Sir Richard Sykes, head of British pharmaceutical giant Glaxo Smith Kline (GSK), used the term “pirate” to describe generic pharmaceutical manufacturers in 2001.Footnote 1 His wrath was particularly aimed at one company, the Chemical, Industrial & Pharmaceutical Laboratories (Cipla), founded in Mumbai in 1935.Footnote 2 In 2001 the AIDS epidemic, with its epicenter in sub-Saharan Africa, was the leading global health concern and nearly two million people were dying annually.Footnote 3 GSK was among the four leading manufacturers of antiretroviral drugs, and its market position was threatened by Cipla's ability to offer good-quality generic versions of Combivir (a cocktail of drugs containing AZT and 3TC, two non-nucleoside reverse transcriptase inhibitors for the treatment of HIV) for around US$350, compared with GSK's initial price tag of nearly $15,000 (a price that was subsequently revised down for developing nations but remained significantly higher than the Cipla version).Footnote 4 The problem of HIV infection had by then become a global pandemic, and new funds were being mobilized by the Joint United Nations Programme on HIV/AIDS (UNAIDS), which was founded in December 1995. Cipla argued that not only would it offer the drugs for a fraction of the cost, but it would offer them at an identical quality—countering one of the biggest objections of large pharmaceutical companies against generic manufacturers in low-income countries: that the quality of generic drugs was suspect and untrustworthy.Footnote 5 While Cipla's claims were bold at the time (especially coming from India, with its then notoriously weak pharmaceutical regulatory regime), and have sometimes been disputed, the assertion that they could produce reliable medicines in fact rested on years of growth and evolution.Footnote 6 Attention to creating drugs that could meet international standards for purity and reliability, which the company referred to internally with the shorthand “quality,” had been a centerpiece of Cipla's marketing strategy and investment for years.
This article provides an analysis of the inception and evolution of “quality,” and its cost, as Cipla evolved from a small pharmaceutical company in Mumbai to a multinational. This article also provides a new perspective on why producing to international standards has been central to the rise of Cipla. While much has been written on the practice of medicine in the British colonial era and the changing landscape of indigenous medicine in India during the Raj, there is little discussion on how Indian pharmaceutical enterprises have viewed quality control.Footnote 7 This article aims to fill this gap. There is already a considerable body of literature on the issue of intellectual property (IP) and patents in Indian businesses, particularly in the pharmaceutical industry.Footnote 8 However, what makes Cipla's history distinctive is the convergence of nationalism, the changing landscape of Indian patent laws, the integration of technology, and the opportunity to capitalize on global goodwill during the most high-profile public-health crisis of the last forty years.
To understand the historical context of Cipla's efforts to compete in the market for AIDS medicines, it is helpful to consider the long and controversial history of chemical and drug manufacturing in modern India, as well as the persistent problem and perception of adulteration as a central feature of chemical products made in India. In colonial India, most drugs were imported from Europe, but beginning as early as 1900 the Swadeshi (“of one's own country”) movement encouraged indigenous manufacturing, including in pharmaceuticals.Footnote 9 Between 1905 and 1907, chemists such as P. C. Ray, who founded Bengal Chemicals and Pharmaceutical Works (BCPW), and B. D. Amin, who founded Alembic Chemical Works, urged the creation of a scientifically managed pharmaceutical industry in India. The Swadeshi movement included an effort to professionalize pharmacists and manufacture more reliably pure medicines, with vigorous campaigns for standardization in the Indian press in the interwar years. Nonetheless, the first official government-issued regulation, the Drug Act, was not passed until 1944.Footnote 10
Indian companies producing medicines at the time of independence, in 1947, accounted for very little of the total market; 80 to 90 percent of medicines sold in India were manufactured by Western multinational companies (MNCs). In the 1960s, the Indian government established state-owned companies to encourage local production of pharmaceuticals. In 1970, Indian patent law changed to allow patents on manufacturing processes rather than on particular drug formulations, thus allowing imported medicines to be reverse-engineered by Indian companies. Basically, Indian companies were able to copy the patents for best-selling Western drugs, with the help of the government-owned Indian Institute of Chemical Technology in Hyderabad. This permitted the rapid growth of Indian pharmaceutical companies, including Ranbaxy, which was started in 1961, and Wockhardt, incorporated in 1973.Footnote 11 By 2005 the situation of 1947 had been reversed, with less than 20 percent of drugs in India being imported from MNCs.Footnote 12
Cipla, founded in 1935, has participated in and endured all of these stages of the industry's development. One factor in the company's persistence has been its sustained focus on improving the purity and reliability of its products. The evolution of this strategy at Cipla can be divided into three main stages. Between its founding and 1970, Cipla was a small and growing business, headed by Dr. Khwaja Abdul Hamied, an Indian nationalist motivated significantly by self-reliance and anticolonialism. This period encompassed the end of British colonial rule and the breakup of the country into India and Pakistan in August 1947. From 1970 until 1985, Yusuf Hamied, Khwaja Hamied's son, was in charge. This was the formative phase wherein Indian patent law was changed, new companies appeared on the market, and Yusuf Hamied established internal structures to make quality the centerpiece of growth. Between 1985 and 2001, Cipla became a global brand with international certifications of its plants. This was also the period in which Cipla started to actively participate in global efforts to combat the HIV epidemic and to challenge large pharmaceutical MNCs, benefiting largely from antiglobalization and antiestablishment sentiments in Africa.
