) than any other notothenioid (Eastman Reference Eastman2017). The biology of these species was unknown until recently due to their rare occurrence, generally restricted to deep waters (Gon Reference Gon1990). More attention was devoted to their physiology, such as the morphology of brain and sense organs (Eastman & Lannoo Reference Eastman and Lannoo2003), and composition of antifreeze glycopeptides (Wöhrmann Reference Wöhrmann1996).
Species composition and distribution of demersal fish fauna of the Weddell Sea is relatively well known, having been investigated during several expeditions of FS Polarsirkel and RV Polarstern during the 1980s (e.g. Kock et al. Reference Kock, Schneppenheim and Siegel1984, Ekau Reference Ekau1988, Schwarzbach Reference Schwarzbach1988). Two main areas were surveyed by bottom trawling, off Vestkapp and in Gould Bay, in the eastern and southern Weddell Sea, respectively. Bathydraco macrolepis and B. marri were rarely caught, each representing < 2.0% of total catches of bathydraconids, whereas A. nudiceps accounted for c. 20% of total catches (Ekau Reference Ekau1988, Schwarzbach Reference Schwarzbach1988). Regarding their biology, there are some data available on diet but very few on reproductive traits. Based on stomach contents analysis, A. nudiceps fed mainly on various taxa of crustaceans, such as amphipods, isopods, mysids, cumaceans and copepods, as well as on polychaetes (Schwarzbach Reference Schwarzbach1988). The reproductive effort of females was estimated for a single specimen of A. nudiceps and a few specimens of B. marri (Ekau Reference Ekau1991, Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993). Absolute and relative fecundities were 200 eggs/female and 16.2 eggs/g for A. nudiceps, and 1549–2208 eggs/female and 34.0–46.6 eggs/g for B. marri, respectively.
During two recent cruises of the RV Polarstern conducted in summer in the southern Weddell Sea, we collected some specimens of A. nudiceps, B. macrolepis and B. marri off Halley Bay and along the western side of the Filchner Trough, an interesting, poorly investigated area in the southernmost part of the Weddell Sea. To improve the knowledge of the life history traits of these rare species, the present study attempts for the first time to 1) estimate the individual age of fishes through the interpretation of growth increment patterns of sagittal otoliths, and 2) study the gonad cycle of both sexes through histological analyses of gonads. A further aim was to integrate the few available data on the reproductive effort of these species, estimating for each specimen the gonadosomatic index (GSI), the egg size and the absolute/relative fecundities through the macroscopic analyses of gonads.
Materials and methods
Field sampling
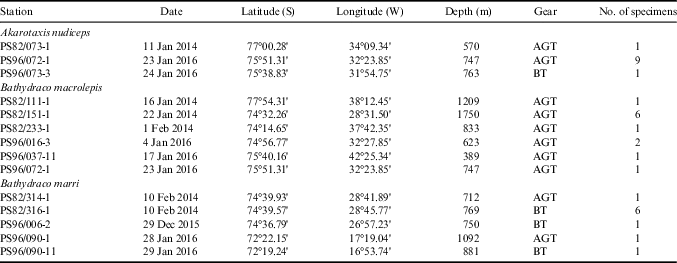
Specimens of A. nudiceps, B. macrolepis and B. marri were collected during the expeditions PS82 (ANT-XXIX/9) and PS96 (ANT-XXXI/2) of RV Polarstern, carried out in the southern Weddell Sea in summer 2013–14 and 2015–16 (Knust & Schröder Reference Knust and Schröder2014, Schröder Reference Schröder2016). Most of the catches were obtained on the continental shelf and upper slope off Halley Bay and along the Filchner Trough (Fig. 1). Details of sampling stations and fish caught are reported in Table I. The fish samples were collected by bottom trawling, using otter bottom trawl (BT) and Agassiz trawl (AGT) nets with 40 and 10 mm cod-end mesh size. The mouth opening of the bottom trawl was 2.5–3.2 m×16–18 m. BT and AGT nets were towed at 3 knots for 30 minutes and at 1 knot for 10 minutes, respectively.

Fig. 1 Map of the study area, showing positive sampling stations for Akarotaxis nudiceps (green crosses), Bathydraco macrolepis (red crosses) and Bathydraco marri (black crosses) located in the south-eastern Weddell Sea.
Table I Sampling data of Antarctic dragonfishes collected during the Polarstern cruises PS82 and PS96 in the southern Weddell Sea.
