INTRODUCTION
The Cirratulidae is a widespread family of sedentary deposit-feeding polychaetes (Penry & Jumars, Reference Penry and Jumars1990; Blake, Reference Blake, Blake, Hilbig and Scott1996; Dorgan et al., Reference Dorgan, Jumars, Johnson and Boudreau2006), most of which inhabit shallow layers of sedimentary environments from the intertidal zone to the deep sea (George, Reference George1964a; Blake, Reference Blake, Blake, Hilbig and Scott1996, Reference Blake, Sardá, San Martín, López, Martín and George2006; Levin & Edesa, Reference Levin and Edesa1997; Rouse, Reference Rouse, Rouse and Pleijel2001). Recent taxonomic revisions of this problematic family (Blake, Reference Blake, Petersen and Kirkegaard1991, Reference Blake, Blake, Hilbig and Scott1996) demonstrate the lack of clearly-defined morphological characters to distinguish between species and sometimes genera; ontogenic modification during growth and development further obscures the taxonomy in several species (George & Petersen, Reference George, Petersen, Petersen and Kirkegaard1991; Díaz-Díaz & Salazar-Vallejo, Reference Díaz-Díaz, Salazar-Vallejo, de León, Bastida-Zavala, Carrera-Parra, García-Garza, Peña-Rivera, Salazar-Vallejo and Solís-Weiss2009). Accurate re-descriptions of some ‘old cosmopolitan’ species within their reported distributions are required to define expected differences in widely-distributed populations (Chambers, Reference Chambers2000; Chambers et al., Reference Chambers, Lanera, Mikac, Giangrande, Gambi and Rouse2011); furthermore, the existence of sibling species complexes is expected (Christie, Reference Christie1985; Chambers & Woodham, Reference Chambers and Woodham2003). Moreover, early juvenile stages of both bitentaculate and multitentaculate cirratulids are known only for a few species (Petersen, Reference Petersen1999; Halt et al., Reference Halt, Rouse, Petersen, Pleijel, Rouse and Pleijel2006). The population dynamics and ecology of a few cirratulid species has been investigated with respect to long temporal (years, several seasons) and large spatial (regional) scales, mainly in pristine or human-impacted sediments (Gibbs et al., Reference Gibbs, Langston, Burt and Pascoe1983; Oyekan, Reference Oyekan1987; Gherardi et al., Reference Gherardi, Sciscioli, Lepore, Todisco and Giangrande2007; Elías & Rivero, Reference Elías and Rivero2011). In contrast, manipulative studies on colonization patterns, settlement preferences and distribution within the sediment at small scales are lacking.
This work is a part of a larger experimental investigation of the small spatio-temporal colonization of sediment-dwelling macrofauna from its source habitat into an introduced bare substratum, in a semi-confined marine environment. The main aims of this paper are: (1) a comparison between local adult cirratulid assemblages of the natural sediment (source area) and the extent of colonization of the artificial substratum; (2) a description of the morphology and settlement patterns of an unknown early juvenile stage here defined as Cirratulidae gen. sp.1.
MATERIALS AND METHODS
Study area
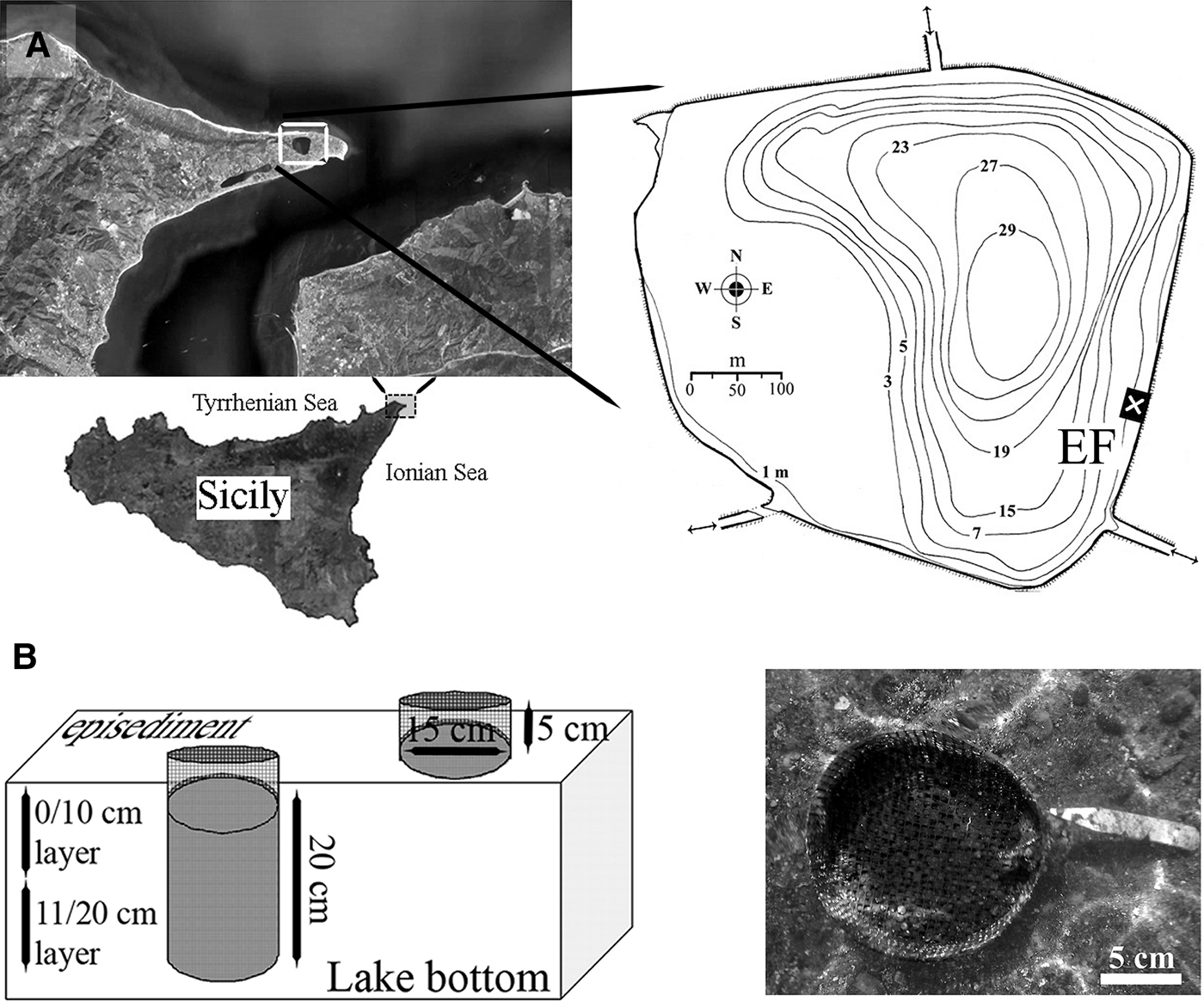
The Faro Lake (38°16′07″N15°38′13″E; 0.26 km2 surface) is a small temperate meromictic (i.e. where the layers of water do not inte1rmix) marine basin of 29 m maximum depth, located in the Oriented Natural Reserve of Peloro Cape, NE Sicily (Figure 1A). Hydrodynamic exchanges are driven by the tidal regime of the nearby Messina Strait through two small channels. The Faro water mass is almost permanently stratified, with the upper 10–15 m of mixed water and the deeper sulphide-rich anoxic layer having very reduced water movement. The fresh water supply is mainly limited to atmospheric precipitation. Shallow water salinity ranges from 26–34 psu (February–March) to 35–36 psu (August–September); mean temperature is 10–11°C and dissolved oxygen content, 6.5–8.0 ml l−1 during winter, ranging from 29 to 30°C and from 3.5 to 3.8 ml l−1 during summer, respectively. Surface redox potential varies from +120 mV to +190 mV; pH ranges from 7.9 to 8.6 (Saccà et al., Reference Saccà, Guglielmo and Bruni2008). Both water column and sediments have a moderate content of organic matter, which supports a relevant microbial productivity (Leonardi et al., Reference Leonardi, Azzaro, Azzaro, Caruso, Mancuso, Monticelli, Maimone, La Ferla, Raffa and Zaccone2009). Anthropogenic pressure is moderately high, due to adjacent coastal urbanization and the presence of mollusc aquaculture.

