Introduction
Mass aggregations of some epibenthic organisms are commonly known to serve as a habitat for a variety of motile, sessile and sedentary fauna providing food and refuge from predators or adverse environmental conditions (Gutiérrez et al., Reference Gutiérrez, Jones, Byers, Arkema, Wolanski and McLusky2011). These properties have been shown for the populations of ascidians (Monteiro et al., Reference Monteiro, Chapman and Underwood2002; Castilla et al., Reference Castilla, Lagos and Cerda2004), sponges (Abdo, Reference Abdo2007; Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Konstantinou and Voultsiadou2016), algae and seagrass (Crowe et al., Reference Crowe, Cusson, Bulleri, Davoult, Arenas, Aspden, Benedetti-Cecchi, Bevilacqua, Davidson, Defew, Fraschetti, Golléty, Griffin, Herkül, Kotta, Migné, Molis, Nicol, Noël, Pinto, Valdivia, Vaselli and Jenkins2013; McCloskey & Unsworth, Reference McCloskey and Unsworth2015), corals (Curdia et al., Reference Curdia, Carvalho, Pereira, Guerra-Garcia, Santos and Cunha2015; Ponti et al., Reference Ponti, Grech, Mori, Perlini, Ventra, Penzalis and Cerrano2016), tubicolous worms (Albano & Obenat, Reference Albano and Obenat2009; Gravina et al., Reference Gravina, Cardone, Bonifazi, Bertrandino, Chimienti, Longo, Marzano, Moretti, Lisco, Moretti, Corriero and Giangrande2018), shared populations of barnacles and ascidians (Yakovis et al., Reference Yakovis, Artemieva, Fokin, Varfolomeeva and Shunatova2007). The environmental modification caused by these organisms and their impact on the associated and surrounding fauna are so significant that these organisms have been termed ecosystem engineers, bioengineers or foundation species (Jones et al., Reference Jones, Lawton and Shachak1994, Reference Jones, Gutiérres, Byers, Crooks, Lambrinos and Talley2010; Crain & Bertness, Reference Crain and Bertness2006). The well-known representatives of engineering species are bivalves, most notably various species of mussels: Mytilus edulis L. and Mytilus trossulus Gould (Tsuchia & Nishihira, Reference Tsuchiya and Nishihira1985, Reference Tsuchiya and Nishihira1986; Günther, Reference Günther1996; Commito & Rusignuolo, Reference Commito and Rusignuolo2000; Khaitov et al., Reference Khaitov, Artemyeva, Gornykh, Zhizhina and Yakovis2007; Arribas et al., Reference Arribas, Donnarumma, Palomo and Scrosati2014), Mytilus galloprovincialis Lamarck (Emrić, Reference Emrić1996), Mytilus californianus Conrad (Suchanek, Reference Suchanek1992), Perumytilus purpuratus (Lamarck) (Tokeshi, Reference Tokeshi1995; Thiel & Ullrich, Reference Thiel and Ullrich2002); Mytilus edulis platensis d'Orbigny and Perna perna (L.) (Borthagaray & Carranza, Reference Borthagaray and Carranza2007), Semimytilus algosus (Gould) (Tokeshi, Reference Tokeshi1995; Tokeshi & Romero, Reference Tokeshi and Romero1995), Septifer virgatus (Wiegmann) (Seed, Reference Seed1996) and others.
A wide geographic distribution of mussels, a large number of published studies and the ecological as well as commercial importance of these molluscs have created the notion of their high efficiency as engineering species (Gutiérrez et al., Reference Gutiérrez, Jones, Strayer and Iribarne2003). One of the outcomes of ecosystem engineers' activity is high species diversity of associated organisms that can serve as an estimation of engineering species efficiency. A number of studies have now been conducted to determine the factors that promote high species diversity and abundance of associated fauna in the populations of engineering species. In the majority of these studies, the spatial or structural complexity produced by the population of engineering species, i.e. the physical component, is treated as the leading factor in the formation of the associated fauna. The role of the fact that the space is organized by a living organism is not separated from the overall effect or interpreted as a much less significant factor (Myers & Southgate, Reference Myers and Southgate1980; Dean, Reference Dean1981; Crooks & Khim, Reference Crooks and Khim1999; Lee et al., Reference Lee, Fong and Wu2001; Abdo, Reference Abdo2007; Palomo et al., Reference Palomo, People, Chapman and Underwood2007). Some studies, however, have demonstrated an effect of the physiological activity of engineering species on both species composition and the abundance and biomass of the associated fauna (Lee et al., Reference Lee, Fong and Wu2001; Khaitov & Brovkina, Reference Khaitov and Brovkina2014). In patches of live freshwater mussels Limnoperna fortune (Dunker), the density and biomass of oligochaetes is higher than in non-living imitations of these patches, which is explained by the accumulation of mussel faeces and pseudofaeces that serve as a source of food for the worms (Sardiña et al., Reference Sardiña, Cataldo and Boltovskoy2008). According to Norling & Kautsky (Reference Norling and Kautsky2007), the organisms inhabiting patches of Mytilus edulis receive 24–31% of the required energy through mussel biodeposition.
The mussel communities, however, are not always ‘high biodiversity centres’, especially in those cases, when they form endobenthic, rather than epibenthic, beds, in which mussels are partially buried in soft sediments (Buschbaum et al., Reference Buschbaum, Dittmann, Hong, Hwang, Strasser, Thiel, Valdivia, Yoon and Reise2009).
The question of whether or not different engineering species under similar abiotic conditions determine the composition and structure of the associated fauna in the same fashion remains little explored. The available information is scarce and ambiguous. Yakovis et al. (Reference Yakovis, Artemieva, Fokin, Varfolomeeva and Shunatova2007) have pointed to the differences in the composition of the associated vagile fauna in the epibioses of the shells of dead Serripes groenladicus depending on whether they are dominated by Balanus crenatus Bruguière or Styela spp. This contrasts with the evidence that the biomass of the most abundant representatives of the associated fauna in the White Sea assemblages do not depend on which species predominates in the fouling: the mussel Mytilus edulis or the solitary ascidian Styela rustica (Khalaman, Reference Khalaman1998). Costello & Myers (Reference Costello and Myers1987) have reported that the amphipods associated with the sponges Halichondria panicea (Pallas) and Hymeniacidon perleve (Montagu) living off the coast of Ireland have similar species composition and abundance. Significant differences between associated faunas of the sponges Agelas oroides (Schmidt) and Aplysina aerophoba (Nardo) inhabiting underwater caves in the Aegean Sea have been reported by Gerovasileiou et al. (Reference Gerovasileiou, Chintiroglou, Konstantinou and Voultsiadou2016).
There have been relatively few comparative studies on ‘efficiency’ of engineering species, i.e. the comparison of species richness, density and biomass of the associated organisms that have been supported by different engineering species (Costello & Myers, Reference Costello and Myers1987; Tokeshi, Reference Tokeshi1995; Seed, Reference Seed1996; Chapman et al., Reference Chapman, People and Blockley2005; Prado & Castilla, Reference Prado and Castilla2006; Abdo, Reference Abdo2007; Carvalho et al., Reference Carvalho, Curdia, Pereira, Guerra-Garcia, Santos and Cunha2014; Gerovasileiou et al., Reference Gerovasileiou, Chintiroglou, Konstantinou and Voultsiadou2016). The results of the studies conducted in the Baltic Sea indicate that the ‘efficiency’ of the mussel Mytilus edulis and the brown alga Fucus vesiculosus L. is approximately equal and their shared community has an enhanced positive effect on the associated fauna (Koivisto & Westerbom, Reference Koivisto and Westerbom2010).
An accurate comparison of ‘efficiency’ of different engineering species is complicated by a number of factors. The faunal community associated with the same species can be different depending on the location and environmental conditions (Costello & Myers, Reference Costello and Myers1987; Lintas & Seed, Reference Lintas and Seed1994; Duarte & Nalesso, Reference Duarte and Nalesso1996; Svane & Setyobudiandi, Reference Svane and Setyobudiandi1996; Adami et al., Reference Adami, Tablado and López Gappa2004; Abdo, Reference Abdo2007; Khaitov et al., Reference Khaitov, Artemyeva, Gornykh, Zhizhina and Yakovis2007; Sepúlveda et al., Reference Sepúlveda, Camus and Moreno2016). In particular, the species composition, abundance and biomass of the fauna associated with the mussel Mytilus edulis, depend on whether the mussel population is a natural mussel bed or an artificial suspended or bottom culture (Murray et al., Reference Murray, Newell and Seed2007).