After each tow, fish were sorted, measured individually to the nearest mm (total length, TL and standard length, SL) and weighed in g (total weight, TW). Sex was determined by visual inspection, and gonad stage of development was assigned according to a five-point scale of maturity for notothenioids (Kock & Kellermann Reference Kock and Kellermann1991). Gonads were removed from each specimen, weighed in grams (total gonad weight, GW) and stored in Dietrich solution for histological analysis. In spawning capable females, right or left half gonads were randomly selected and fixed in 10% seawater formalin solution for fecundity estimation. Sagittal otoliths were removed from the skull, cleaned by adhering tissue and stored dry in vials.
Laboratory procedures
In A. nudiceps, half gonads preserved for fecundity estimates were dissected and the whole content soaked in a Petri dish. In Bathydraco species, a subsample (c. 50%) was separated, weighed and soaked as above. Oocytes were separated using pointed needles under a stereomicroscope and photographed with a LEICA DFC 420 video camera. All oocytes were counted and maximum diameter was measured with an accuracy of 0.1 mm using the Leica Application Suite (LAS) software. All the species investigated exhibited group-synchronous ovary development, as two main cohorts of oocytes were easily distinguished by size (Fig. 2) (Wallace & Selman Reference Wallace and Selman1981).

Fig. 2 Size frequency distribution of oocytes in the ovaries of spawning capable females of a. Akarotaxis nudiceps, b. Bathydraco macrolepis, and c. Bathydraco marri.
The maturation process of gonads was studied through histological analyses of gonads in both sexes. Following a standard procedure, gonads were dehydrated and embedded in paraplast. From each gonad sample, 7 µm thick transverse sections were mounted on slides and stained with haematoxylin and eosin for microscopic analysis with a light microscope (Leica DM4000B) set at 5–40× magnification. According to Brown-Peterson et al. (Reference Brown-Peterson, Wyanski, Saborido-Rey, Macewicz and Lowerre-Barbieri2011), gonads of both sexes were classified into five phases based on their histological features: 1) immature, 2) developing, 3) spawning capable, 4) regressing, 5) regenerating.
From all the specimens caught, a single sagittal otolith was randomly selected, burned at 350°C and mounted on slides using a thermoplastic resin (Crystalbond™ 509). Otoliths were then ground to obtain transverse sections reaching the core, and polished with alumina powder to eliminate scratches. Once the inner pattern was revealed, the otolith section was viewed under reflected light using a stereomicroscope (Leica M205C). Each pair of contiguous hyaline and opaque zones was considered to be an annulus, presuming they were deposited once per year, and counted (North Reference North1988). Two blind readings for each otolith were performed by an experienced reader a week apart, and the mean value calculated as the individual age estimate.
Data analysis
Due to the low number of samples, the length–weight relationship was estimated for each species by pooling together males and females. The exponential model applied was TW=aTLb, where TW was the total body weight (g), TL was the total length (mm) and a and b were growth parameters. Isometric growth departure (i.e. b≠3) was assessed by applying a t test to the equation t=(b – 3) SE-1, where SE is the standard error of b (Sokal & Rohlf Reference Sokal and Rohlf1995).
The gonadosomatic index (GSI) was calculated individually as the proportion of gonad weight (GW) to total body weight (TW). As a measure of gonad investment, the total fecundity (Ftot) was estimated in spawning capable females according to the gravimetric method (Murua et al. Reference Murua, Kraus, Saborido-Rey, Witthames, Thorsen and Junquera2003). Density of vitellogenic oocytes (number of oocytes per g) was calculated in the weighed subsample and multiplied by the total gonad weight. The relative fecundity (Frel) was assessed as the number of vitellogenic oocytes per gram of total body weight (Witthames et al. Reference Witthames, Thorsen, Murua, Saborido-Rey, Greenwood, Dominguez, Korta and Kjesbu2009).
According to Campana (Reference Campana2001), the average percentage error (APE) and the coefficient of variation (CV) were evaluated for quantifying ageing precision.
Results
Akarotaxis nudiceps
Overall, seven females of 120–140 mm TL and 8–13 g TW, three males of 115–130 mm TL and 8–9 g TW and a single juvenile of 95 mm TL and 4 g TW were collected by Agassiz and bottom trawls deployed at 570–763 m. The length–weight relationship was TW=0.000011 TL2.82 (n=11, r 2=0.91), with an isometric growth (t=0.65, df=9, P=0.52). Age estimates were in the range 6–11 and 7–9 years for females and males, respectively (Fig. 3). The average percentage error (APE) and the coefficient of variation (CV) were 3.9% and 5.6%, respectively.