The Foundation
The 1930s saw a significant growth of Indian-owned business, assisted by the imposition of tariff protection. Indian business leaders rose in terms of their social status and political influence, particularly in major cities such as Kolkata (then called Calcutta), Ahmedabad, and, most prominently, Mumbai (Bombay). A number of these business leaders became associated with the growing nationalist movement. As Claude Markovits has argued, “the greater politicization of businessmen in the 1930s was linked to changes in the structure of Indian capital as well as in the pattern of nationalist politics.”Footnote 13 The change in nationalist politics had to do with unsuccessful civil disobedience movements, the changing face of Indian national congress, and the increasing awareness among domestic Indian capitalists that their role in the future independent nation would include doing things for which they had historically had to rely on outsiders. While a diversity of opinions and business approaches were used by Indian merchants and local manufacturers, the Swadeshi movement, aimed at boycotting British goods and products, generally facilitated the growth of a new generation of Indian businessmen. The image of Mahatma Gandhi—probably the most prominent leader of the Indian independence movement, who had also been named Time magazine's “man of the year” in 1931—would appear on posters in bazaars, spinning cotton in traditional cotton weaving machines.
Against the backdrop of this political landscape, Cipla came into being, twelve years before the independence of India. At the time, entrepreneurs were beginning to respond to Gandhi's call for Indian self-reliance in industry, including health care.Footnote 14 Cipla's inception was very much rooted in this time of high nationalist zeal and a growing interest among businesses to demonstrate self-reliance. Khwaja Abdul Hamied, a businessman from Aligarh, in Uttar Pradesh, studied briefly in Britain and then moved to Berlin, where he completed his PhD in chemistry. Even before he left to study in Europe, he had interacted with Gandhi and other leaders of the Indian freedom movement. Hamied's interactions with Gandhi started in 1920, when Gandhi stayed with Hamied's uncle during the noncooperation movement against British rule. Hamied later recalled, “It was during this stay of Mahatma Gandhi at my uncle's residence that I had the privilege of serving Gandhiji and remaining in his company for over a week. I thus came to know Gandhiji intimately.”Footnote 15 Hamied, an entrepreneur at heart, first tried his luck in selling typewriters and sewing machines, but he was looking for something that would combine his passion for industrial pharmaceuticals and business. In 1935, in Mumbai, he established Cipla. The company mirrored Hamied's own interests in pharmaceuticals and had the potential to be meaningful in the anti-imperial struggle of the time.Footnote 16 Khwaja Hamied often said that he inherited his interest in pharmaceuticals from his family's business tradition, though there is little evidence for that claim. What is clearer is the family's long tradition of developing modern institutions. Khwaja Hamied was a nephew of Sir Syed Ahmed Khan, the Muslim reformer who founded Aligarh University, intended to help Muslims to move from traditional madrassas that Syed felt were the reason for the social struggles of Muslims during the British Raj.Footnote 17 In his life, Syed Ahmed Khan faced significant criticism from the orthodox Ulama about his modernist ideas and nationalist causes. Cipla's nationalism was encouraged by a visit, in 1939, from Mahatma Gandhi. Gandhi's visit is still celebrated as a high mark in Cipla's history.Footnote 18
In its early years, Cipla was mainly concerned with making small-scale pharmaceuticals and focusing on local demands in Mumbai. Sales were modest, as the firm was unable to compete with British imports and operated largely in losses. In 1938, frustrated by continued losses, Khwaja Hamied briefly considered shutting down the business; however, the company's fortunes turned soon after. During World War II the number of British imports plummeted and the demand for locally produced medicines increased overnight. Cipla was able to provide, at a low cost, what the British firms could no longer supply.Footnote 19 Over the next decade Cipla acquired additional property adjacent to its office in Mumbai and imported machines. Its capital increased from Rs 600,000 to Rs 3 million. This allowed for a new factory to be built alongside the old one in 1951. As the company expanded, it continued its nationalist message. A poster from 1948 highlights both an emphasis on quality and a strong nationalist zeal (Figure 1). Not only does the poster address the ideal character of a model citizen, it also notes that Cipla was as good as the best in the world. It is important to note the claim of “supreme quality” in this early advertisement—but also that this claim could not be checked by Indian authorities at the time, since the existing provisions in Indian law did not require any quality checks, either pre- or post-market.Footnote 20

Figure 1. Cipla advertisement. (Courtesy of Cipla Archives, Mumbai, India.)