The composition and quantitative characteristics of the associated fauna are influenced by interannual (Khalaman & Naumov, Reference Khalaman and Naumov2009; Naumov et al., Reference Naumov, Khalaman and Fokin2009; Khalaman, Reference Khalaman2013) and seasonal (Tokeshi, Reference Tokeshi1995; Adami et al., Reference Adami, Tablado and López Gappa2004; Costa et al., Reference Costa, Mansur and Leite2015; Dias et al., Reference Dias, Curdia, Cunha, Santos and Carvalho2015) fluctuations in the number of species comprising this fauna and by the population state of the engineering species, in particular, the ageing process of the population (Tsuchia & Nishihira, Reference Tsuchiya and Nishihira1985, Reference Tsuchiya and Nishihira1986; Khalaman, Reference Khalaman1989; Günther, Reference Günther1996; Büttger et al., Reference Büttger, Asmus, Asmus, Buschbaum, Dittmann and Nehls2008).
The fouling communities that form on the suspended mussel farms in the White Sea provide, in our opinion, a convenient model for the comparative analysis of ‘efficiency’ of some engineering species. The biotechnology for growing mussels (Mytilus edulis) employed in the White Sea does not require collecting mussels from natural habitats. Clean artificial substrates (collectors) are left suspended in the sea shortly before the mass settlement of mussels, which in the White Sea occurs in July (Berger et al., Reference Berger, Dahle, Galaktionov, Kosobokova, Naumov, Rat'kova, Savinov and Savinova2001). The settlement of mussel larvae onto the artificial substrates and formation of the mussel population as well as formation of populations of associated organisms proceed in a natural fashion (Oshurkov, Reference Oshurkov and Scarlato1985, Reference Oshurkov1992; Khalaman, Reference Khalaman2001a). In the course of long-term succession, an alternative perennial fouling community dominated by the solitary ascidian Styela rustica L. can be formed on some of the substrates. As a rule, after 4–15 years of exposure the mussel population is replaced by a population of Styela rustica (Oshurkov, Reference Oshurkov1992; Khalaman, Reference Khalaman2001b, Reference Khalaman2013). This process accelerates by elimination of mussels in consequence of the predation or accidental falling of heavy mussel patches. The result is that neighbouring substrates, spaced only half a metre apart, can differ in the dominant species of fouling community. It should be noted that the reverse replacement ascidia with mussels is possible too. Thus, mussel (Mytilus edulis) and ascidian (Styela rustica) communities can be regarded as alternative stable points in succession of fouling communities in shallow waters of the White Sea (Khalaman, Reference Khalaman2005, Reference Khalaman2013). Additionally, the substrates can sometimes be colonized by a few isolated individuals of the sponge H. panicea. Halichondria panicea normally grow on the pre-existing populations of mussels and ascidians. Some of these sponges can reach significant sizes (Khalaman & Komendantov, Reference Khalaman and Komendantov2011). All the above-mentioned fouling communities have their direct analogues in the White Sea benthos. These are in fact the same epibenthic communities of natural hard ground, or close to them, but formed on artificial substrates (Oshurkov, Reference Oshurkov1992; Khalaman, Reference Khalaman2001a, Reference Khalaman2001b, Reference Khalaman2005). A methodological advantage of this biological model is that different communities within one mussel farm are being formed and exist under similar environmental conditions and on the same type of substrate, with the substrates being exposed in the sea over the same period of time. In our opinion, it seems more correct to compare the ‘efficiency’ of various engineering species in such standardized conditions than in natural bottom communities, where the compared species usually occupy different locations, substrates and depths (Golikov et al., Reference Golikov, Scarlato, Galtsova and Menshutkina1985; Oshurkov, Reference Oshurkov1992; Plotkin et al., Reference Plotkin, Railkin, Gerasimova, Pimenov and Sipenkova2005). In addition, the lifespan of natural populations of these species is unknown. All these circumstances are factors that can significantly affect the comparison result. In our opinion, obtaining correct comparative assessments is an important task. This should lead to a better understanding of the capabilities of the various ecosystem engineers, as well as to the ability to predict changes in coastal ecosystems that may occur when one dominant competitor is replaced by another. Study on a biological model such as fouling communities has its own ‘internal’ meaning, because anthropogenic substrates (artificial reefs, breakwaters, pontoons, piers, mariculture structure etc.) and fouling communities that inhabit these substrates have become a significant component of marine coastal ecosystems (Reznichenko, Reference Reznichenko1978; Svane & Petersen, Reference Svane and Petersen2001; Airoldi et al., Reference Airoldi, Turon, Perkol-Finkel and Rius2015).
As there is the aforementioned well-established opinion that mussels are highly effective as an ecosystem engineer, we have tried to test whether mussels are indeed more effective than other engineering species in this respect, at least in fouling communities. Thus, in this study, we have tested the following hypotheses:
(1) The species structures of the associated vagile fauna in the White Sea fouling communities are different if the base of fouling is formed by the mussel Mytilus edulis, the solitary ascidian Styela rustica or the sponge Halichondria panicea.
(2) Mytilus edulis is the most efficient engineering species. Species richness, species diversity, density and biomass of the associated vagile fauna are higher in the mussel fouling community than in those formed by the ascidian Styela rustica and the sponge Halichondria panicea.
Materials and methods
Study area and sampling
Sampling was conducted in 1988–2009 from mussel culture farms located in the Kandalaksha Bay of the White Sea. The complete database contains about 500 samples. The non-mussel fouling communities were detected on most of mussel culture farms. However, some hydrobiological surveys contain only single samples of non-mussel fouling communities, which cannot be considered representative data. Therefore, in this study the samples were analysed only for those hydrobiological surveys that contained more than three samples of other communities in addition to mussels (107 samples). These are the five hydrobiological surveys that were carried out on four mussel culture farms (Figure 1). In all farms, the artificial substrates (collectors) were of strips of Capron netting with a mesh size of 0.01 m, 3 m long by 0.2 m wide suspended vertically in the upper layer of water. The samples of fouling communities were collected at depths of 0.5–2.5 m. Each sample was a 0.1 m section of the substrate. The samples were washed with clean seawater through a series of sieves. The mesh size of the finest sieve was 0.5 mm. All the organisms found were identified to species level and their number and wet weight were measured to the nearest 1 mg. For further analysis, the biomass and density were recalculated per 1 linear metre of artificial substrate. The technique and sampling methods used in this study are described in more detail in Khalaman (Reference Khalaman2001a) and Khalaman & Komendantov (Reference Khalaman and Komendantov2011).