Fig. 1. Study site: (A) the coastal marine lake of Faro (NE Sicily, central Mediterranean Sea) and location of the experimental field (EF); lake channels (double arrows) are shown; (B) schematic (left) and real view (right) of artificial modules (cylinders) and their position with respect to the sandy bottom.
Experimental design and sampling procedure
The study area was 7 × 4 m, at 1.2 m mean depth and was located at the ESE lake shore. It extended over a broadly homogeneous bottom, with the sediment consisting of coarse sands mixed with bioclastic fragments (cobbles 11.96%; gravel 21.93%; coarse sand 52.88%; medium sand 11.16%; fine sand 2.03%; silt/clay 0.04%), poorly to scarcely sorted (mean σϕ 1.28), with a less conspicuous mud fraction (silts and clays from 0.02% to 0.10%). Sediments were highly reduced, with a mean redox potential discontinuity at 12.5 cm (early spring) and 5.4 cm (early summer); the mean pH was 7.76 and mean Eh −315.49. Organic matter ranged from 2.8 to 4.3% of total organic carbon; the carbonate content was from 1% to 14.5%.
The experimental substratum consisted of artificial expanded fire clay granules (EFCG), from 4.0 to 8.0 mm in diameter (0.31 g cm−3), washed and packaged into cylindrical modules of 20 × 15 cm plastic net, with a mesh of 5 mm. The estimated average free interstitial volume was 1.37 l (about 42% of the total volume). On 16 May 2008, eighteen modules were tagged and almost entirely buried into the natural sediment using a plastic core (Figure 1B). After 15, 30 and 60 days, quantitative samples were obtained by removing two cylinders at random (six replicates), together with two random cores of equal volume (3.5 l) of natural undisturbed sediment inside the experimental field. Another two replicates of this latter substratum were also sampled 15 days before the positioning of modules. Sampling design was asymmetrical, as fewer sediment replicates were chosen to reduce disturbance in a limited experimental field. The cylinders and sediment cores were removed and divided into upper (0–10 cm) and lower (11–20 cm) layers to evaluate the vertical distribution of fauna. Both the artificial and natural substrata were fixed with 10% buffered isotonic formalin stained with rose Bengal. In the laboratory, the natural sediment and artificial substratum were elutriated, to remove the organisms. About 150 mL of the sample was placed in a glass funnel and washed out by means of an upward-directed still water flux. Lower-density supernatant particles were sieved on a steel ASTM-USA series of 1.0 and 0.33 mm meshes. The fauna was identified to the lowest taxonomic level and stored in 70% ethanol. Specimens of the family Cirratulidae were treated separately.
Total abundance (N) and average density (N/L) were determined for the cirratulid assemblage. Short time comparisons between the lake sediments and the colonization of the granular artificial substratum, at two different vertical layers, were performed with a Student's t-test on independent groups, with Bonferroni's correction of significance for multiple tests. An early and uncommon juvenile stage of an undetermined cirratulid species, Cirratulidae gen. sp.1, was investigated in more detail and is discussed in this paper. A detailed morphological description of 24 specimens of representative sizes was carried out using a Zeiss stereo- and optical light microscope, equipped with an ocular micrometer. Microphotographs and drawings were performed by a camera AxioCam ICc 3 (3.3 Mpxl resolution) from 16× to 1000× (oil-immersion) magnification.
Morphometric analysis
The external morphology of the Cirratulidae gen. sp.1 juvenile stage has been described and its characteristics quantified. The general body shape, with the morphology of the anterior branchial region and the posterior portion, according to the segmental variation, is provided. Twelve complete specimens from both the lake sediments and from the artificial substratum were selected as representatives of each sub-population size. The number of segments, total body length, pharynx diameter, length and width of prostomium, peristomium, chaetigers 1, 2, 5, 10, 15 and the last two chaetigers and pygidium, as well as noto-, neuropodial chaetal length and the dimensions of the branchial filaments at base and tip were assessed as mean ±95% confidence limits and minimum/maximum values (α 0.05, N = 24). Among these quantitative variables, highly inter-correlated measures (Pearson r > ±0.8) for chaetiger 1, and highly variable parameters with respect to the contraction of fixed specimens, such as the peristomium, buccal opening and last two chaetigers, were excluded from the subsequent analyses. Body length, dimensions (length and width) of the prostomium, chaetigers 2, 5, 10, 15 and the pygidium were selected for multivariate analysis. Due to the homogeneity of measures, variables were not standardized. Euclidean distance, after normalization, was applied as a resemblance measure among specimens in the morphometric multivariate space. Principal component analysis (PCA) (Chatfield & Collins, Reference Chatfield and Collins1980; Clarke & Warwick, Reference Clarke and Warwick2001) with the thirteen most explicable measures as overlaid vectors was carried out using the PRIMER-E v 6.2 dedicated software package (PRIMER-E Ltd, Plymouth Marine Laboratory, UK).