Fig. 3 Photomicrographs of sagittal otolith of 12-year-old Akarotaxis nudiceps. a. Proximal side showing the whole otolith morphology, and b. transverse section showing the pattern of opaque and translucent zones (black dots).
Based on the histological analyses, females were categorized into three phases of gonad development. Two specimens were developing, with GSI of 0.16 and 1.06%. Ovaries consisted of chromatin nucleolar (mean size ± SD, 76±28 µm), perinucleolar (164±30 µm), cortical alveoli (377±86 µm) and early vitellogenic (655±55 µm) oocytes (Fig. 4a & b). Two specimens were spawning capable, with GSI of 9.22 and 9.65%. Total and relative fecundities were 183 and 244 eggs/female, 14.1 and 18.8 eggs/g, respectively. Ovarian follicles consisted of a few sparse chromatin nucleolar (79±8 µm), perinucleolar (169±40 µm) and cortical alveoli (477±78 µm) oocytes, as well as several late vitellogenic (2248±140 µm) oocytes completely filled by yolk granules just before coalescence, characterized by a wide multi-layered zona radiata and granulosa cells (Fig. 4c & d). All other females were regressing (GSI 0.74–0.91%), with ovaries composed of chromatin nucleolar (93±20 µm), perinucleolar (191±41 µm), cortical alveoli (560±111 µm), atretic oocytes and postovulatory follicles at different stages of resorption (Fig. 4e). All males were in spawning capable condition (GSI 0.20–0.70%), with cysts of spermatogonia, spermatocytes and spermatids, as well as spermatozoa filling lobules lumina and spermiducts (Fig. 4f).

Fig. 4 Light photomicrograph of gonad histological sections of Akarotaxis nudiceps at different development phases. a. and b. Developing females, c. and d. spawning capable females, e. regressing females, and f. spawning capable males. A: atretic oocytes, CA: cortical alveoli, CN: chromatin nucleolar, EV: early vitellogenic, g: granulosa layer, LV: late vitellogenic, n: nucleus, PN: perinucleolar, POF: post-ovulatory follicles, Sc: spermatocytes, Sg: spermatogonia, St: spermatids, Sz: spermatozoa, Yg: yolk granules, Zr: zona radiata.
Bathydraco macrolepis
A total of four females of 220–282 mm TL and 40–111 g and eight males of 110–234 mm TL and 5–50 g were collected exclusively by Agassiz trawl over a wide depth range (389–1750 m). The length–weight relationship was TW=0.00000099 TL3.25 (n=12, r 2=0.98), with an isometric growth (t=2.12, df=10, P=0.06). Age estimates were 8–11 and 5–11 years for females and males, respectively (Fig. 5). APE and CV were 4.8% and 6.8%.

Fig. 5 Photomicrographs of sagittal otolith of 8-year-old Bathydraco macrolepis. a. Proximal side showing the whole otolith morphology, and b. transverse section showing the pattern of opaque and translucent zones (black dots).
Females were assigned to three phases of gonad development. A single specimen was immature (0.16% GSI), with small paired ovaries composed of a few scattered oogonia, chromatin nucleolar (mean size ± SD, 57±10 µm) and perinucleolar (123±34 µm) oocytes (Fig. 6a). Two females were spawning capable, with GSI of 6.34 and 7.31%. Total and relative fecundities were 1844 and 2414 eggs/female, and 19.4 and 21.7 eggs/g, respectively. Ovarian follicles consisted of a few chromatin nucleolar (80±21 µm), perinucleolar (149±26 µm) and cortical alveoli (355±44 µm) oocytes, representing the batch to be spawned in the next spawning season; the bulk of the ovaries comprised late vitellogenic oocytes (2248±140 µm) completely filled by yolk granules just before coalescence, with a characteristic multi-layered zona radiata (Fig. 6b & c). A single regressing female (GSI 0.75%) had ovaries composed of chromatin nucleolar (76±15 µm), perinucleolar (161±42 µm) and cortical alveoli (558±63 µm) oocytes, as well as atretic oocytes in resorption (Fig. 6d). Three small-sized males were immature, with a GSI range of 0.01–0.03%. Testicular lobules were filled exclusively by cysts of spermatogonia, without evident lumina (Fig. 6e). All other males were in developing stage with a GSI range of 0.14–1.32%. Testes consisted mainly of spermatocytes and spermatids, as well as a few cysts of spermatogonia located at their periphery (Fig. 6f).