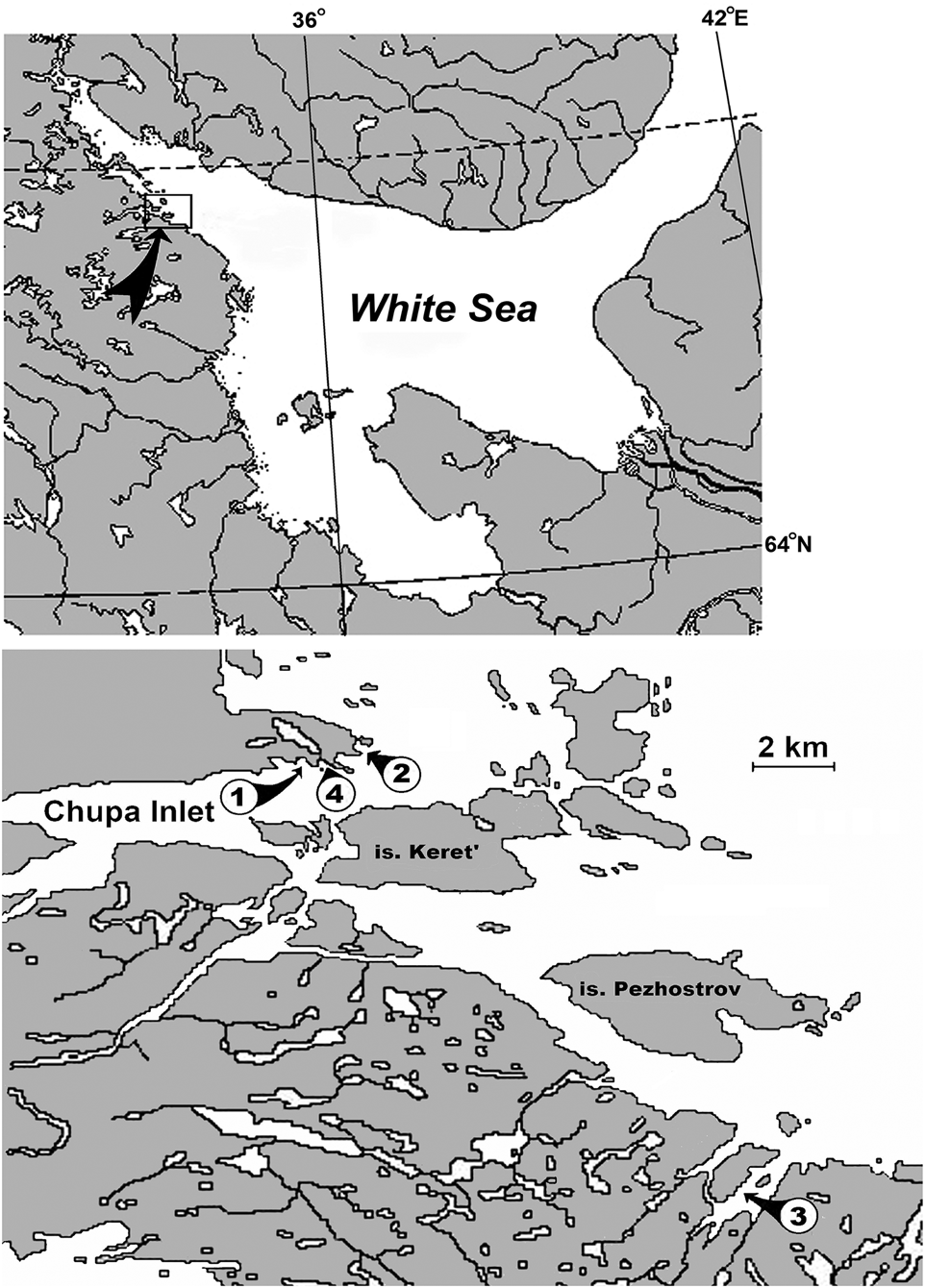
Fig. 1. Study area. (1) Kruglaya Bay; (2) Ivanovskaya Bay; (3) Nikolskaya Inlet; (4) Krivozerskaya Bay.
For the purposes of this study, the fouling was categorized as mussel-dominated, if the weight of the mussels Mytilus edulis in the sample was more than 50% of the total weight of fouling organisms. The fouling was classified as ascidian-dominated, if the solitary ascidian Styela rustica accounted for more than 50% of the weight of the fouling in the sample. The sponges (Halichondria panicea or Halichondria panicea + Halisarca dujardini Johnston) were considered dominant in the fouling community, if they covered 100% of all substrate in the sample and have overgrown all other sessile organisms or if the sponge biomass accounted at least for 50% of the weight of all organisms in the sample.
Nine samples were collected in June 1988 from a culture farm in Kruglaya Bay. Of those, four samples were classified as mussel-dominated and five as ascidian-dominated. The period of substrate exposure at the time of sampling was 5 years. Additionally, 18 samples were collected from Ivanovskaya Bay in June 1988. Of those, nine were mussel-dominated, five were ascidian-dominated and four samples had the sponges Halichondria panicea and Halisarca dujardini as dominant species. The period of substrate exposure was 4 years.
Culture farms in Nikolskaya Inlet were sampled in late June 1993 and in mid-July 1994. The periods of substrate exposure at the time of sampling were 4 and 5 years, respectively. Fifteen samples were collected in 1993 (10 with mussel-dominated and five with ascidian-dominated fouling) and 25 in 1994 (nine with mussel-dominated and 14 with ascidian-dominated fouling).
In late June to early July 2009, the samples were collected from an abandoned culture farm in Krivozerskaya Bay. The period of substrate exposure was about 10 years. In total, 40 samples were collected: 12 were mussel-dominated and 18 were ascidian-dominated. The remaining 10 samples included the sponge Halichondria panicea, which covered the entire population of either mussels (4 samples) or ascidians Styela rustica (6 samples). Most sponge-covered mussels or ascidians remained alive.
Comparative analysis of assemblages of the associated vagile fauna
The analysis was based on Bray–Curtis similarity matrices calculated separately for each of the five hydrobiological surveys. The similarity was calculated between the samples, with the biomasses of the vagile species used as parameters. Before the analysis, the biomasses were transformed using root-square transformation. The resulting similarity matrices were used for multidimensional scaling (MDS) and one-way PERMANOVA (Anderson, Reference Anderson2005) that were performed separately for each of the five hydrobiological surveys. The factor whose action was tested was the dominant engineering species. For Kruglaya Bay (1988) and Nikolskaya Inlet (1993, 1994), the factor had two gradations: mussel-dominated (Mytilus edulis) and ascidian-dominated (Styela rustica) fouling. For Ivanovskaya Bay (1988), three gradations were assigned for the factor: mussel-dominated (Mytilus edulis) and ascidian-dominated (Styela rustica) fouling and the fouling community formed by cohabiting sponges Halichondria panicea and Halisarca dujardini. For Krivozerskaya Bay (2009), the factor had four gradations: mussel-dominated (Mytilus edulis) and ascidian-dominated (Styela rustica) fouling and the fouling communities, in which the sponge Halichondria panicea covered the mussel or ascidian populations (H. panicea + M. edulis and H. panicea + S. rustica).
Additionally, we carried out the two-way PERMANOVA in which the first factor was the engineering species and the second was the hydrobiological survey. Since not all the hydrobiological surveys we used in the study hold data about sponge-dominated fouling, the two-way PERMANOVA was performed only for those samples that were dominated either by mussels or ascidians. The first factor (the engineering species) had two gradations: M. edulis and S. rustica. The second factor had five gradations according to the number of hydrobiological surveys: Kruglaya Bay (1988), Ivanovskaya Bay (1988), Nikolskaya Inlet (1993), Nikolskaya Inlet (1994) and Krivozerskaya Bay (2009).
Homogeneity of variance was checked by means of the PERMDISP procedure (Anderson, Reference Anderson2001). The contribution of a given species to dissimilarities between the assemblages of the associated vagile fauna characteristic of different engineering species was evaluated using the SIMPER procedure.
For the species whose contribution to dissimilarities between the assemblages of the associated fauna could reach 10% or more, the influence of the following factors to the biomass of these species was evaluated using two-way ANOVA. (1) Engineering species. This factor had two gradations: mussel-dominated (Mytilus edulis) community and ascidian-dominated (Styela rustica) community. (2) Sites and times of sampling. This factor had five gradations according to the number of hydrobiological surveys used in the study: Kruglaya Bay (1988), Ivanovskaya Bay (1988), Nikolskaya Inlet (1993), Nikolskaya Inlet (1994) and Krivozerskaya Bay (2009).
Analysis of ‘efficiency’ of engineering species
The following parameters were determined for each sample to evaluate the ‘biological capacity’ or ‘efficiency’ of different engineering species with respect to the associated vagile fauna.
-
H’w – Shannon–Weaver Index H’w = pi Σlog 2 pi, where pi is the proportion of the total weight of vagile animals (W) made up by weight of vagile species i (wi): pi = wi/W;
-
H’n – Shannon–Weaver Index H’n = pi Σlog2 pi, where pi is the proportion of the total number of individuals of vagile animals (N) made up by specimens of vagile species i (ni): pi = ni/N;
where S is number of vagile species; Wv is total weight of vagile animals; and N is total number of individuals of vagile animals.
In epibenthic communities, the sedentary and sessile organisms create space, which is occupied by the vagile fauna. Patches of the attached organisms living on sections of artificial substrate of the same length can have different volumes. For this reason, we believe that specific indices, i.e. the indices corrected for the volume of these patches, are a more adequate measurement of ‘biological capacity’ for the population of a given engineering species. The total biomass of sedentary and sessile organisms in each individual sample was used as an approximate estimation of this volume. The following specific indices were calculated for each sample.
-
S/Ws – number of vagile species per 100 g of weight of sessile and sedentary organisms;
-
Wv/Ws – weight of vagile animals per 100 g of weight of sessile and sedentary organisms;
-
N/Ws – number of individuals of vagile animals per 100 g of weight of sessile and sedentary organisms.