RESULTS
Small-scale distribution of the cirratulid assemblage
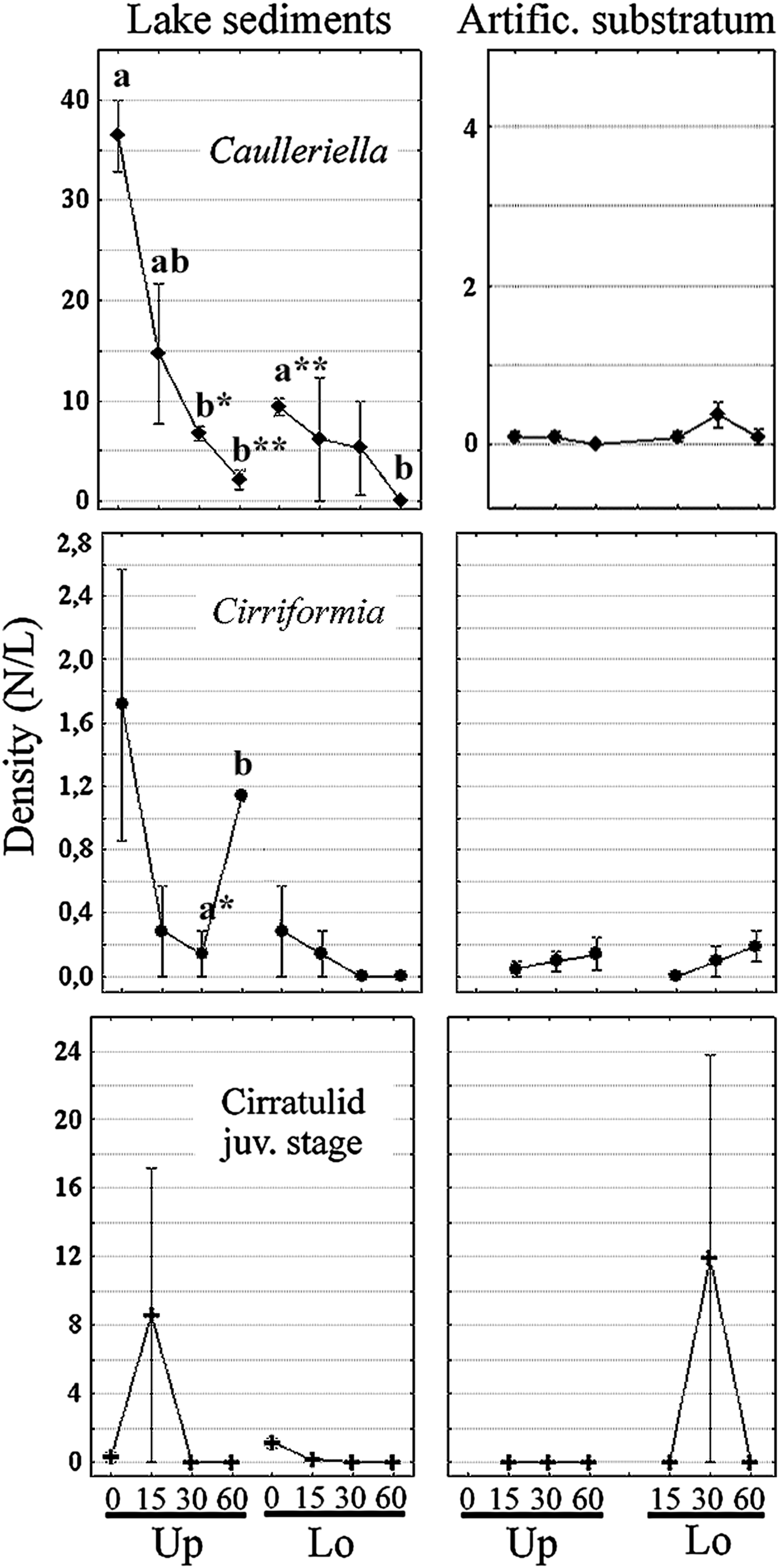
During the experiment, the local adult cirratulid assemblage was composed of only two species, Caulleriella bioculata (Keferstein) and Cirriformia tentaculata (Montagu). In the experimental field, a total of 566 individuals of the former species occurred in the natural sediment with frequency of 1.0 sample−1, and a low aggregation effect (total mean abundance 70.8, SD 66.8). A clear seasonality was observed for this species; 83% of individuals were restricted from late March to middle of May (numerous individuals with fertile gonocoele), abruptly decreasing from June to July both in the upper (May vs July, t = 9.2, P-level < 0.01) and lower layer (t = 11.0, P < 0.01) (Figure 2). A mean density of 15.0 ± 10.2 N L−1 for C. bioculata, 74% of population occurred in the upper sediment layer (SD 14.7), whereas 5.2 ± 3.8 N L−1 (SD 5.5) were in the deeper layer, although this differential distribution was not statistically significant (t = 1.76, P = 0.10). Only 16 adults penetrated into half of the artificial cylinders after 15 days, but with little aggregation behaviour (SD 0.76). The density of immigrants was very low for the whole experiment and was not significant. A mean of 0.06 ± 0.06 N l−1 (SD 0.12) colonized the upper layer, whereas 0.19 ± 0.14 N l−1 (SD 0.29) colonized the lower layer (t = −1.69, P = 0.10), after 30 days of colonization.

Fig. 2. Short-time trend of the Lake Faro cirratulid assemblage. Mean density (±SE) is compared between the source area of sediments and short-time colonization of the artificial modules with respect to vertical distribution (Up/Lo). Significant differences are indicated in bold lower case letters (t-test, P < 0.05*, 0.01**).
Twenty-six individuals of C. tentaculata were found in the sediment with a frequency of 0.75 sample−1 and a moderately aggregated distribution (total mean abundance 3.3, SD 3.5). Most of the individuals were distributed in the upper sediment layer (92%), with a mean of 0.8 ± 0.6 N l−1 (SD 0.84) vs 0.1 ± 0.1 N l−1 in deeper sediments (SD 0.21; t-statistic = 2.32, P = 0.03) (Figure 2). A small temporal trend was not significant for this species. Fertile individuals were observed, though not constantly, during the whole investigation. Thirteen adults of C. tentaculata colonized half of the artificial modules after 30 days, and showed a moderate, although not significant, increase during summer. In the new micro-habitat, individuals were distributed along the whole vertical extent of the substratum, with a mean 0.1 ± 0.08 N l−1.
In the same environment, the early juvenile stage of Cirratulidae gen. sp.1 was found in the natural sediment within a narrow time window. Ten individuals were found in the middle of May and 61 individuals at the end of May; no individuals were found from June to July. Most individuals (87%) were distributed in the upper sediment layer, with a mean of 2.2 ± 4.2 N l−1 and a strong aggregation in time (SD 6.0). Density was reduced to 0.3 ± 0.4 N l−1 in the lower layer, with a relevant aggregated distribution (SD 0.5) (Figure 2). The sample frequency was 0.38 sample−1 and the SD was 21.2 due to the high aggregation effect (settling cohorts). In the granular artificial substratum, a total of 250 early juveniles were found in one sample replicate at the second sampling time (June), 30 days after positioning of the bare substratum. In this case, the frequency was 0.06 sample−1 and the SD increased to 58.9. In this micro-habitat, all juveniles settled in the lower layer (4.0 ± 7.9 N l−1). Due to the stressed inter-replicate variation, the Student's t-test was not significant among sampling times or vertical distribution of individuals.