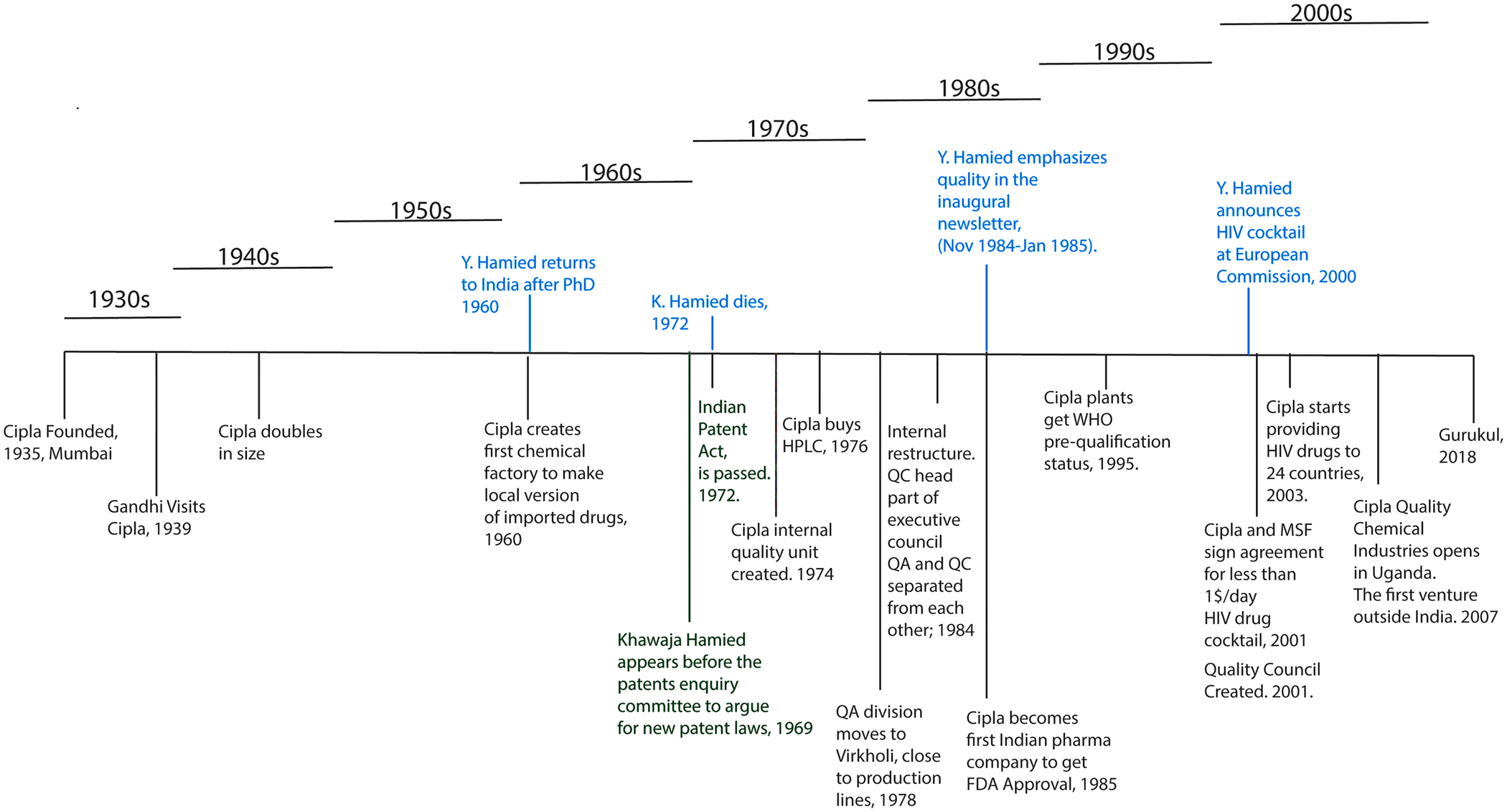
Figure 2. Cipla inception, growth, and timeline of major milestones with regard to quality. (Figure by authors.)
In 1960 came the first major turning point in Cipla's business model, with the creation of a chemical factory to produce Indian versions of imported drugs. The new chemical manufacturing division was headed by Khwaja Hamied's Cambridge-educated son, Yusuf, who had just returned to India after finishing his PhD in organic chemistry in Britain. The post–World War II period had seen major shifts in the pharmaceutical industry in the United States, Europe, and Japan. The literature from that period suggests a strong emphasis both on quality in manufacturing and production and on evaluating the cost of quality (a term coined by Joseph Juran).Footnote 21 At that time, most of the chemicals needed for drug manufacture were imported from Europe, with no local production. Cipla's factory to manufacture these chemicals locally was a novel venture for Indian pharmaceutical companies and one that was highly controversial even at that time.Footnote 22 In postcolonial India, both finished products and raw materials were imported exclusively from abroad (largely from Britain but also increasingly from the United States). There was little emphasis on the local manufacturing of products already available in the market under foreign brands. At that time, the import duty on final drugs was 100 percent and that on the raw material for pharmaceutical ingredients (called active pharmaceutical ingredients, or API) was nearly 150 percent.Footnote 23 Thus, there was a disincentive to import materials and produce local drugs. However, there was a strong financial, and nationalist, incentive to create a chemicals division that could create locally produced materials for locally made pharmaceuticals. This would, however, require a change in patent laws, since the local R&D sector was not coming up with new formulations. Parallel to creating this manufacturing venture, Khwaja Hamied cofounded (and subsequently headed) the Indian Drug Manufacturers Association (IDMA), an industry group that also acted as a lobbying arm of the growing industry.Footnote 24 The main charter of IDMA was the promotion of local manufacturing and the promotion of self-reliance. It was, once again, a vehicle to promote the nationalist cause and create trust in local products.