The comparative evaluation of ‘efficiency’ of engineering species, i.e. their ability to support the diversity and abundance of the associated fauna, was performed by comparing the mean values of the parameters listed above.
In all cases, comparisons of the means were made using post-hoc LSD test. Cochran's C test was used to check homogeneity of variance. Normality of distribution was tested using Kolmogorov–Smirnov test. If the conditions of normality of distribution and homogeneity of variance were not satisfied, the data were log-transformed. Significance level was set at Р = 0.05. Mean values were given with their standard errors. Analyses were carried out using STATISTICA (Version 7.0) and PRIMER 6 (Version 6.1.13) & PERMANOVA + (Version 1.0.3) software.
Results
Comparison of assemblages of the vagile invertebrates associated with different engineering species (mussels, ascidians, and sponges) in fouling communities
The structures of the fouling communities of both sedentary and sessile engineering species and the associated vagile fauna are shown in Figure 2.
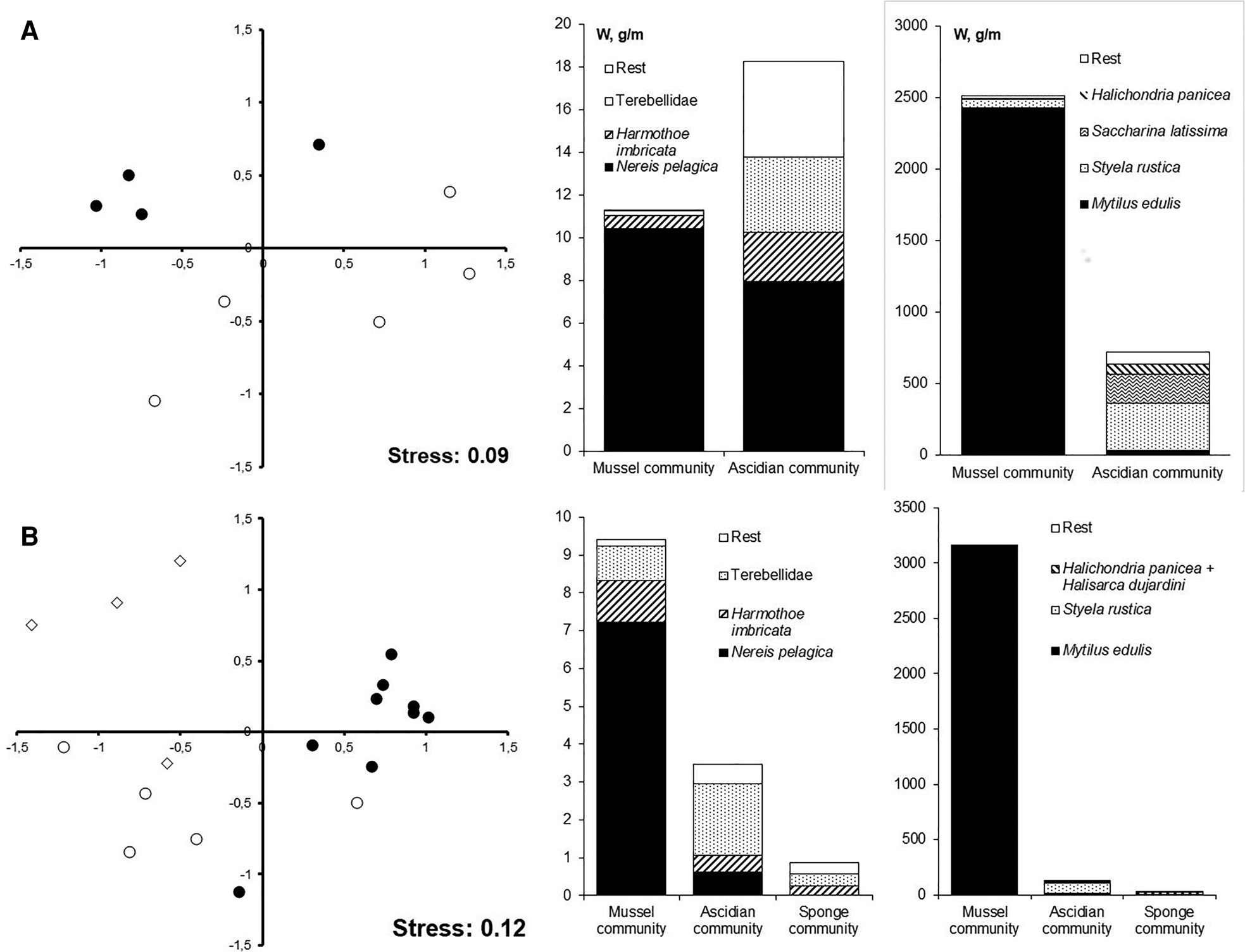
Fig. 2. Characteristics of the fouling communities in the mussel culture farms. (A) Kruglaya Bay; (B) Ivanovskaya Bay. Left column: MDS ordinations of the assemblages of the vagile fauna associated with the following engineering species: mussel (Mytilus edulis), filled circle; ascidia (Styela rustica), open circle; sponge (Halichondria panicea + Halisarca dujardini), open diamond. Middle column: structure of assemblage of vagile fauna. Right column: structure of assemblage of sedentary and sessile organisms.
In total, 42 species of vagile animals were found in the fouling communities studied (Table 1). The similarity analysis for the assemblages of vagile invertebrates conducted using PERMANOVA has yielded the following results. In the samples collected from Kruglaya Bay, ecosystem engineers had a significant effect on the assemblage composition (Pseudo-F = 3.48; P = 0.045). The assemblage of the vagile invertebrates associated with the mussel Mytilus edulis was significantly different from that living with the ascidian Styela rustica (Figure 2A) (P = 0.037). The same factor was also statistically significant for the fouling communities in the mussel culture farm of Ivanovskaya Bay (Pseudo-F = 7.35; P = 0.001) (Figure 2B). A pairwise comparison has shown that the mussel-associated vagile fauna at this site was significantly different from the vagile inhabitants of both the populations of the ascidian Styela rustica (P = 0.004) and those of the sponges Halichondria panicea + Halisarca dujardini (P = 0.002). Differences between the fauna associated with ascidians and that associated with sponges were also statistically significant (P = 0.042).
Table 1. List of vagile species that were found in fouling communities
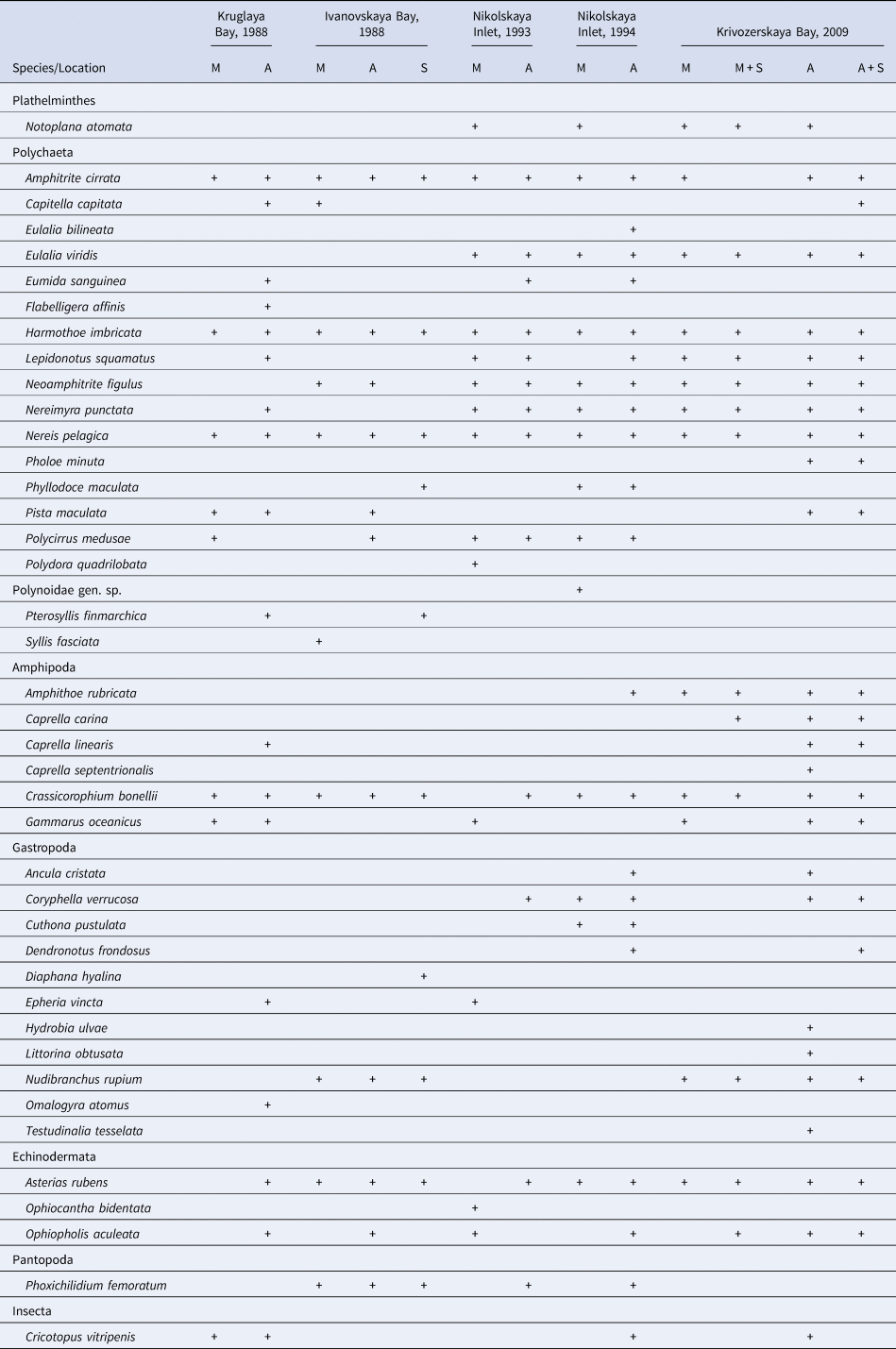
M, mussel (Mytilus edulis) community; A, ascidian (Styela rustica) community; S, sponge (Halichondria panicea + Halisarca dujardini) community; M + S, mussel (M. edulis) + sponge (H. panicea) community; A + S, ascidia (S. rustica) + sponge (H. panicea) community.