Description of the early juvenile stage (Cirratulidae gen. sp.1)
GENERAL BODY SHAPE
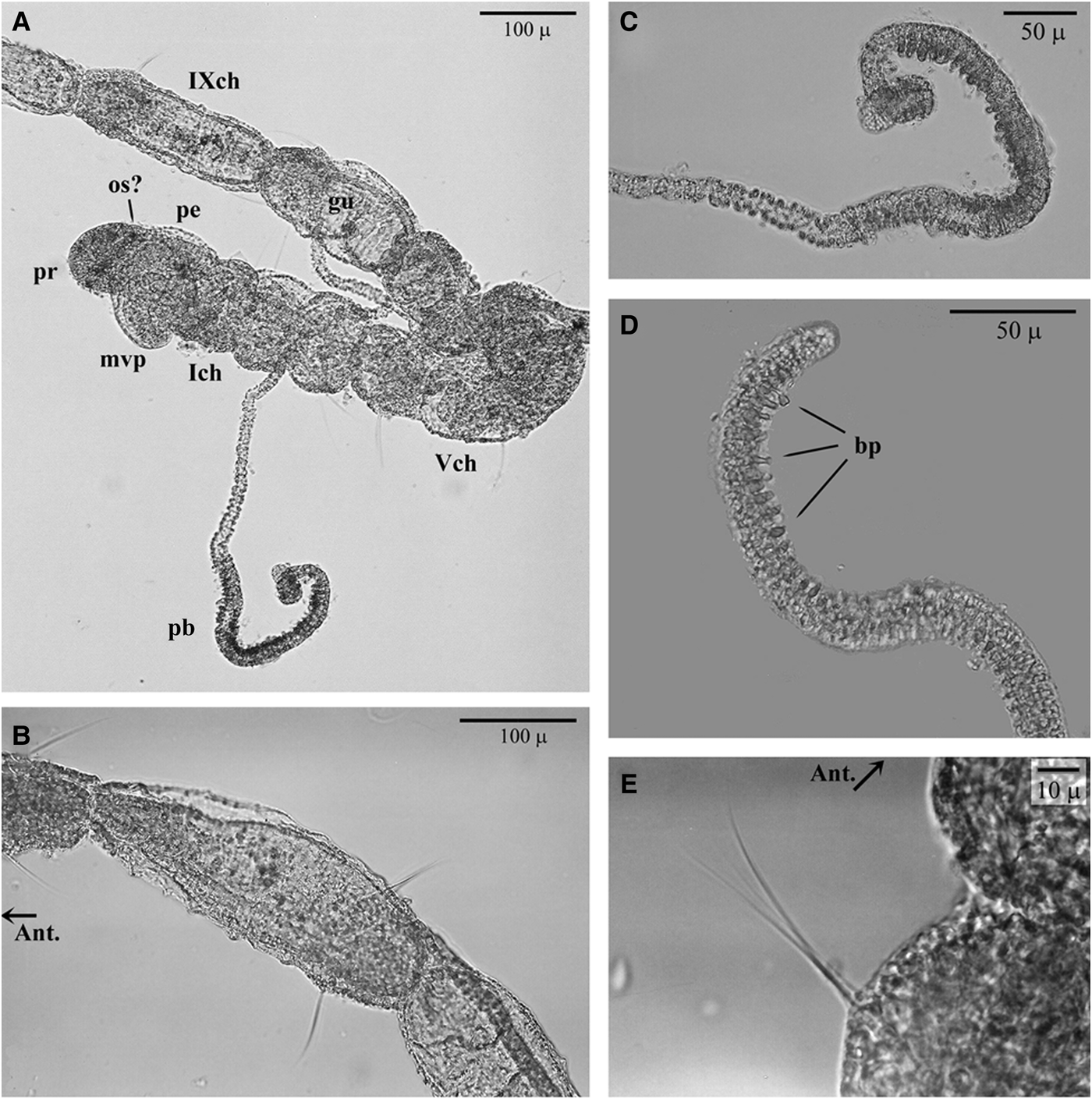
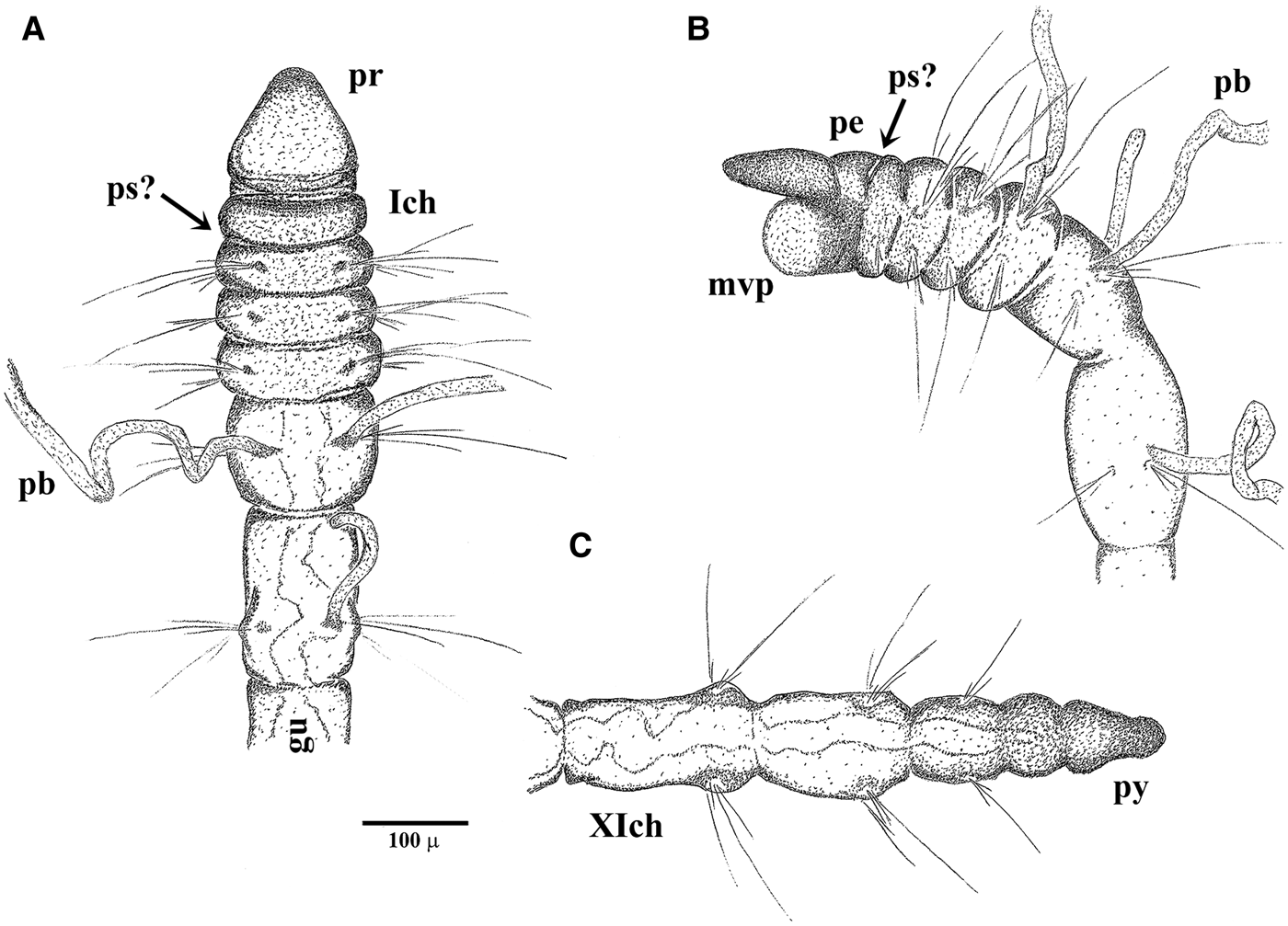
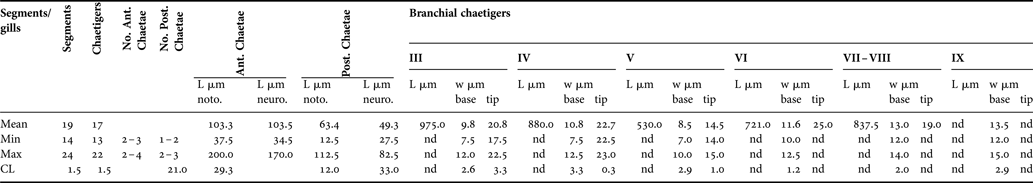
The early juveniles have 14–24 segments (or 13–22 chaetigers) (Table 1). The prostomium is broadly rounded; some individuals have small and irregular denser pigmentation, which might be interpreted as simple ocular spots (Figure 3). The achaetous peristomium is as long as wide and more or less distinguishable from the second segment, due to eversion of the pharyngeal apparatus in response to the fixation technique. The pad-like pharynx is everted in most specimens, with the surface covered by minute papillae (Figures 3A, 4B & 5B). Palps are absent in all fixed specimens, but some individuals show more or less visible pits in the zone between the posterior peristomium and the first chaetiger (see Figure 4A, B) that might be scars of palp insertion. The first chaetiger is shorter than wide. Chaetigers 2–4 have the same length and width and are sub-spherical to sub-oval in shape, thus