Fig. 6 Light photomicrograph of gonad histological sections of Bathydraco macrolepis at different development phases. a. Immature female, b. and c. spawning capable females, d. regressing female, e. immature males, and f. developing males. A: atretic oocytes, CA: cortical alveoli, CN: chromatin nucleolar, g: granulosa layer, L: lumen, LV: late vitellogenic, n: nucleus, OO: oogonia, PN: perinucleolar, Sc: spermatocytes, Sg: spermatogonia, St: spermatids, Yg: yolk granules, Zr: zona radiata.
Bathydraco marri
Overall, seven females of 206–249 mm TL and 29–63 g and three males of 185–208 mm TL and 25–32 g were collected by Agassiz and bottom trawl in relatively deep waters (712–1097 m). The length–weight relationship was TW=0.0000098 TL2.82 (n=10, r 2=0.82), with an isometric growth (t=0.41, df=8, P=0.69). Age estimates were 8–10 and 9–11 years for females and males, respectively (Fig. 7). APE and CV were comparatively low, at 1.1% and 1.6%.

Fig. 7 Photomicrographs of sagittal otolith of 9-year-old Bathydraco marri. a. Proximal side showing the whole otolith morphology, and b. transverse section showing the pattern of opaque and translucent zones (black dots).
Based on histological analysis of gonads, a single female was developing (GSI 0.64%), with ovarian follicles made up of chromatin nucleolar (mean size ± SD, 58±10 µm), perinucleolar (142±28 µm) and cortical alveoli (610±48 µm) oocytes, as well as vitellogenic oocytes at an early stage of maturity (610±48 µm) (Fig. 8a). Two specimens were spawning capable, attaining GSI of 7.36 and 8.96%, with ovaries composed of chromatin nucleolar (81±14 µm), perinucleolar (163±45 µm) and cortical alveoli (399±52 µm) representing the reserve batch for future spawning, and late vitellogenic oocytes (1629±117 µm) to be spawned in the current season (Fig. 8b & c). Total and relative fecundities were 1489 and 1994 eggs/female, and 31.7 and 33.8 eggs/g, respectively. The remaining females had ovaries in a regressing phase, with GSI of 0.60–1.11%. Ovarian follicles consisted exclusively of chromatin nucleolar (73±11 µm), perinucleolar (153±36 µm) and cortical alveoli (464±78 µm) oocytes, with evident postovulatory follicles dispersed in the ovarian stroma (Fig. 8d & e). All males in the sample were developing, with GSI of 0.33–1.26%. Seminiferous lobules were composed of spermatogonial cysts located at testes periphery, spermatocytes and spermatids (Fig. 8f).

Fig. 8 Light photomicrograph of gonad histological sections of Bathydraco marri at different development phases. a. Developing female, b. and c. spawning capable females, d. and e. regressing females, and f. developing males. CA: cortical alveoli, CN: chromatin nucleolar, EV: early vitellogenic, g: granulosa layer, LV: late vitellogenic, n: nucleus, PN: perinucleolar, POF: post-ovulatory follicles, Sc: spermatocytes, Sg: spermatogonia, St: spermatids, Yg: yolk granules, Zr: zona radiata.
Discussion
The spatial distributions of the species investigated in this study closely resemble those previously reported in the Weddell Sea (e.g. Ekau Reference Ekau1998, Schwarzbach Reference Schwarzbach1988). In particular, A. nudiceps was found in the southernmost area along the Filchner Trough, while B. macrolepis and B. marri showed different distributions, being collected in the south-western and north-eastern parts of the investigated area, respectively. Looking at the map of distribution, B. marri was present in deep waters on steep continental slope areas, whereas B. macrolepis exhibited a disjunct vertical distribution, as reported elsewhere (DeWitt Reference DeWitt1985). Most of the specimens (72%) were collected by Agassiz trawl, probably due to the smaller mesh size.