For the samples collected from Nikolskaya Inlet in 1993, the significance level of the engineering species factor was close to the critical threshold (Pseudo-F = 2.46; P = 0.052), but the assumption of homogeneity of variance was not satisfied (P = 0.002). The samples collected from mussel and ascidian fouling communities do not form separate groups in the scatter plot that shows the results of multidimensional scaling (Figure 3A). For the samples collected from the same culture farm during the following year (1994), the influence of the engineering species factor was highly significant (Pseudo-F = 5.77; P = 0.001) (Figure 3B). The mussel-associated vagile fauna was significantly different from ascidian-associated fauna (P = 0.001).
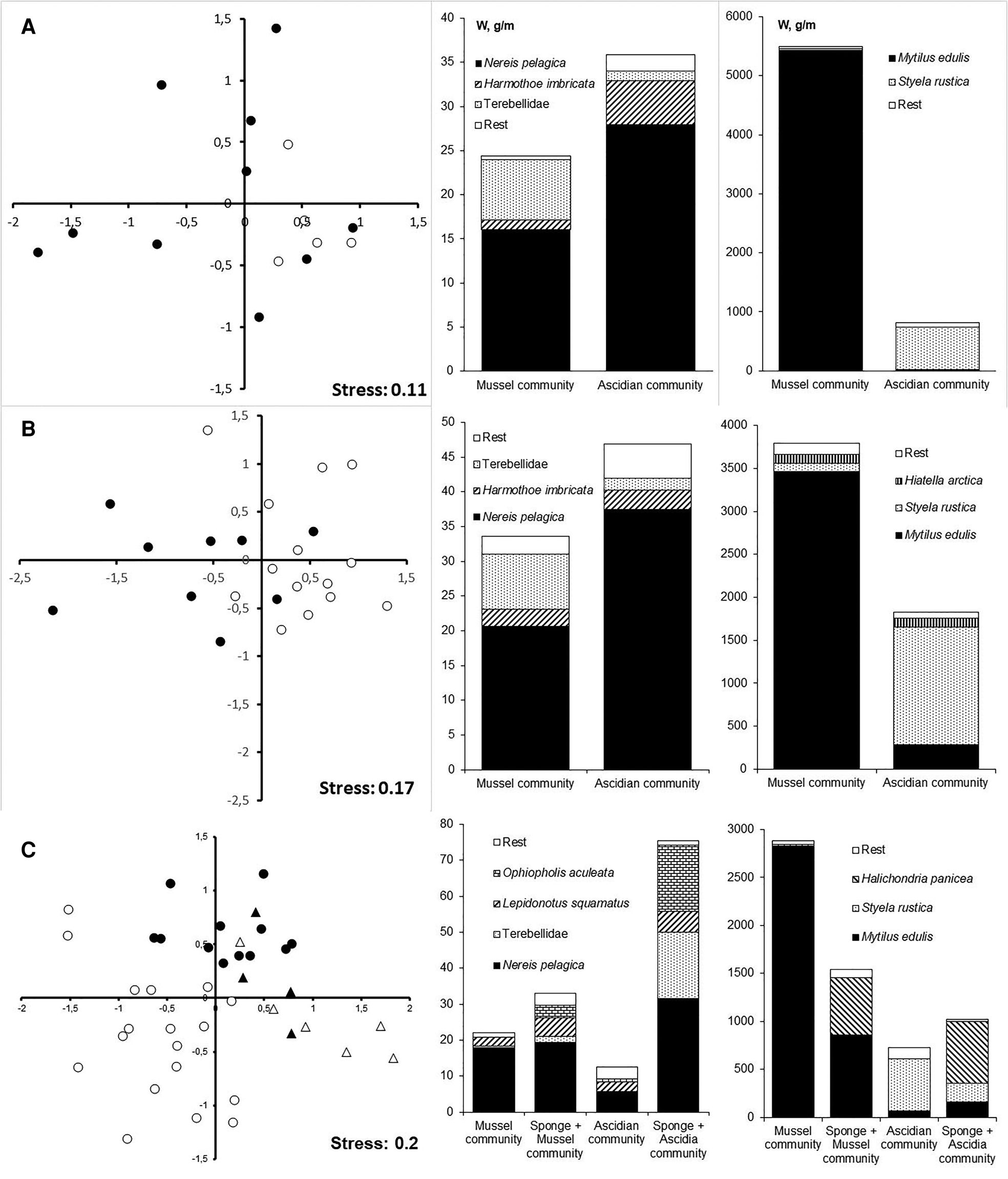
Fig. 3. Characteristics of the fouling communities in the mussel culture farms. (A) Nikolskaya Inlet, 1993; (B) Nikolskaya Inlet, 1994; (C) Krivozerskaya Bay. Left column: MDS ordinations of the assemblages of the vagile fauna associated with following engineering species: mussel (Mytilus edulis), filled circle; ascidia (Styela rustica), open circle; sponge (Halichondria panicea) + mussel (Mytilus edulis), filled triangle; sponge (Halichondria panicea) + ascidia (Styela rustica), open triangle. Middle column: structure of assemblage of vagile fauna. Right column: structure of assemblage of sedentary and sessile organisms.
For the associated fauna found in the fouling communities of the abandoned farm in Krivozerskaya Bay, the engineering species factor was statistically significant (Pseudo-F = 6.09; P = 0.001) (Figure 3C). A pair-wise test showed that the vagile fauna of the M. edulis community differed from that in the community of the ascidians S. rustica (P = 0.001). In places where the sponge Halichondria panicea has overgrown the ascidians S. rustica the associated fauna was different from that living among those S. rustica that were not covered by the sponge (P = 0.001). By contrast, the associated fauna of mussel patches did not depend in any significant way on whether or not the mussels were covered by the sponge but level of difference was close to the significance threshold (P = 0.054). In fouling where the sponge H. panicea was present, the associated vagile fauna showed little dependence on the population being overgrown either by mussels or ascidians (P = 0.161).
Consequently, in each mussel farm, the fouling communities differed from one another not only in the type of dominant engineering species (mussels M. edulis, ascidians S. rustica or sponges H. panicea or H. panicea + H. dujardini) but also in the assemblages of the associated vagile fauna, with the average dissimilarity among assemblages of the vagile fauna being moderate (58 ± 3.3%).