The initiative to create a company in India that could produce drugs that would meet international standards attracted both controversy and praise and became one of the major components of national discourse on patent laws in the late 1960s. Khwaja Hamied appeared before the newly formed patents enquiry committee of Parliament on behalf of the IDMA in 1969, and his deposition was instrumental in formulating the 1970 Patent Act (eventually passed in 1972).Footnote 25 The most controversial provision of the patent act was its focus on the process and not the product.Footnote 26 The new law meant that as long the process was unique, any company could make a patented product. This meant that Indian firms could copy globally available products as long as they developed unique processes to make them—the crux of much later controversy. The emphasis on national pride and self-reliance remained the primary thrust of Khwaja Hamied's arguments.Footnote 27 During the same period, the environment became more hostile in general to non-Indian manufacturers that would do business in India, and this hostility extended to the pharmaceutical sector, thereby erasing some of Cipla's multinational competition.Footnote 28
Commitment to Quality
The second period of Cipla's growth saw several new initiatives. First, in 1972, Yusuf Hamied took over as CEO of the company upon the death of his father. Yusuf Hamied was more cognizant than was his father of recent trends in the pharmaceutical industry, having been exposed to them during his time in Europe and seen the change in pharmaceutical practices in the post–World War II period. A significant boom occurred in the development of anti-infectives, and global demand for these products (particularly antibiotics) was increasing.Footnote 29
Similarly, the introduction of technology was significantly impacting the process of drug manufacture. Batch processes, and advances in synthetic organic chemistry to improve natural product synthesis, were improving the output in pharmaceutical companies.Footnote 30 New regulatory frameworks focused on testing drugs for efficacy were also introduced in Europe and the United States for better quality control. These new sets of laws greatly depended on new technologies to ensure quality. As a result of Yusuf Hamied's exposure to recent innovations in Europe, associated with development of complex anti-infectives and implementing quality metrics and quality control during manufacturing, he created an internal unit focused on quality in the early 1970s. The internal unit reported directly to the CEO and had its own separate budget along with its own new standard operating procedures that emphasized hygiene, safety, and quality metrics. Yusuf Hamied would personally interview nearly all of the applicants, asking them about various chemical processes and emphasizing the need for quality. Many recruits who were interviewed by the CEO remember a discussion on quality.Footnote 31 This was a departure from the practices of the elder Hamied, who was less hands-on in interviews and whose writings do not show any strong focus on quality. The change apparently percolated to the lower ranks as well. While practices such as using the burners in the lab for cooking or warming up food had previously been acceptable, a stronger, stringent code of conduct was established that would make such practices, and related informality, unacceptable in the new labs.
Another major change in Cipla's culture was the acquisition of new technology. Through a series of investments, Cipla was able to acquire cutting-edge instrumentation from Germany and the United Kingdom to focus on quality control and quality assurance.Footnote 32 While leading pharmaceutical companies had started to use high-performance liquid chromatography (HPLC) instruments in the late 1960s, there was no precedent for using such instruments in manufacturing-related quality assurance in India.Footnote 33 With no local manufacturing sector, few HPLCs were available globally outside western Europe, the United States, and Japan. Furthermore, the costs of such instrument acquisition, and the infrastructure for parts, services, and labor needed to support the performance of such an instrument, were substantial. Unfortunately, Cipla archives do not include receipts showing how much each instrument cost, and the exact labor costs are not available, but it is reasonable to assume that the outlay for these instruments and associated consumables was significant. The technical resources, in terms of both spare parts and trained staff, that were needed to operate and maintain were also in short supply, and none existed in India at the time. There were no publications or reports from any Indian manufacturers or researchers using HPLC in the 1960s or early 1970s. Cipla's acquisition, therefore, marked a major departure from existing practices driven in part by Yusuf Hamied's own deep interest in, and passion for, technology. In an interview, he said, “as a scientist or whatever you may call it, I kept abreast with what was going on in the area of AIDS, reading medical journals and various scientific publications, and in 1996–97, I came across an article in one of the medical journals called HAART. And this article said that a combination of three drugs controlled HIV infection.”Footnote 34
In 1974, Cipla also created new internal protocols for safety and quality assurance and created a separate division for quality assurance in Mumbai; four years later, the quality assurance (QA) division was moved to the suburb of Vikhroli where the drugs were being manufactured. The construction of a new division, with a need to interface directly with manufacturing, was both unprecedented and costly. It required additional transport between the two centers and new internal procedures. In 1984, the QA division (focused on manufacture) was separated from the quality control (QC) division (focused on post-market monitoring of quality), thereby creating two independent units to monitor quality for pre- and post-market compliance, a process that differed significantly from other Indian pharmaceutical companies of the time. This also enabled two separate sets of experts who would look at drug quality and decreased the chance of poor-quality drugs reaching the market.Footnote 35
Perhaps the biggest event of this period was the emergence of Cipla's interest in international markets. Cipla had made several attempts to get U.S. Food and Drug Administration (FDA) approval for several of its drugs in the late 1970s but all of them failed. Lower quality standards in India compared with FDA requirements regarding ingredients, hygiene, manufacturing, and quality assurance processes had blocked Cipla's entry into regulated markets. In 1985, Cipla became the first Indian company to receive FDA approval for its drug manufacturing facilities.Footnote 36 Other Indian companies were also seeking to enter international markets, including Ranbaxy Laboratories, Cipla's competitor, which started building its state-of-the-art facility in 1987 in Toansa and received FDA approval the following year.