giving a bead-like appearance to the anteriormost region (Figures 3A & 5B). Chaetigers 5 to the last fourth (third in some specimens) are progressively longer than they are wide, with a ‘sausage’ shape (anterior) or long-stick shape (posterior). The last third of each segment is more or less enlarged due to the position of parapodial lobes (Figure 3E). The pygidium lacks appendages, and has a slight mid-terminal constriction (see Figure 4C).

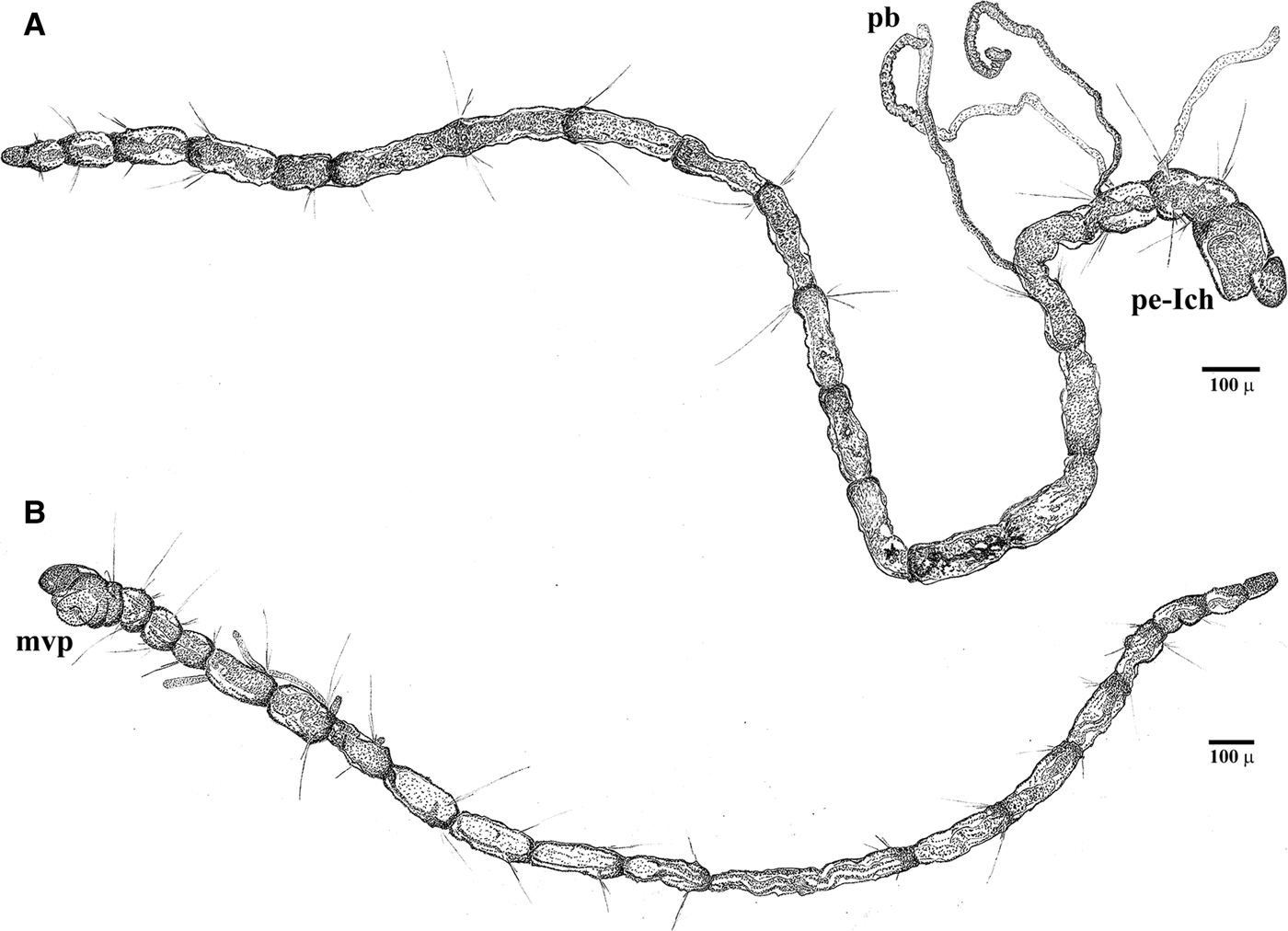
Fig. 3. Light microscopy images of the Cirratulidae gen. sp.1 juvenile stage settled in the experimental substratum: (A) paratype_04, head and nine anterior segments, with a papillate branchial filament and (B) its magnification; (C) paratype_05, distal portion of the papillate branchiae, chaetiger 3; (D) paratype_05, anterior fine capillary chaetae, chaetiger 2 and (E) mid-region, chaetigers 10–11. Ant., anterior side; bp, (glandular) branchial papillae; ch, chaetiger; gu, gut canal; mvp, mouth, partially everted ventral pharynx; os, ocular spot(?); pb, long papillate branchia; pe, peristomium; pr, prostomium.

