Introduction
Cholesterol granulomas of the petrous apex are expansile, cystic lesions containing cholesterol crystals surrounded by foreign body giant cells, fibrous tissue reaction and chronic inflammation.
The literature describes two possible mechanisms of origin for cholesterol granulomas of the petrous apex. An older theory asserts that mucosal swelling combined with gas resorption results in negative pressure and haemorrhage into the temporal bone air cells.Reference Jackler and Cho1 A more recent theory, presented by Jackler and Cho, asserts that exuberant pneumatisation of the temporal bone exposes marrow-filled spaces of the petrous apex.Reference Jackler and Cho1 The resulting coaptation of the marrow and mucosa produces a proclivity towards haemorrhage. Haemorrhage is triggered and clot develops, resulting in obstruction of the petrous apex outflow tract. The resulting degradation of haemosiderin and cholesterol causes an inflammatory granulomatous reaction. Recently, this latter theory has gained greater acceptance.
The clinical presentation of petrous apex cholesterol granuloma can vary depending on its extent.Reference Mosnier, Cyna-Gorse, Grayeli, Fraysse, Martin and Robier2 A previous review of our institution's management of 34 patients with petrous apex cholesterol granuloma revealed that the most common presenting symptom was hearing loss (64.7 per cent), followed by vestibular symptoms (56 per cent), tinnitus (50 per cent), headache (32.3 per cent), facial twitching (23.5 per cent), facial paraesthesia (20.6 per cent), otorrhoea (11.8 per cent), diplopia (5.9 per cent) and facial weakness (2.9 per cent).Reference Brackmann and Toh3 Sanna and colleagues noted that hearing loss and vertigo are present in approximately 50 per cent of their patients with petrous apex cholesterol granuloma; they also reported tinnitus (36.6 per cent), headache (32.5 per cent), trigeminal neuralgia (25 per cent), diplopia (16.6 per cent) and facial weakness (17.5 per cent).Reference Sanna, Dispenza, Mathur, De Stefano and De Donato4
Accurate radiological diagnosis of petrous apex cholesterol granuloma is essential for correct diagnosis and subsequent treatment. The differential diagnosis of petrous apex cholesterol granuloma includes a variety of similar entities; however, careful examination of the radiological features will identify distinguishing features which enable accurate diagnosis.
Generally, patients with symptoms are managed surgically, while non-surgical management is advocated for asymptomatic patients. Surgical management of cholesterol granuloma is performed primarily by drainage procedures via the translabyrinthine, infralabyrinthine, middle fossa, transsphenoidal or, more commonly, the infracochlear approach. The main goal of these procedures is to establish a permanent outflow drainage pathway so that cholesterol granuloma expansion does not cause a recurrence of symptoms.
This paper aims: (1) to review the relevant radiological features of petrous apex cholesterol granuloma and to highlight those relevant to differential diagnosis; and (2) to review the histopathological and radiological findings associated with surgical drainage of these lesions.
Materials and methods
This study was approved by the institutional review board of the St Vincent Medical Center, Los Angeles, California, USA (approval number 11-014).
Radiological review of diagnostic features
The radiological features of petrous apex cholesterol granulomas and other lesions in the differential diagnosis are reviewed below in a pictorial fashion. Radiological images were obtained from patients seen at the House Clinic, Los Angeles. The differential diagnosis of petrous apex cholesterol granuloma includes asymmetrical pneumatisation (marrow), effusion, mucocele, cholesteatoma, arachnoid cyst and, rarely, cephalocoele. Features of both computed tomography (CT) and magnetic resonance imaging (MRI) will be discussed.
Radiological review of surgical management
We also review the radiological features of surgically managed petrous apex cholesterol granuloma, using examples from patients seen at the House Clinic. Both CT and MRI features are discussed.