On the one hand, Cipla's earlier FDA approval opened new market opportunities for the company and, at the same time, required the creation of compliance protocols to prepare for international audits for the U.S. and European markets. It gave Cipla a business and marketing edge over its competitors. On the other hand, maintaining international standards in Cipla's plants added significant costs in personnel, equipment, processes, and internal governance.Footnote 37 With limited international brand recognition, the cost of international certification was significant in the initial period. In preparation, and then in subsequent compliance, Cipla had to reshape its internal organization. It could no longer opt to grow and fulfill orders rapidly and potentially compromise quality. As a result, it reorganized to separate quality entirely from several other business decisions.Footnote 38 The QA and QC teams had already been separated, and strict regulatory compliance was now established as a prerequisite for any business decisions. All the while, Indian compliance requirements did not require such strict quality guidelines. Even today, Indian manufacturers find it significantly more expensive to comply with more extensive FDA compliance guidelines than to adhere to domestic regulatory requirements.
This led to the now infamous remark by G. N. (Gyanendra Nath) Singh, India's top drug regulator, in 2015 that “if Indian companies had to follow FDA compliance requirements, nearly all would close down.”Footnote 39 Cipla was able to get FDA approval in part through compliance with stricter standards of manufacturing and in part through internal restructuring. In 1984, Cipla reorganized its executive council and the head of QA became part of this central executive board, the highest governing body.Footnote 40
Global Events
The third period associated with the evolution of quality, starting in 1985, was shaped both by global events and by Cipla's increasing global footprint in Africa and beyond. The most important aspect of this period was the global HIV/AIDS epidemic that, in many ways, shaped Cipla's subsequent structure and affected its future operations. The earliest reports of HIV in the U.S. Centers for Disease Control and Prevention (CDC) literature (and subsequently in the popular press) suggest 1981 as the first time the world became aware of the disease.Footnote 41 Initially, the cause and the biological mechanism of the disease were uncertain. Studies from that time show that the classification initially ranged from rare disease to cancer or lung disease and ultimately to autoimmune disease.Footnote 42 Broader public awareness did not emerge until the mid-1980s.Footnote 43 After intense pressure from public-health commissioners in his native California, U.S. President Ronald Reagan mentioned AIDS for the first time in 1985.Footnote 44 The same year, Congress allocated $70 million to AIDS research and the Pentagon announced that it would screen its recruits for HIV.Footnote 45
In India in the late 1980s and early 1990s, there was both a lack of awareness of and little political support to tackle the HIV/AIDS problem. The Indian government, despite India representing the highest number of HIV patients in the region (classified as South and Southeast Asia by the World Health Organization), referred to the AIDS epidemic in India as a problem in its “early stages.”Footnote 46 Cipla was actively growing and had launched its own internal campaign to engage a broader group of its employees in its core priorities, including the emphasis on production that met international quality standards. This emphasis on quality would later converge with global action on HIV and AIDS. In 1984 Cipla launched its first newsletter (called CIPLOG, a play on words where “log” refers to people in Hindi), which focused on the firm's core values and was aimed at its employees and their families.Footnote 47 Writing in the inaugural issue (November 1984–January 1985), Yusuf Hamied reminded workers of Cipla's historical commitments and brought up the issue of self-reliance: “Right from Inception, a major objective of our enterprise has been to contribute to the national endeavor towards self-reliance and self-sufficiency in the drug industry. In other words, we should not only make the latest drugs available, but also manufacture the active ingredients from basic stage.”Footnote 48 Yusuf Hamied's message stressed that quality products from an Indian enterprise meant that Indians were fully capable of doing what had historically been the domain of Western MNCs. A strong sense of self-sufficiency and national pride imbued the words of Yusuf Hamied when he wrote that “our fruitful association with CSIR's National Chemical Laboratory, Pune attracted nation-wide attention in 1983, when we manufactured for the first time in India two widely used anti-cancer drugs, vinblastine and vincristine from a common garden plant, Vinca Rosea.”Footnote 49 Regarding quality, he said in the same letter, “We are constantly scanning the globe for newer drug formulations needed in India which we can successfully manufacture and market. Once we decide to proceed with a particular product, an appropriate process has to be developed for the manufacture of the bulk drug and its formulations. High quality standards have been the hallmark of Cipla products. Accordingly the next step is to arrive at quality specifications and in-process controls at every stage of manufacture.”Footnote 50
The CIPLOG newsletter was published every quarter from November 1984 through 1995. Over ten years starting in 1984, the term “quality” (or a similar word such as “international standards”) would appear ninety-nine times in the newsletter. In the February–April 1987 issue, Hamied wrote, “Obviously our attempts will be to develop a product which is superior to or matches the best.” The August–October 1987 newsletter focused exclusively on the “pursuit of quality” and emphasized, among other things, Cipla's strong interest in and commitment to acquiring and using the latest technology.Footnote 51 In this particular case, as with its acquisition of HPLC in the 1970s, Cipla was breaking new ground, with computerization across all its plants.Footnote 52 The quality issue, once again, was connected to self-reliance. Writing in the August–October 1986 issue of CIPLOG, Hamied opined about the recent FDA approval: “for our company, it was another expression of our continuing commitment to self-reliance, self-efficiency and promotion of indigenous research and development.”Footnote 53