Fig. 4. Morphotype I in the natural lake sediments: (A) holotype_02, dorsal view and (B) paratype_02, lateral view, anterior portion with possible scars of palp insertion (ps?); (C) paratype_04, hind portion and pygidium (py); gut canal is partially distinguishable. See Figure 1 for other abbreviations.

Fig. 5. Morphotype II settled in the artificial substratum (entire specimens): (A) holotype_01, twenty chaetigers, dorso-lateral view; first chaetiger is not contracted and not distinguishable from the peristomium (pe-Ich); papillate branchiae (pb) on chaetigers 3–5; (B) paratype_01, seventeen chaetigers, ventro-lateral view; finely papillate pharyngeal pad everted (mvp); pre-pygidial segment achaetous.
Table 1. General morphometry of early juveniles of Cirratulidae gen. sp.1 sampled in the Faro Lake (Mediterranean Sea). L, length; w, width.

BRANCHIAE
Due to their extreme fragility, paired filamentous branchiae are visible in some segments of most specimens, limited to the anterior region from chaetigers 2 or 3 to 7, rarely 9, and often not paired (Table 1; Figures 3A, 4A, B & 5A). Branchiae reach 788.7 ± 149.9 µm mean length. The anterior-most filaments are thinner in their basal portion (mean width 11.2 ± 1.5 µm), but clearly enlarged in the mid-distal portion (20.4 ± 3.5 µm). In this latter portion, the branchial surface appears to be papillate, due to the presence of large (possibly glandular) cells that stain positive in Rose Bengal (Figure 3B, C). The following filaments are not distally club-shaped and the smooth surface lacks papillae.
PARAPODIA AND CHAETAL FORMULA
In some specimens, the second segment bears only one ventro-lateral short bundle of 1–2 very small capillary chaetae. This neuropodium is not clearly distinguishable in all specimens, in which it appears to be achaetous, as is the peristomium. All the following chaetigers are biramous, with divergent parapodial rami, which bear 2–3, rarely 4 simple capillary chaetae in each bundle in the anterior and middle body region, and 1–3 chaetae in the posterior region; one or two chaetae among them are clearly longer (Figure 3D, E). Parapodia from chaetigers 1 to 5–6 are located in the centre of each segment (Figure 3D); from chaetiger 7 to the following chaetigers, the chaetal bundles emerge in the posterior third of each segment (Figure 3E). All chaetae are narrow and do not exhibit any expansion or serration even at highest magnification. Simple chaetae from chaetigers 2–3 up to the last fourth (or third) posterior chaetiger measure from 34 to 200 µm (Table 1); in the middle-posterior region, chaetigers 8–12, the capillary chaetae progressively lengthen from 49 to 112 µm. The notochaetae are slightly longer than the neurochaetae and the pre-pygidial segment is achaetous in some specimens (see Figure 4C), indicating an active growing region.
All individuals are deposited at the ‘Laboratorio del Benthos, Dipartimento di Scienze Biologiche e Ambientali’, University of Messina, coded as BAEM.PRA05-07.POLY.Cirratjuv001-321.
Morphological remarks on morphotypes (Cirratulidae gen. sp.1)
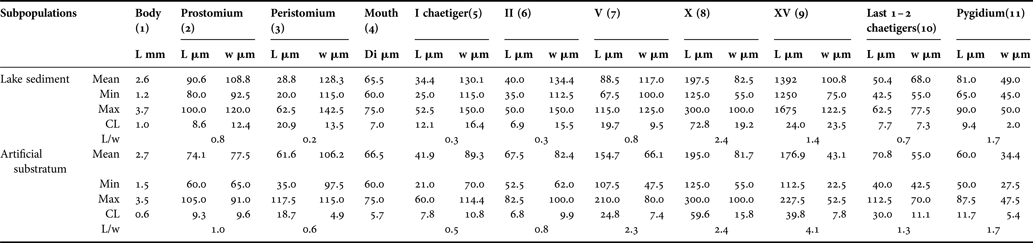
The juveniles of Cirratulidae gen. sp.1 from natural sediments showed some morphological differences compared to individuals that colonized the experimental substratum. All specimens which inhabited sediments were more brownish, probably due to their distribution in the upper sediment layer; the prostomium appeared characteristically triangular-shaped, clearly wider at the base (Figure 4). The anterior part was more contracted; the peristomium and chaetigers 1–5 were significantly wider than they were long (Table 2). In contrast, all specimens that settled in the introduced substratum had a paler body colour, with an oval-flattened prostomium, as long as it was wide, not enlarged at the base (Figure 5); the anterior segments were more extended, as long as they were wide or even slightly longer (Table 2). The middle body region was comparable between the sediment and the artificial substratum types both in shape and length, as demonstrated by the mean length-to-width ratio. The posterior region, except for the pygidium, was also moderately thinner in individuals that settled into the artificial substratum. However, the total body length was not significantly different among individuals in the two habitats, except that those in the natural sediments were more variable and only slightly shorter. With regard to branchial filaments, parapodia and chaetae, their position, extension and shape were similar in specimens from the two habitats.
Table 2. Comparison of two morphotypes of Cirratulidae gen. sp.1 juvenile stage, sampled in natural sediment and into the artificial substratum (cylinders). Di, diameter; L, length; w, width. Numbers in parentheses are vectors in the multivariate principal component analysis plot.