Temporal bone histopathology
A temporal bone specimen was obtained from a patient [that had undergone] surgical drainage of a petrous apex cholesterol granuloma. This temporal bone was sectioned in a plane parallel to the long axis of the drainage stent (angiocatheter) to enable analysis of histopathological changes associated with surgical drainage and stent placement. The temporal bone was fixed, decalcified, embedded in celloidin and sectioned to a thickness of 20 µm. Every 10th section was stained with haematoxylin and eosin and mounted on a glass slide; the other 9 sections were stored for other evaluations.
Results
Radiological differential diagnosis
Pathological conditions relevant to the radiological differential diagnosis of cholesterol granuloma of the petrous apex include asymmetrical pneumatisation, petrous apex effusion, mucocele, cholesteatoma, arachnoid cyst, cephalocoele and petrous apicitis. In Figures 1 to 7, we present a pictorial review of these entities.
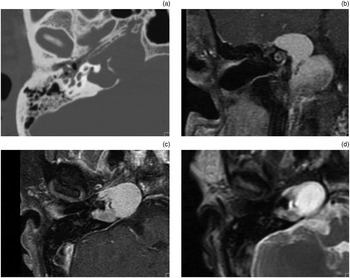
Fig. 1 Radiological images of petrous apex cholesterol granuloma. (a) Axial computed tomography image showing erosion of trabeculae and smooth expansion in the petrous apex. (b) Coronal, T1-weighted, pre-contrast magnetic resonance imaging (MRI) showing a non-enhancing, expansile lesion in the petrous apex with high signal intensity. (c) Axial, T1-weighted, post-contrast MRI showing an expansile lesion with high signal intensity which does not enhance. (d) Axial, T2-weighted MRI showing an expansile petrous apex lesion with high signal intensity.
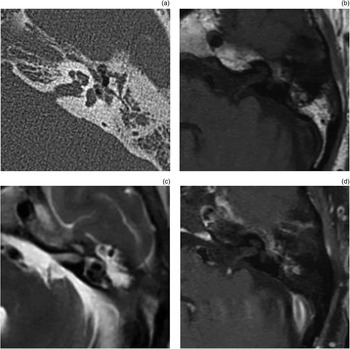
Fig. 2 Radiological imaging of petrous apex marrow. (a) Axial computed tomography image showing a non-expansile lesion in the left petrous apex. (b) Axial, T1-weighted, pre-contrast magnetic resonance imaging (MRI) showing a left-sided lesion isointense to fat (bright signal). (c) Axial, T2-weighted MRI showing a left-sided lesion isointense to fat (dark signal). (d) Axial, T1-weighted, post-contrast MRI with fat suppression, showing suppression of the fat signal in the marrow.

Fig. 3 Radiological imaging of petrous apex effusion. (a) Axial computed tomography image showing preservation of intact trabeculae (arrows). (b) Axial, T1-weighted magnetic resonance imaging (MRI) showing a non-expansile lesion with low signal intensity. (c) Axial, T2-weighted MRI showing a non-expansile lesion with high signal intensity. (d) Axial, T1-weighted, post-contrast MRI showing a non-enhancing lesion.

Fig. 4 Radiological imaging of petrous apex mucocele. (a) Axial, T1-weighted magnetic resonance imaging (MRI) showing bilateral petrous apex mucoceles exhibiting smooth expansion and low signal intensity. (b) Axial, T2-weighted MRI showing bilateral petrous apex mucoceles with characteristic high signal intensity (isointense to fluid). (c) Axial, diffusion-weighted MRI showing that these lesions are not diffusion-restricted, which distinguishes them from petrous apex epidermoids.

Fig. 5 Radiological imaging of petrous apex epidermoid (right ear). (a) Axial computed tomography image showing an expansile lesion in the right petrous apex. (b) Axial, T1-weighted magnetic resonance imaging (MRI) showing an expansile lesion in the right petrous apex with low signal intensity. (c) Axial, T2-weighted MRI showing an expansile lesion in the right petrous apex with high signal intensity. (d) Axial, T1-weighted, post-contrast MRI showing a non-enhancing lesion.