According to the SIMPER procedure, the greatest contribution to dissimilarities between the assemblages of the vagile species inhabiting the populations of different engineering species was made by the most abundant species of polychaetes. The average contribution of Nereis pelagica L. was 29 ± 4.2%, that of Harmothoe imbricata (L.) was 10 ± 1%, and the representatives of the family Terebellidae (Ampitrite cirrata Müller and Neoamphitrite figulus (Dalyell)) contributed on average 14 ± 3% and 11 ± 2%, respectively. Among crustaceans, the most significant contribution to dissimilarities between assemblages (5 ± 1.7%) was made by the amphipod Crassicorophium bonellii H. Milne Edwards. Special note should be made of the brittle star Ophiopholis aculeata (L.), which was encountered primarily in the fouling communities where relatively large individuals of the sponge Halichondria panicea were present. These samples were collected from Krivozerskaya Bay in 2009. The biomass of O. aculeata in the fouling communities of Halichondria panicea + Mytilus edulis and Halichondria panicea + Styela rustica was 5 ± 2.1 g m−1 and 18 ± 6.6 g m−1, respectively, while in the pure populations of the ascidian Styela rustica it was much lower, 0.9 ± 0.51 g m−1 (P = 0.0001) and in the communities formed by the mussel Mytilus edulis alone, the brittle stars were entirely absent. The contribution of Ophiopholis aculeata to dissimilarities between the assemblages of the associated fauna was therefore sufficiently large (12 ± 3.5%).
However, no preference toward any specific engineering species (mussels, ascidians or sponges) was found in the vast majority of the vagile animals. In particular, the two-way ANOVA showed that for a number of species the influence of the factor of taxonomic identity of engineering species (mussel or ascidian) was statistically insignificant, while the influence of time and place of sampling was significant (Table 2). The exception was the polychaete Lepidonotus squamatus (L.), the biomass of which was somewhat higher in the populations of the ascidians Styela rustica (1.4 ± 0.27 g m−1) than in the fouling communities formed by the mussel Mytilus edulis (0.7 ± 0.23 g m−1) (Table 2). It is noteworthy that the interaction of factors proved to be statistically significant for most vagile species (Table 2). This situation can be illustrated by the example of the largest and commonly occurring polychaete, Nereis pelagica. According to the samples taken from Ivanovskaya (1988) and Krivozerskaya (2009) Bays, the biomass of N. pelagica was higher in the aggregations of the mussel M. edulis than in those of the ascidian S. rustica (Figures 2B and 3C): respectively, 7 ± 1.6 g m−1 and 0.6 ± 0.52 g m−1 (P = 0.008) for Ivanovskaya Bay and 18 ± 4 g m−1 and 6 ± 1.3 g m−1 (P = 0.004) for Krivozerskaya Bay. The biomass distribution for Nereis pelagica in the samples from Nikolskaya Inlet showed the opposite trend (Figure 3A, B): it was 16.8 ± 4 g m−1 and 28 ± 8.6 g m−1 (P = 0.017) in 1993 and 21 ± 7.3 g m−1 and 37 ± 7.7 g m−1 (P = 0.06) in 1994 for the mussel and ascidian populations, respectively. No statistically significant differences were found for Kruglaya Bay (P = 0.19) (Figure 2A).
Table 2. Results of two-way ANOVA on factors affecting the associated vagile fauna
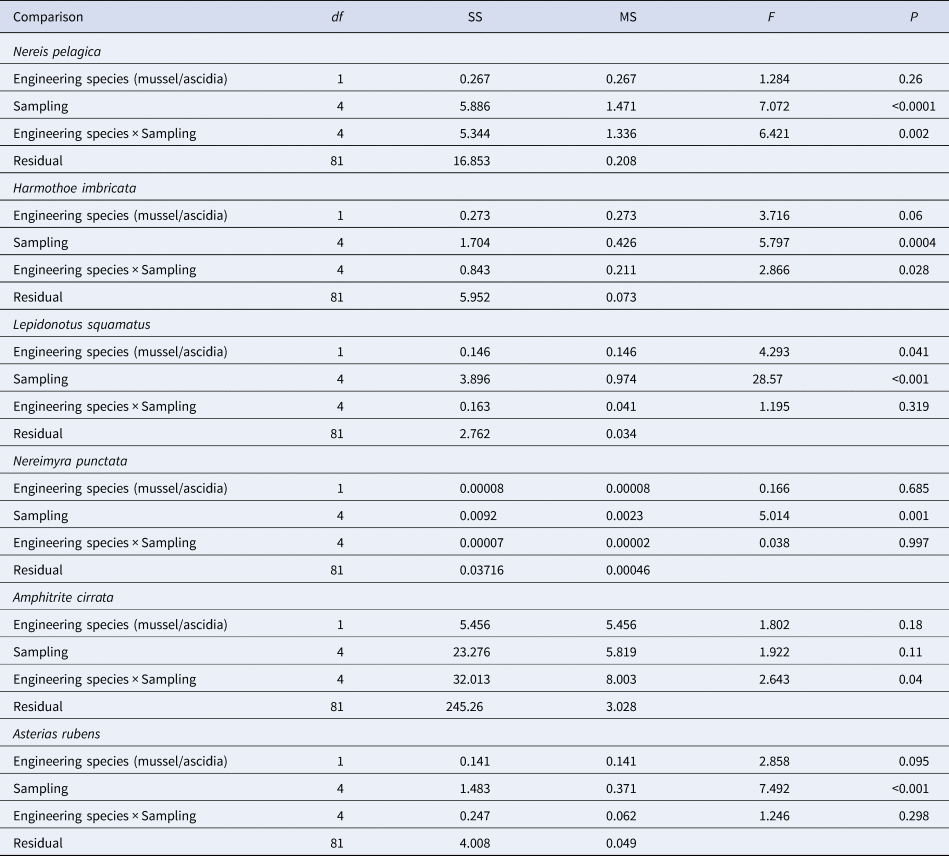
In general terms, this situation can be clearly seen from the results of the two-way PERMANOVA performed for all fouling samples included in this study, in which Mytilus edulis or Styela rustica was the dominant species. The factor of taxonomic identity of the engineering species as well as the factor of time and place of sampling had a statistically significant effect on the assemblage of vagile fauna (Pseudo-F = 9.23; P = 0.001 and Pseudo-F = 9.7; P = 0.001, respectively). The interaction of these factors was also statistically significant (Pseudo-F = 4.79; P = 0.001). However, according to the pair-wise test, there were no significant differences between the assemblages of vagile fauna associated with mussels collected from Kruglaya and Ivanovskaya Bays (P = 0.11) and between the samples collected at different times from Nikolskaya Inlet (P = 0.182). Assemblages of vagile fauna associated with Styela rustica showed no significant differences only between the samples collected in 1993 and 1994 from Nikolskaya Inlet (P = 0.154).
Taking all of the above into consideration, it can be concluded that under every specific location and time the assemblages of the vagile fauna associated with a certain engineering species exhibit their own specific differences.
Comparative analysis of the ‘biological capacity’ for the populations of different engineering species (mussels, ascidians and sponges) in fouling communities
The comparison of mean values of the Shannon–Weaver diversity index, where the species biomass (H’w) was used as a quantitative measure of species yielded the following results. Species diversity of the associated vagile fauna was usually higher in the populations of Styela rustica than in those of Mytilus edulis. The exception were the samples collected from Nikolskaya Inlet, for which no statistically significant differences were found (Figure 4A). In the fouling communities formed by sponges, the species diversity of the associated fauna was also higher than in the mussel communities, but showed no statistically significant differences from the fouling communities dominated by the ascidian Styela rustica (Figure 4A).