The newsletters also provide a window into a shift in focus and give an early indication of Cipla's interest in the global market. Phrases such as best “value for money” and “here begins quality” appear in stories on new markets, particularly in the United States.Footnote 54 Cipla began to court international donors and international partners aggressively, through letters from both Hamied and Dr. A. J. Gogtay (who eventually became Cipla's chief medical officer). They wrote to leading labs and to professional organizations focusing on blood disorders (thalassemia), ensuring them that “Cipla guarantees the identity, purity, potency and stability of the drug made according to current international Good Manufacturing Practices (GMP) regulations at the time these are dispatched.”Footnote 55
The newsletters were temporarily discontinued because of communal riots in Mumbai in December 1992 and January 1993. The riots created an unstable political environment in India, and Cipla decided to cancel nearly all of its sports events, celebrations, and annual days and to cease public events during the period to avoid any undue attention. The newsletter, which was both a source of corporate news and a report of various family-oriented activities, lost momentum. Many in the company felt that, given the political climate, continuing with the newsletter was not in the best interest of the organization, though the specific arguments for discontinuing the newsletter are unclear. While publication of the newsletter was suspended and little was being celebrated about the growth of the company, oral history records show that even during this period there were internal discussions about quality and its strategy. By 1995, several of Cipla's plants had been certified by the WHO and the FDA but the company remained relatively unknown internationally and its global business was rather small. In 1995, the total exports of all of India's pharmaceutical industry were valued at less than US$600 million, and Cipla controlled no more than 20 percent of the market share.Footnote 56
In the late 1990s, Cipla's interest in global markets and the international momentum against HIV converged as a result of global attention to HIV both as an epidemic and as a symbol of racial inequality in the United States, Europe, and Africa. Cipla was manufacturing AZT in the early 1990s but had been unable to make it affordable to Indian patients.Footnote 57 A number of factors, ranging from U.S. government interest in new funding, interest in the biology and epidemiology of the disease, and support from international donors, as well as movements driven by citizen activists, playwrights, and gay rights activists, made HIV the biggest global health issue of the decade. In 1996, Time magazine's “person of the year” was David Ho, an AIDS researcher. Oral history records and interviews with Cipla's head of quality control suggest that Yusuf Hamied, who had long argued for accessible drugs, saw an opportunity with HIV in late 1990s.Footnote 58 During this time, new initiatives were being launched both within the United Nations system and at the national and local levels.Footnote 59 The creation of the Joint United Nations Programme on HIV/AIDS (UNAIDS) in 1995 also made new funding available.Footnote 60 It is important to note that the issue of accessible and affordable drugs was on the minds of UN leadership and public-health practitioners well before Hamied came on the scene. On July 8, 1996, the San Francisco Chronicle specifically addressed this subject: “The major focus of yesterday's opening ceremony was political. It concentrated on governments that still drag their feet on the AIDS front, and on major pharmaceutical companies whose new drug combinations—however effective in drugs so far—drive expenses for people with AIDS to US$ 10,000–15,000 a year. . . . [Peter] Piot voiced anger . . . at roadblocks to distribution of the latest medicine to those infected, ‘most of these drugs could be made accessible . . . if governments had the right drug policies and if doctors prescribed appropriately.’”Footnote 61
The issue of accessible drugs was being reiterated on multiple forums not just by UN staff and epidemiologists but also by world leaders, including President Jacques Chirac of France.Footnote 62 In response to the sensitivity about drug access, the UNAIDS Drug Access Initiative (DAI) was launched in 1997.Footnote 63 This was a partnership between UNAIDS and brand-name pharmaceuticals to bring the price down to 40 percent of the existing market price.Footnote 64 There were, however, challenges related to infrastructure and implementation and reluctance from large pharmaceuticals to create global price reductions. A Merck & Co. executive, for example, recalled that “they came to talk to us in about 1997 about whether we would provide Crixivan at a discount for use in Uganda. We were skeptical. Our argument about the Drug Access Initiative at the time was that, even if we made our medicine available for free, the infrastructure wasn't there, so people wouldn't be able to use the medicines effectively.”Footnote 65 With time, skepticism faded; Merck itself became a part of the initiative by 2000. However, there were broad issues of IP, access, and in-country costs that remained unresolved and that frustrated both the UNAIDS officials and large brand pharmaceuticals.Footnote 66
In 1999, generic companies started entering the AIDS market, driven largely by support from antiglobalist voices arguing for a more open and “anti-colonial” approach to HIV and AIDS.Footnote 67 There were, however, concerns about the quality of generic drugs, and the UN agencies were reluctant to embrace generics because of a lack of strong data showing comparable quality between generics and name-brand drugs.Footnote 68 Recognizing the frustration of nongovernmental agencies with the UN and its partner organizations—something that UN staff noted as well—Hamied publicly floated the idea of an $800 (per year) combination therapy at a September 2000 meeting in Brussels.Footnote 69 At that time, while prices varied across the developing world, the average patient in the developing world was set back roughly $10,000 to $12,000 annually.Footnote 70