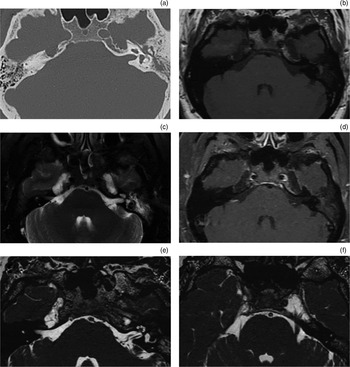
Fig. 6 Radiological imaging of petrous apex cephalocoele. (a) Axial computed tomography image showing bilateral smooth erosion extending from Meckel's cave to the petrous apex. (b) Axial, T1-weighted, pre-contrast magnetic resonance imaging (MRI) showing bilateral lesions isointense to cerebrospinal fluid (CSF) (low-signal intensity). (c) Axial, T2-weighted MRI showing bilateral lesions isointense to CSF (high signal intensity). (d) Axial, T1-weighted, post-contrast MRI showing bilateral lesions which do not enhance. (e) Axial, constructive interference in the steady state (‘CISS’) MRI focused on the right petrous apex cephalocoele, showing a lesion isointense to CSF. (f) Axial, constructive interference in the steady state MRI focused on the left petrous apex cephalocoele, showing the trigeminal nerve embedded within the cephalocoele.

Fig. 7 Radiological imaging of petrous apicitis (right ear). (a) Axial, T1-weighted, pre-contrast magnetic resonance imaging (MRI) showing few specific findings in the petrous apex; note the presence of fluid in the mastoid (asterisk). (b) Axial, T2-weighted MRI showing increased signal intensity in the petrous apex (white arrow). (c) Axial, T1-weighted, post-contrast MRI showing rim enhancement of the area of inflammation in the petrous apex (black arrow). (d) Coronal, T1-weighted, post-contrast MRI showing high signal intensity in the petrous apex (white arrow).
Radiology of surgical management
As previously discussed, the surgical management of petrous apex cholesterol granuloma often entails surgical drainage. It has been hypothesised that stent placement at the time of surgery will prolong the effectiveness of such drainage procedures.
Radiological examination of patients who have undergone surgical management of petrous apex cholesterol granuloma indicates that the patency of the drainage pathway is maintained, thus delaying the need for further intervention for many years. The CT image presented in Figure 8(a) clearly shows a drainage catheter lying within the infracochlear drainage pathway. The CT image presented in Figure 8(b) shows evidence of new bone formation along the infracochlear drainage tract, notably around the stent.

Fig. 8 (a) Axial computed tomography (CT) image taken 14 months after surgery, in a 36-year-old woman with a right-sided petrous apex cholesterol granuloma who originally presented with right-sided hearing loss, dizziness, tinnitus and headache and who underwent an infracochlear drainage procedure; the image shows maintenance of the petrous apex outflow tract by a stent. (b) Post-operative, coronal CT image in a 28-year-old man with a left-sided petrous apex cholesterol granuloma, who originally presented with left-sided hearing loss, headache, facial weakness and dizziness and who underwent an infracochlear drainage procedure; the image shows maintenance of the petrous apex outflow tract by a stent, and also demonstrates bony growth along the petrous apex outflow tract around and abutting the stent. This patient remained symptom-free at last follow up (117 months), with no expansion in cyst size.
Figure 9 shows characteristic MRI features of a surgically drained petrous apex cholesterol granuloma. The procedure aims to provide a path for surgical drainage. The drainage tract, indicating the drainage pathway provided and maintained by the stent, can often be visualised using constructive interference in the steady state (‘CISS’) MRI or T2-weighted MRI (Figure 9c).