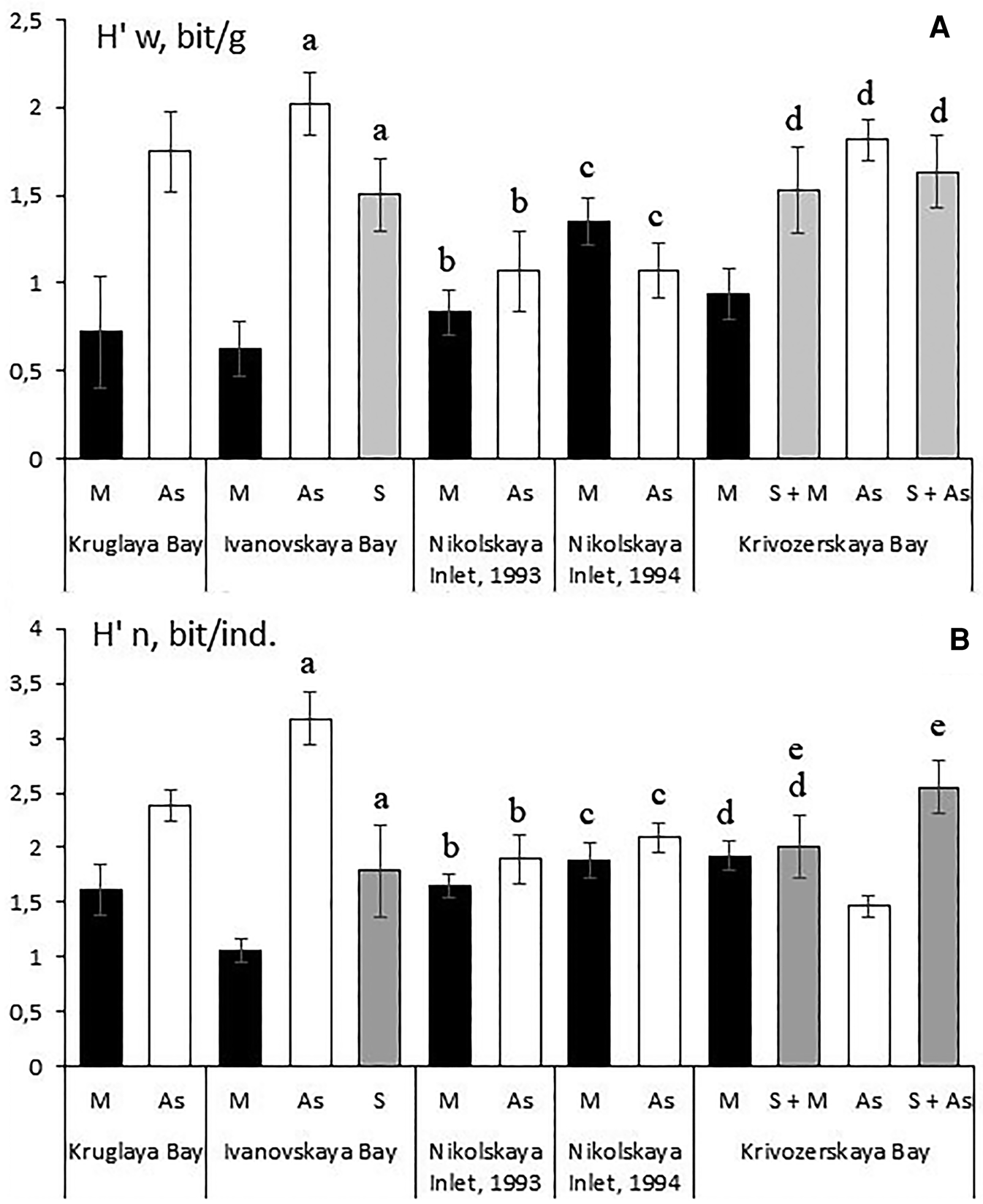
Fig. 4. Species diversity per sample (Shannon–Weaver Index) for the assemblage of the vagile fauna associated with different engineering species in the fouling communities of the mussel culture farms studied. (A) Species diversity calculated from the biomass of the species (H’w). (B) Species diversity calculated from population density of species (H’n). M, mussel (Mytilus edulis) community; As, ascidian (Styela rustica) community; S, sponge (Halichondria panicea + Halisarca dujardini) community; S + M, sponge (Halichondria panicea) + mussel (Mytilus edulis) community; S + As, sponge (Halichondria panicea) + ascidian (Styela rustica) community. The same letters indicate the values that do not have statistically significant differences.
The use of species diversity index by number of individuals (H’n) has produced similar results (Figure 4B), except that in Krivozerskaya Bay the species diversity of the vagile fauna associated with Styela rustica was lower than in the populations of mussels and sponges (Figure 4B). This is explained by low evenness (0.48 ± 0.031) that was caused by an exceptionally high abundance of a small amphipod Crassicorophium bonellii in the ascidian community (1233 ± 253 ind. m−1), which was one or two orders of magnitude higher than those of other vagile species. It should be noted that the population density of this amphipod in the mussel fouling community was much lower (52 ± 16 ind. m−1; P = 0.0007).
The species richness of the vagile fauna per sample (S) was higher in the populations of Styela rustica than in those of Mytilus edulis. The only exception were the samples collected from Nikolskaya Inlet in 1993, in which these differences were statistically insignificant (Figure 5A). It is worth mentioning that among the samples collected by the authors there were samples of mussel fouling similar to those used in the present study, which contained only a single species of the vagile fauna. As a rule, this species was represented by the polychaeta Nereis pelagica. In the samples of those fouling communities, which were dominated by the ascidian Styela rustica, the number of vagile species was in all cases no less than four.
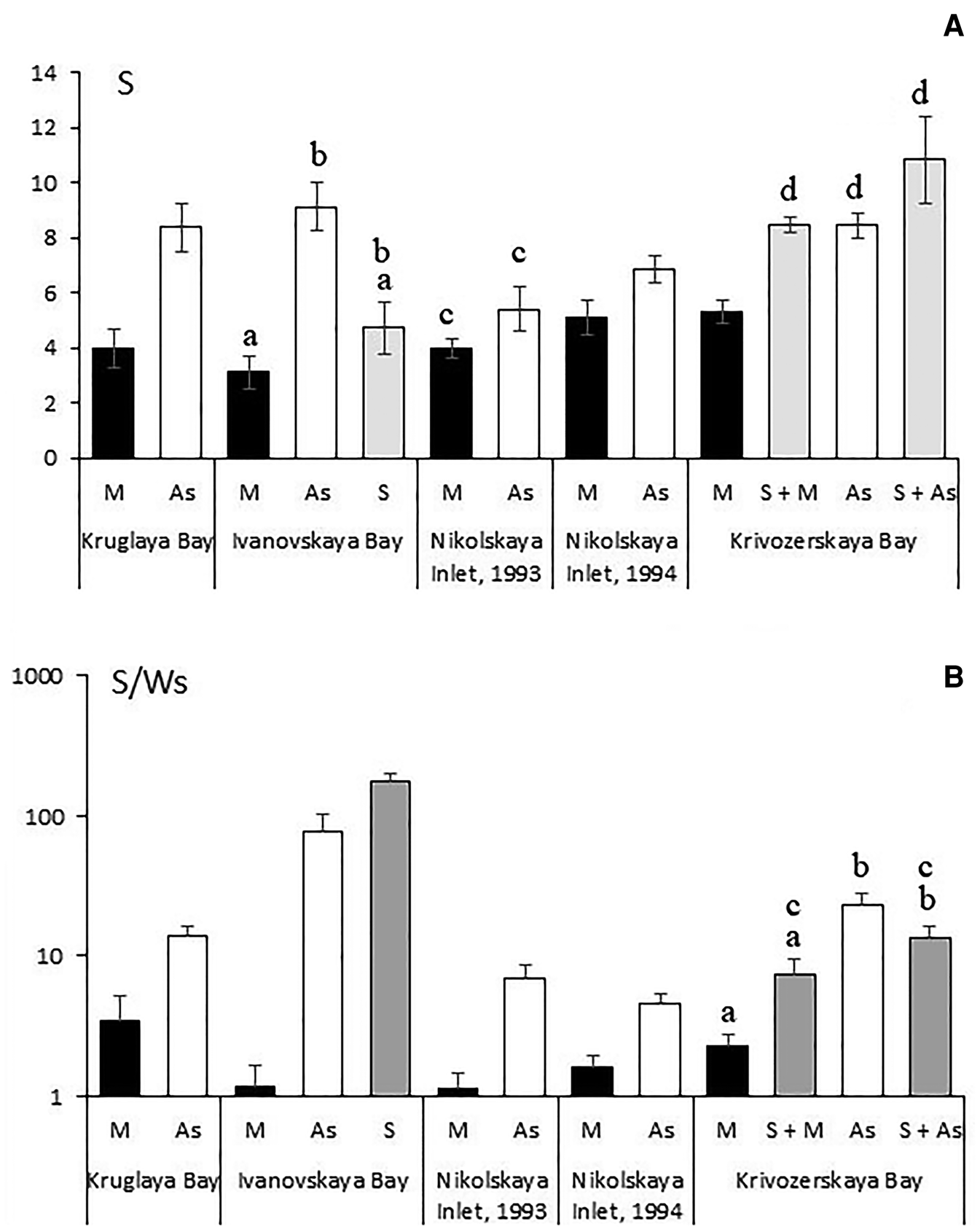
Fig. 5. Species richness of the vagile fauna per sample (S) (A) and per 100 g of total weight of sedentary and sessile organisms (S/Ws) (B) in fouling communities that were combined by different engineering species. Abbreviations as in Figure 4. The same letters indicate the values that do not have statistically significant differences.