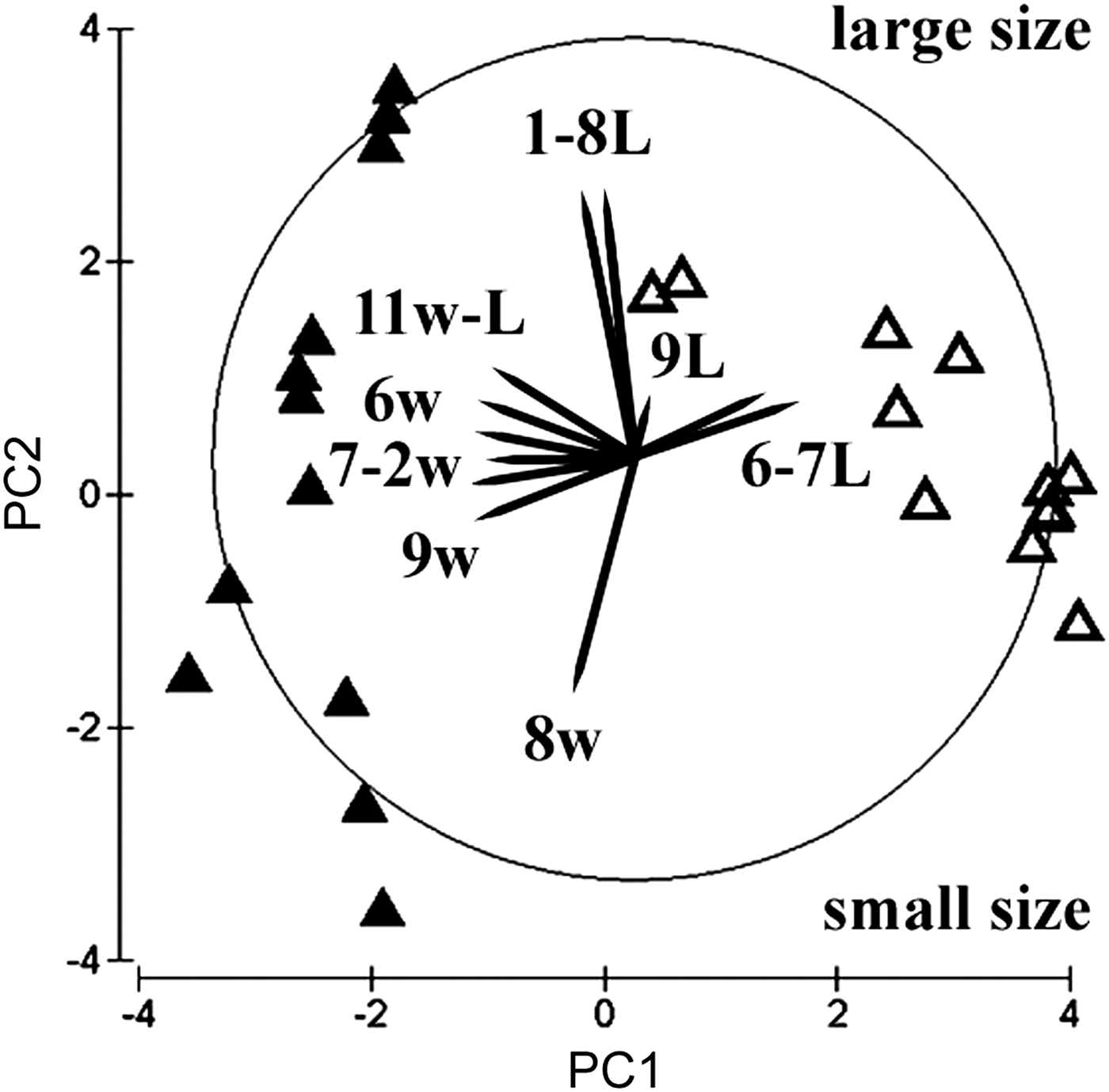
The multivariate principal components plot discriminates the two sub-populations well (Figure 6), according to the first and second PC axes, which explain almost 74% total variation (Table 3). The PC1 arranges the juveniles of the natural sediment according to an increase in anterior body width, whereas an increase in length of chaetigers 2 and 5 is responsible for the arrangement of juveniles into the artificial substratum. The PC2, in contrast, arranges specimens according to total body size and to the length of the tenth chaetiger, whereas an increase in the width of this segment is more evident in the natural sediment sub-population. Juveniles of natural sediments are therefore more variable in body size and show a greater 2-D spreading in the PCA plane. In contrast, the other sub-population is more homogeneous in size (possibly only one cohort), but more variable in terms of prostomial, chaetigers 2 and 5, and pygidial dimensions. Only larger specimens of the two types appear to converge in their morphomentric appearance. The PC3 (not shown) explains only 11% variation and arranges the specimens mainly according to the length of the fifteenth posterior chaetiger and, secondarily, according to the width of the tenth chaetiger in the middle body region.

Fig. 6. Multivariate 2D principal component analysis plot of the two morphotypes of Cirratulidae gen. sp.1 juveniles (N = 24), settled in the lake sediments (full triangles) and in the artificial modules (empty triangles). Explicable vectors are overlaid as Arabic numbers of Table 2 for length (L) and width (w).
Table 3. Eigenvectors of principal component analysis on thirteen normalized morphometric variables. Most responsible vectors of Figure 6 are underlined.

DISCUSSION
Small scale patterns of colonization
Within the sediment, cirratulids are very slow-moving worms. The adult stage is poorly dispersive and colonization occurs mainly by larval and early juvenile developmental phases (Palmer et al., Reference Palmer, Allan and Butman1996), although benthic invertebrates that inhabit high-energy environments can move and disperse into new available habitats through water currents (e.g. Hall & Frid, Reference Hall and Frid1997). Seasonal cirratulid blooms with population densities of thousands individuals m−2 are reported in northern Europe for bitentaculate species, e.g. Chaetozone setosa, Caulleriella caputesocis and Aphelochaeta marioni, also in organically-enriched sediments (Gibbs, Reference Gibbs1971; Hily, Reference Hily1987). Cirriformia tentaculata is also considered an opportunistic species of nutrient-rich mud flats; northern populations have a restricted summer breeding period, but they reach lower maximum densities (George, Reference George1964b; Gibbs, Reference Gibbs1971). This manipulative experiment demonstrated that adults of Caulleriella bioculata and C. tentaculata have different colonization patterns of the newly available artificial substratum. Notwithstanding its small body size and its higher density in the source habitat, the immigration of the former species into the new microhabitat is more rapid but very limited, whereas adults of the larger C. tentaculata are able to actively colonize the granular substratum. The adult stage of this latter species, therefore, appears to be more effective in colonizing the new experimental substratum by active immigration from neighbouring sediments, than previously thought for a larger ‘sedentary’ polychaete species. Rapid recolonization of sea bottom sediments, disturbed by repeated disposals, is likely to be driven by adult immigration with respect to the lengthy larval settlement (e.g. Blake et al., Reference Blake, Maciolek, Ota and Williams2009).