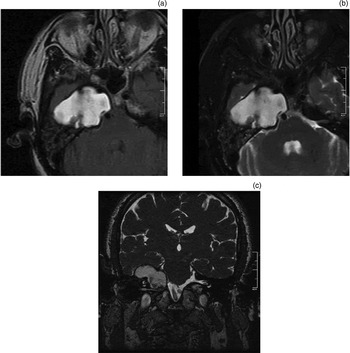
Fig. 9 Magnetic resonance imaging characteristics of petrous apex cholesterol granuloma: (a) high signal intensity on axial, T1-weighted image; (b) high signal intensity on axial, T2-weighted image; (c) drainage catheter leading from the petrous apex cholesterol granuloma cyst cavity to the middle-ear cavity, on coronal, T2-weighted image.
Histopathology of surgical management
A temporal bone specimen from a patient with surgically managed petrous apex cholesterol granuloma was obtained and sectioned parallel to the plane of catheter drainage. This specimen provided an opportunity to examine the histopathological changes associated with surgical drainage of petrous apex cholesterol granuloma. Figure 10(a) shows the partially sectioned temporal bone, with the cholesterol granuloma draining into the mastoid via an angiocatheter. Figure 10(b) shows fatty replacement of the cholesterol granuloma cyst by adjacent haematopoietic bone marrow. Figure 10(c) shows the angiocatheter draining the lesion. In addition, connective tissue is noted to surround the lesion. Closer examination reveals new bone formation around the site of the angiocatheter (Figure 10d).
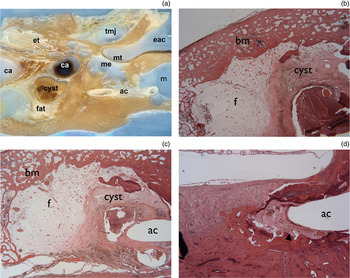
Fig. 10 Photomicrographs of temporal bone from a patient with petrous apex cholesterol granuloma. (a) Temporal bone section showing drainage of the granuloma (cyst and fat) into the mastoid (m) via an angiocath (ac), and also showing the temporomandibular joint (tmj), eustachian tube (et), external auditory canal (eac), tympanic membrane (mt), middle ear (me), and carotid artery with one segment containing clotted blood (ca) (H&E; ×5). (b) Adjacent section showing replacement of cyst cavity by fat (f), with haematopoietic bone marrow (bm) adjacent to the cholesterol granuloma cyst (H&E; ×20). (c) Section showing angiocatheter (ac) draining the cholesterol granuloma cyst (H&E; ×10). (d) Section showing new bone (arrowhead) surrounding the angiocatheter (ac) (H&E; ×20).
Discussion
Differential diagnosis
An accurate understanding of the radiological features of petrous apex cholesterol granuloma and its differential diagnosis is essential for determining appropriate treatment. Cholesterol granulomas are non-enhancing, expansile, petrous apex lesions which demonstrate erosion of bony trabeculae on CT imaging and which produce high signal intensity on T1- and T2-weighted MRI (Figure 1). Cholesterol granulomas exhibit high signal intensity on fluid-attenuated inversion recovery (‘FLAIR’) MRI and low signal intensity on diffusion-weighted MRI. Petrous apex marrow does not enhance, is non-expansile, and is isointense to fat on T1- and T2-weighted MRI (Figure 2). The fat suppression created on T1-weighted MRI will thus suppress the signal intensity for petrous apex marrow. Likewise, marrow has a low signal intensity on diffusion-weighted MRI and may not be evident.
Petrous apex effusions are non-enhancing, non-expansile, petrous apex lesions which demonstrate low signal intensity on T1-weighted MRI and high signal intensity on T2-weighted MRI (Figure 3). Preservation of bony trabeculae on CT is an important feature distinguishing petrous apex effusion from petrous apex cholesterol granuloma. Effusions demonstrate low signal intensity on both fluid-attenuated inversion recovery and diffusion-weighted MRI sequences.
Petrous apex mucoceles are expansile, non-enhancing lesions which do not show preservation of bony trabeculae on CT, and which demonstrate low to intermediate signal intensity on T1-weighted MRI and high signal intensity on T2-weighted MRI (Figure 4). Mucoceles demonstrate low signal intensity on fluid-attenuated inversion recovery and diffusion-weighted MRI sequences. Petrous apex mucoceles can be expansile and may exhibit occasional rim enhancement.