As in many other notothenioids (e.g. White Reference White1991), sagittal otoliths proved to be suitable hard structures for age determination of bathydraconids. The consistency between age estimates, based on the low values of indices of precision (Campana Reference Campana2001), supported the reliability of the ageing procedure. For each species, the larger specimens sampled were close to the maximum size reported in literature, which is 130, 250 and 230 mm SL for A. nudiceps, B. macrolepis and B. marri, respectively (Gon Reference Gon1990). Therefore, the maximum age estimate shared by these species probably represents their maximum theoretical age or longevity, falling within the range reported for other bathydraconids aged so far, such as Gerlachea australis (14 years) and Parachaenichthys charcoti (9 years) (La Mesa et al. Reference La Mesa, Catalano, Kock and Jones2012, Reference La Mesa, Calì, Donato, Riginella and Mazzoldi2018). Although the low number of specimens precluded the application of any growth model to the age–length data, A. nudiceps can be considered a slow growing species compared to the species of Bathydraco, attaining a similar maximum age at a considerably smaller size. Consistently, it is an extremely sluggish bottom-dwelling species adopting a ‘sit and wait’ feeding strategy, which requires minimum energy expenditure (Schwarzbach Reference Schwarzbach1988, La Mesa et al. Reference La Mesa, Caputo and Eastman2007).
The reproductive strategies of the species investigated fell within those characterizing most notothenioids. The occurrence of two well-separated modes of oocytes, with the presence of pre-vitellogenic and early vitellogenic oocytes in spawning capable females, supported the common feature of a prolonged gametogenesis. Moreover, all species exhibited low fecundity and relatively large eggs at spawning (Kock & Kellermann Reference Kock and Kellermann1991). Nevertheless, some differences were found either at interspecific level (Akarotaxis vs Bathydraco) or between populations of the same species (A. nudiceps) living in different areas.
The reproductive investment was very similar between the two species of Bathydraco, with slight differences possibly linked to body size-dependent constraints. Compared to B. marri, B. macrolepis attained a larger maximum size and weight, but a similar total fecundity and size of late vitellogenic oocytes. As a result, the relative fecundity was significantly higher in B. marri than in B. macrolepis. The presence of late vitellogenic oocytes in spawning capable females of both species indicated they would be ready to spawn within a few weeks of the sampling period, i.e. in late summer–early autumn, contrasting with previous data (Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993). This spawning season is also supported by the occurrence of regressing females in both species, with post-ovulatory follicles in B. marri. Interestingly, the gonad investment of females in terms of egg size and fecundity estimated in the two species of Bathydraco closely resembles that reported from the Weddell Sea for other bathydraconids of comparable size, such as Gerlachea australis and Racovitzia glacialis (240 mm SL) (Ekau Reference Ekau1991, Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993, La Mesa et al. Reference La Mesa, Calì, Donato, Riginella and Mazzoldi2018).
The reproductive traits of Bathydraco were rather different from those of A. nudiceps, which is the smallest bathydraconid (Gon Reference Gon1990). Compared with Bathydraco, A. nudiceps produced relatively large eggs associated with a strikingly low fecundity, releasing fewer than 250 eggs/female. It was at the lower end of total fecundities estimated in bathydraconids, and comparable to those reported for the small-sized species of Artedidraco from the Weddell Sea (Duhamel et al. Reference Duhamel, Kock, Balguerias and Hureau1993, Meneghesso et al. Reference Meneghesso, Riginella, La Mesa, Donato and Mazzoldi2017). As a consequence of the relatively large egg size and low fecundity, some degree of parental care may be suggested in A. nudiceps, as already stated for Artedidraco (e.g. La Mesa et al. Reference La Mesa, Caputo, Rampa and Eastman2006). Finally, A. nudiceps collected in the Ross Sea (La Mesa et al. Reference La Mesa, Caputo and Eastman2007) showed slightly higher fecundities (260 eggs/female and 31.5 eggs/g) and smaller egg size (1.6–2.0 mm) than those in the Weddell Sea, probably within the natural variability between geographically distant populations.
Acknowledgements
We would like to thank all of the crew members and personnel aboard RV Polarstern during the cruises PS82 and PS96, for their valuable support during field sampling activities. We are much indebted to two anonymous reviewers, whose comments improved the early draft of the manuscript. This study was financially supported by the Italian National Antarctic Research Program (PNRA) within the project 2013/C1.07.
Author contributions
MLM conceived the study and wrote the paper; ER participated in field activities providing fish samples; and FD and CM performed the histological analysis of gonad samples. In addition to individual contributions, each author contributed significantly to data analyses and manuscript editing before submission.