A clear seasonal dynamic is shown by Caulleriella, whereas densities of Cirriformia are more variable at a small spatial scale and not clearly related to the short-term seasonal change. For this latter species, colonization increases during the summer season, even though the warming of shallow waters joined to the organic deposits of deeper layers might cause a hypoxic condition, which negatively affects other taxa. Caulleriella bioculata shows a wider vertical distribution in the compacted sediments and is able to exploit the organically-enriched sediments at different sediment depths (Nowell et al., Reference Nowell, Jumars, Self, Southward, Lopez, Taghon and Levinton1989). In contrast, small-scale distribution of C. tentaculata is more confined to the upper sediment layer, within which individuals can reach the sediment/water interface with their tentacular appendages. However, both species are able to settle deeper into the artificial substratum due to the greater intergranular void space and consequent interstitial water circulation. This experimental observation suggests that some bitentaculate as well as multitentaculate cirratulids are potentially able to expand their local vertical distribution in response to the grain size and compactness of sediments they inhabit.
Cirratulids have a great variety of (a-)sexual reproduction strategies even among related congeneric species. In his review, Wilson (Reference Wilson1991) has estimated at least eight different sexual reproductive modes for eighteen cirratulid species. Nevertheless, virtually all the investigated species have yolk-rich and non-feeding lecithotrophic larvae, direct development (Christie, Reference Christie1985; Halt et al., Reference Halt, Rouse, Petersen, Pleijel, Rouse and Pleijel2006) or viviparity, known for some species of Caulleriella, Chaetozone and Dodecaceria (see Petersen, Reference Petersen1999). In the experimental field, the spatio-temporal distribution of the observed juveniles is strongly clumped both in natural and artificial microhabitats. Their temporal and vertical distribution patterns are more comparable to those of C. bioculata (compare the first and third graph of Figure 2).
The problematic identity of the juvenile stage
Published data support the relevant morphological changes during the development of cirratulids. Both chaetal and branchial arrangements may vary from juvenile to adult stages (George & Petersen, Reference George, Petersen, Petersen and Kirkegaard1991; Blake, Reference Blake, Blake, Hilbig and Scott1996). In the multitentaculate genus Cirriformia, the substitution of bifid hooks by capillary chaetae has been observed (George, Reference George1964a; Blake, Reference Blake1975), whereas bidentate hooks in juveniles of Caulleriella zetlandica change to unidentate awl-shaped spines according to Woodham & Chambers (Reference Woodham, Chambers, Dauvin, Laubier and Reish1994). Moreover, during post-larval ontogeny, branchial filaments are the first anterior appendages which develop before palps in Cirriformia tentaculata, Cirriformia moorei and some Aphelochaeta species (Wilson, Reference Wilson1936; Cazaux, Reference Cazaux1972; Blake, Reference Blake1975; Petersen, Reference Petersen1999), and asymmetrical development of branchiae and palps occurs in Dodecaceria juveniles (George & Petersen, Reference George, Petersen, Petersen and Kirkegaard1991). Even the first post-prostomial segments might be problematic. Rouse (Reference Rouse, Rouse and Pleijel2001) suggests that during development the peristomium may split into additional achaetous segmental rings, determining a change of position of the first branchial pair, with the subsequent appearance of palps. The fact that cirratulids have the first achaetous segment is questionable (Blake, Reference Blake, Blake, Hilbig and Scott1996), and in (re-)described species of Atlantic and Pacific Aphelochaeta, Caulleriella and Chaetozone, the true first segment is chaetigerous, although preceded by some achaetigerous annulations (Chambers, Reference Chambers2000; Doner & Blake, Reference Doner, Blake, Sardá, San, López, Martín and George2006, Reference Doner and Blake2009; Dean & Blake, Reference Dean and Blake2007; Elías & Rivero, Reference Elías and Rivero2009).
The very small body sizes, the presence of few and similar thin simple capillary chaetae, the presence of an active pre-pygidial growth zone and the highly clumped distribution in both types of substrata, support the hypothesis that Cirratulidae gen. sp.1 represents an early juvenile stage with peculiar characters, albeit very few descriptions of juvenule or post-larval cirratulids exist. If the presence and insertion of palps is confirmed, Cirratulidae gen. sp.1 could be referred to the bitentaculate genus Aphelochaeta (Blake, personal communication). In contrast, the sub-terminal club-shaped branchiae, restricted to the anterior body, might resemble those of the Dodecaceria taxon, but chaetal morphology is entirely different. A third option is provided by other taxa in the local cirratulid assemblage of non-vegetated soft bottoms of the Faro Lake, represented by the species C. tentaculata and C. bioculata. The investigated substrata are unsuitable as a habitat for Dodecaceria species, which is never found in the vegetated hard surfaces of the lake. Notwithstanding the similarity of these juveniles with respect to the early young stage of true Aphelochaeta (sensu Blake, Reference Blake, Petersen and Kirkegaard1991; figure 2E in Petersen, Reference Petersen1999), adults of this genus have never been found in the Faro brackish basin. The referral of Cirratulidae gen. sp.1 to these two genera is therefore unlikely, on the basis of the known local polychaete fauna. In contrast, the notable occurrence of Cirratulidae gen. sp.1 with bitentaculate C. bioculata suggests a possible relationship between these two taxa. The juvenile metamorphosis would determine the new transformation of noto-, neuropodial bidentate hooks and the appearance of two anal cirri, among the other changes. Another hypothesis is that the observed juveniles are early immature individuals of C. tentaculata. Apalpate juveniles of the related Timarete filigera were described by Claparède and Mecznikov in 1869 (fide Petersen, Reference Petersen1999) for the Gulf of Naples, during the winter season. The external appearance of branchial filaments is slightly comparable to the juvenile stage of Lake Faro samples, but the pointed prostomium and body shape are quite different (see figure 2Q in Petersen, Reference Petersen1999). A relevant difference between Cirratulidae gen. sp.1 and C. tentaculata could only be explained by a strong morphological modification during metamorphosis, which determines the successive appearance of multiple (displaced) palps, the increase and modification of branchial filaments, the development of unidentate spines together with the general body shape and length-to-width ratio changes. Moreover, the demonstrated morphological plasticity of the two sub-groups of juveniles clearly depends on the microhabitat where they settled. The greater interstitial space allowed the young worms to develop a thinner but not necessarily longer body, whilst the compactness of natural sediments is a constraint for a more ‘packed’ body. This microhabitat-dependent variability has to be taken into account for the complete and correct description of less known cirratulid juvenile stages.
ACKNOWLEDGEMENTS
This research project was funded by the PRA University of Messina projects 2005–2007, led by Professor Salvatore Giacobbe. The author wishes to thank Professor James A. Blake (United States of America), Dr Susan Chambers (United Kingdom) and Dr Rodolfo Elías (Argentina) for their useful advice and support in the taxonomy of the investigated taxa.