Instead of having patients take HIV drugs from three different manufacturers at high prices, Hamied proposed, he could provide a single “triple cocktail.” Yet this proposal failed to attract any interest from the global health community. It was still too expensive and, more importantly, Hamied did not have a willing partner who could implement his vision of affordable HIV drugs. Nonetheless, he proposed a price even more audacious than the last one, suggesting that AIDS treatment should be available for less than a dollar a day.Footnote 71 Hamied floated the idea and galvanized momentum; it was another significant research development that brought the idea closer to reality. The Accelerating Access Initiative (AAI)—a collaboration of five research-based pharmaceutical companies and UNAIDS, the WHO, UNICEF, UNFPA, and the World Bank—was able to reduce the prices of HIV medicines by about a third by May 2000, and prices continued to decline. For example, one company, MSD, initially offered a two-thirds discount through AAI, and by early 2001 it had lowered its prices further for the least developed countries. However, these prices were still more expensive than Hamied's proposed price of a dollar a day.Footnote 72
In 2001 Hamied partnered with international medical aid agency Médecins Sans Frontières (MSF). Started in 1971, MSF had won the Nobel Peace Prize in 1999 and was riding high on international approval. Cipla had improved its ability to make a potent AIDS cocktail, though it was not Cipla's formulation; it was a combination of three drugs, made by different manufacturers in the United States, United Kingdom, and Germany. In 2001 the agreement was signed, with the cost of the HIV cocktail at $350 for a year. Cipla, in an effort to ensure that its drugs were of appropriate quality and to dispel the notion that access and quality were at odds, also publicly shared the results of its testing of drugs being marketed in Uganda with international regulators and donor agencies, sending samples to international labs for testing to demonstrate that its formulation made in India was safe.Footnote 73 By comparison, the drugs by GSK and Boehringer Ingelheim GmbH were still more expensive in some countries but on par with Cipla's in some of the lowest-income countries.Footnote 74 The variations in prices across Africa were due to differences in procurement practices, negotiations, volume of drugs imported, and the nature of regulatory frameworks.Footnote 75
Cipla continued to work toward Hamied's simpler dollar-a-day goal, starting negotiations in 2001 with the government of South Africa, the country with the largest HIV burden. Here, once again, Cipla's entry into a market benefited from events that it had not started. In 1997, the South African government had passed its landmark Medicines and Related Substances Control Act that gave the government a legal mandate to override patents in cases of acute national need.Footnote 76 However, and as expected, international pharmaceutical companies challenged this legislation with a lawsuit in South Africa in February 1998.Footnote 77 The plaintiffs argued that the new law was in violation of the World Trade Organization act called Trade Related Aspects of International Property Rights (or TRIPS), to which South Africa was a signatory.Footnote 78 The question was whether the AIDS crisis was a health emergency or an issue of trade and IP infringement.Footnote 79 What eventually tipped the balance were efforts from an activist group called Treatment Action Campaign (TAC).Footnote 80 Formed by three AIDS patients, TAC strongly supported the government's program in overriding patents; however, the nascent partnership between TAC and the government started to sour when the government refused to declare HIV a national emergency and refused to provide a key drug, nevirapine, to HIV patients. The drug was shown to be effective in controlling mother-to-child transmission of HIV. TAC took the government to court, in the case Treatment Action Campaign and Others vs. Minister of Health and others, in Pretoria High Court.Footnote 81 At the same time, supported by outside groups (including Cipla), TAC created a major international campaign for the cause and painted big pharma as the culprits.Footnote 82 The effort gained momentum with support from activist groups in Europe. The pressure on pharmaceutical companies to lower prices was tremendous, and Cipla benefited fully from it.
Cipla was also lobbying the government of South Africa, independent of the TAC case, to allocate to it a compulsory license (a process by which Cipla could sell the drugs even though it did not have the patent), arguing that quality drugs were not being made available to the population at affordable prices.Footnote 83 Cipla had the advantage of not just being a generic company with low R&D costs but also manufacturing in India with low production costs. Hamied had also famously remarked that he was not interested in making money on the backs of HIV patients, so he was able to argue that a compulsory license was intended to benefit the patients and not the company. The stipulation of the license was that the government of South Africa would allow Cipla to make and distribute the HIV cocktail despite not having any IP ownership of the drugs. The discussions between the government of South Africa and Cipla became public in the same week that national protests against high prices for HIV drugs erupted across the country.Footnote 84 Cipla and other companies argued for an exclusive import license and created a significant public campaign for which Cipla paid.. Eventually, under public pressure, the large pharmaceutical companies withdrew their challenge and made it possible for Cipla to export its drug cocktail to South Africa.Footnote 85 While Cipla and Ranbaxy Laboratories benefited tremendously, this success was largely due to the international campaign and the legal challenge mounted by TAC and the subsequent civil disobedience movement created at the local and national levels by TAC and its partners.