Petrous apex cholesteatoma or epidermoids are non-enhancing and demonstrate erosion on CT. Typical radiological features on MRI are shown in Figure 5. Cholesteatomas demonstrate high signal intensity on fluid-attenuated inversion recovery and diffusion-weighted MRI sequences. These features enable differentiation from arachnoid cysts.
Unlike their counterparts in the cerebellopontine angle, arachnoid cysts of the petrous apex are extremely rare, non-enhancing lesions which demonstrate low signal intensity on T1-weighted MRI and high signal intensity on T2-weighted MRI. Low signal intensity is noted on fluid-attenuated inversion recovery and diffusion-weighted MRI sequences.
Petrous apex cephalocoeles have non-specific imaging findings on CT; however, smooth erosion may be seen, which may point to the location of origin. These non-enhancing petrous apex lesions exhibit low signal intensity on T1-weighted MRI and high signal intensity on T2-weighted MRI (Figure 6). Low signal intensity is noted on fluid-attenuated inversion recovery and diffusion-weighted MRI sequences.
Finally, petrous apicitis shows irregular erosion on CT, which enhances with contrast. Petrous apicitis exhibits low signal intensity on T1-weighted MRI, high signal intensity on T2-weighted MRI, and rim enhancement with gadolinium contrast (Figure 7).
Radiology and histopathology of surgical management
Radiological follow up of surgically managed cholesterol granulomas indicates that stent placement may prolong drainage patency. Evidence of new bone formation is noted both along the petrous apex outflow tract and within the cyst cavity. The long-term success of surgical management procedures is due in part to maintenance of drainage (preventing cyst expansion) together with gradual reduction in cavity size (due to fibrosis and neo-osteogenesis).
Analysis of a temporal bone specimen from a patient with surgically managed petrous apex cholesterol granuloma suggests that long-term preservation of drainage pathway patency is possible with stent placement; in this patient's case, a patent drainage pathway was observed approximately 24 years after surgical drainage. However, the presence of new bone formation suggests that these drainage pathways may obstruct over time, due to a combination of bony regrowth, fibrous tissue obstruction and cholesterol granuloma reaccumulation. The placement of a stent at the time of surgical drainage may help prevent eventual obstruction of this drainage pathway.
Thus, multiple lines of evidence suggest the presence of new bone formation around the drainage pathway. The question does arise as to whether the presence of a stent acts as a foreign body which stimulates fibrosis and new bone formation. While new bone formation may be evident in other parts of the cyst, the relatively small calibre of the drainage pathway makes it difficult to determine whether radiological evidence of new bone formation along the drainage pathway is present. However, histopathological examination reveals the presence of bony growth not in areas adjacent to the stent but in areas adjacent to the cyst cavity; this suggests that bony growth occurs for some reason other than proximity to the stent. It has been suggested that ischaemia may lead to new bone formation.Reference Kimura and Perlman5, Reference Keithley, Chen and Linthicum6 We hypothesise that drainage relieves the pressure on the cavity exerted by the cholesterol granuloma. As a result, fibrosis within the cyst cavity may promote an ischaemic environment leading to new bone formation.
Conclusion
The recommended treatment for symptomatic petrous apex cholesterol granuloma is surgical drainage with stenting of the drainage pathway. Appropriate treatment decisions depend on accurate radiological diagnosis of petrous lesions. It is therefore essential to understand the relevant radiological features of the differential diagnoses of petrous apex cholesterol granuloma. Placement of a stent to maintain the drainage pathway may decrease the likelihood of symptomatic recurrence. Histopathological analysis of surgically managed petrous apex cholesterol granulomas reveals new bone formation around the drainage pathway, which, along with drainage pathway obstruction by fibrous tissue and granulomatous reaction, may curtail the long-term patency of surgical drainage.