Cipla used the court ruling to its advantage and by 2003 had registered five different drugs in South Africa and was providing HIV medicines in more than thirty-five countries. Cipla found willing buyers from two new international bodies with significant resources. One was the Global Fund (also known as the Global Fund to Fight AIDS, Tuberculosis, and Malaria), which by 2003 had committed US$4.7 billion to fight HIV/AIDS, malaria, and TB.Footnote 86 The second was the President's Emergency Plan for AIDS Relief (PEPFAR), which had an authorized budget of US$15 billion over a five-year period (2004 to 2008).Footnote 87 Resources from these two organizations allowed national programs to procure large quantities (worth approximately US$189 million) of Cipla drugs.Footnote 88
The expansion to low- and middle-income countries was a centerpiece of Hamied's business model, rooted in a sense of self-reliance and nationalism. He argued that both low cost and high quality were possible in former European colonies.Footnote 89 He certainly was not the only one making the case that drugs made in India were making it possible for Africans with HIV to survive, and Cipla was not the only company operating in Africa (in 2001, Zydus Cadila, Aurobindo, and Sun Pharmaceuticals were also operating in South Africa), but Cipla and Hamied continued to get the lion's share of the limelight. Cipla also continued to enjoy a better reputation during that period than its competitors, including Ranbaxy, which had to withdraw seven of its HIV drugs in 2004 because of quality inconsistencies.Footnote 90
Post-HIV Global Growth
In 2003 Cipla's annual revenues were approximately $350 million.Footnote 91 Over the next fourteen years, its annual revenues increased five-fold.Footnote 92 Since 2001, Cipla, one of the three largest pharmaceutical companies in India, has seen major growth in various parts of the world, particularly in Africa and, more recently, Latin America. In 2004, Cipla entered an agreement with the government of Uganda and created a new venture, called Cipla Quality Chemical Industries Limited (CQCI), which includes its first production facility outside of India.Footnote 93 The focus of this venture, the first of its kind in East Africa, was HIV and malaria. The Cipla plant eventually earned WHO certification, a distinction that no other manufacturing plant in Africa has achieved. However, this has come at a significant cost because of a lack of local capacity and the large number of Cipla employees who shuttle back and forth between India and Uganda. This cost is partially paid by tax incentives by the government of Uganda, but long-term sustainability remains a concern owing to limited technical capacity and challenges associated with education quality.
For Cipla, the term “quality” in the name “Cipla Quality Chemical Industries” was critical to its message and branding in a new market.Footnote 94 Beyond Uganda, Cipla maintains its strong presence in South Africa that dates back to the early days of HIV. Cipla is now the largest manufacturer of drugs in South Africa and the largest supplier of antimalarials in Africa.Footnote 95 At the same time, Cipla has reorganized its internal quality structure in several iterations. In 2001, it created an independent unit to coordinate quality control at all plants and manufacturing sites, called the Quality Council. The council, which is a team that includes heads of quality control at each production site, meets every month and reports to the overall head of QC. This council includes not only members of the QC team but also the head of manufacturing and supply chain. Cipla has created a hundred-point quality scale for all its manufacturing sites that is reviewed quarterly by the head of QC. There is also a quarterly quality review with the CEO, who still takes an interest in issues of quality.Footnote 96 Cipla has started a quality academy and created a Cipla institute for quality called Gurukul (meaning a traditional residence school or a learning academy) in the Indian city of Indore in 2018.Footnote 97 The vision is that all Cipla manufacturing unit technicians will eventually be graduates of this academy. The emphasis on technology has remained a staple of Cipla's quality control efforts, with nearly half of the annual QC budget spent on equipment acquisition and maintenance. It is estimated that in addition to the cost of quality control and quality assurance (which is in the tens of millions of dollars annually) Cipla annually spends upwards of at least $5 million on adding new staff, new equipment, and new training.Footnote 98
Despite its enormous growth in Africa, new markets in Latin America, and acquisition of InvaGen in the United States, Cipla's approach has focused largely on its Indian manufacturing plants and its Indian workforce that is trained to work within India and elsewhere.Footnote 99 Nearly all training for QA staff happens in India, which adds a substantial cost for staff working at African plants. Company leaders in Africa and Latin America agree that Cipla has a reputation for high-quality products but note that this emphasis on quality means its production is slow relative to that of its competitors. At times, Cipla has been unable to meet large international demands owing to its longer production time.Footnote 100 The company's current market performance and reputation are based on its ability to make generics, particularly for low-income markets. As it starts to move into more complex biological drugs for noncommunicable diseases (particularly cancer) it remains unclear how the existing model will perform.Footnote 101
Conclusion
This article has sought to explain how the Indian pharmaceutical company Cipla developed the quality control technology that lay at the heart of its endeavor to manufacture affordable generic drugs. These drugs were instrumental in combating the AIDS epidemic of the early 2000s. While developing and sustaining quality is a complex process, and requires coordination and contributions from individuals and institutions over long periods of time, this analysis suggests that three fundamental pillars within Cipla provided the platform for the company to make the quality argument to its consumers and international regulators. While some of these factors were similar to other businesses in India, such as the Tata Group, and some have been seen in other pharmaceutical companies in India, the combination of these three components, along with decisive action at opportune times, provided Cipla a unique advantage.
First, it was important to create an internal mechanism that worked despite a void of regulatory infrastructure in the environment that otherwise prevented the independent ratification of quality. This mechanism evolved through iterative experimentation and the incorporation of technology. While it is important to note that there have been discussions and often serious concerns about the safety and reliability of generic products in Africa, Cipla by and large maintained a strong reputation for reliable formulation and production.Footnote 102
Second, Cipla took advantage of exogeneous opportunities that came from global events. In particular, the HIV crisis in Africa, arguably the biggest global health challenge of the last four decades, enabled Cipla to internationalize its brand and argue that safety and efficacy could be provided at accessible prices. This improved the company's image and unlocked new business opportunities in markets in Africa and beyond.
Finally, perhaps the factor that most sets Cipla apart from other generic drug manufacturers is the connection of its founders (first Khwaja Hamied and then Yusuf Hamied) with the Indian independence movement and the related drive to economic self-sufficiency of a proud Indian nation. Along with a conventional corporation's aims and objectives, this drive appears to have motivated many of Cipla's actions, including its long-term emphasis on quality, despite the incremental costs incurred in this adherence.