For the samples collected from Krivozerskaya Bay, the number of species per sample in fouling where the sponge Halichondria panicea was present was equal to that in the ascidian community but higher than in the mussel community (Figure 5A). In Ivanovskaya Bay, this index showed no differences between the fouling communities formed by the sponges Halichondria panicea + Halisarca dujardini and those of other engineering species (mussels or ascidians) (Figure 5A).
The specific number of the vagile species (per 100 g of sedentary and sessile organisms) (S/Ws) was in all samples much higher in the fouling communities formed by Styela rustica than in those dominated by the mussel Mytilus edulis (Figure 5B). This result could be expected because the mussel aggregations had a higher weight than the ascidian aggregations (Figure 2 and 3). In Krivozerskaya Bay, the influence of the sponge Halichondria panicea on the volume S/Ws index was statistically insignificant. In Ivanovskaya Bay, however, this value in the fouling community formed by the sponges Halichondria panicea and Halisarca dujardini was much higher than both in mussel and ascidian communities (Figure 5B).
In Ivanovskaya Bay, the highest biomass of the vagile fauna (Wv) was observed for the ascidian fouling communities, while the lowest for the community formed by the sponges Halichondria panicea and Halisarca dujardini (Figure 6A). The latter observation was probably explained by a relatively small biomass of these sponges (Figure 2B). In Kruglaya Bay and Nikolskaya Inlet, the biomass of the vagile fauna in the ascidian fouling community had a trend to be higher than in the mussel community, but this difference was statistically insignificant. In Krivozerskaya Bay, the biomass of the vagile fauna was highest in the community of Halichondria panicea + Styela rustica, but where the sponge was absent the ascidian fouling community in this bay had the lowest biomass of the vagile fauna, being even lower in this index than the community dominated by mussels (Figure 6A).
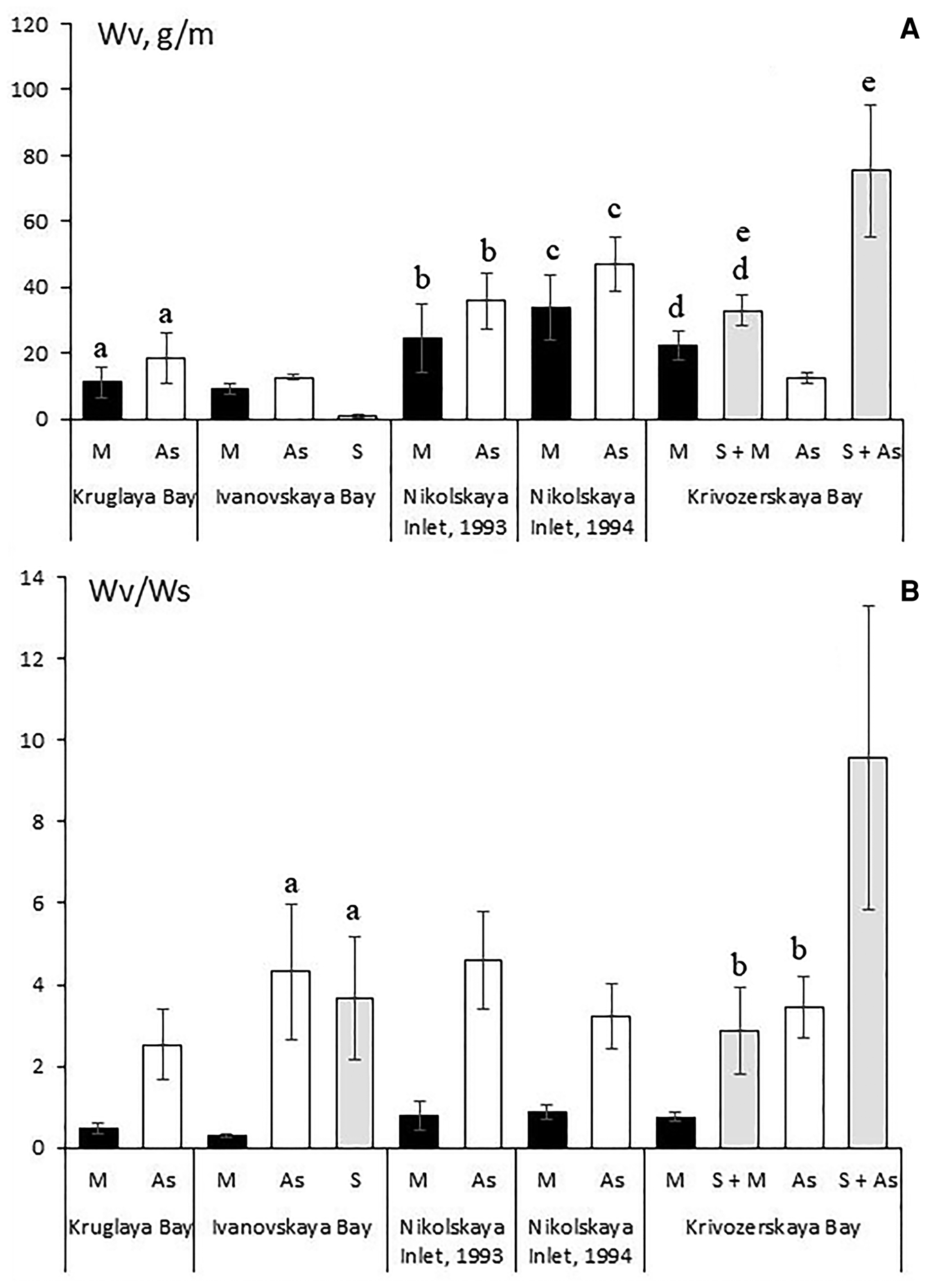
Fig. 6. Biomass of the vagile fauna per sample (Wv) (A) and per 100 g of total weight of sedentary and sessile organisms (Wv/Ws) (B) in the fouling communities that were combined by different engineering species. Abbreviations as in Figure 4. The same letters indicate the values that do not have statistically significant differences.
The values of specific biomass for the vagile species (per 100 g of sedentary and sessile organisms) (Wv/Ws) followed closely the trends described above for the biomass of these species in Kruglaya Bay and Nikolskaya Inlet, but the differences between the mussel and ascidian communities were statistically significant (Figure 6B). In Ivanovskaya Bay, the specific biomass of the vagile fauna in the mussel community was much lower than in the communities of ascidians and the sponges Halichondria panicea and Halisarca dujardini. These values in the last two communities had no statistically significant differences (Figure 6B). In Krivozerskaya Bay, the leading community both in specific biomass and biomass of the vagile fauna was that of Halichondria panicea + Styela rustica. The mussel fouling community, which in this bay exceeded the ascidian community in total biomass of the vagile species, had a value of specific biomass, which was lower than both in the community of ascidians and in that of Halichondria panicea + Mytilus edulis (Figure 6B).
In all the mussel farms studied, the number of individuals in the associated fauna (N) had the highest values in the populations of the ascidian Styela rustica. Statistically significant differences in this index between the mussel and ascidian fouling communities were found in the samples collected from Kruglaya Bay, Nikolskaya Inlet (in 1994) and Krivozerskaya Bay (Figure 7A). In the samples taken from Krivozerskaya Bay, the following inverse trend was observed: while the biomass of the vagile fauna in the community of Halichondria panicea + Styela rustica was higher than in the pure ascidian community (Figure 6A), the population density of the vagile fauna was instead higher in those samples where the sponge was lacking (Figure 7A). It can be explained by the already mentioned high abundance of the amphipod Crassiсorophium bonellii in the ascidian community (1233 ± 253 ind. m−1). In the fouling community formed by Halichondria panicea + Styela rustica, the population density of this species was much lower (128 ± 78 ind. m−1; P = 0.0096). Since Crassicorophium bonellii has a small body size (the maximum weight of a single individual did not exceed 0.001 g), this animal, despite its high population density, has a small contribution to the total biomass of vagile species.
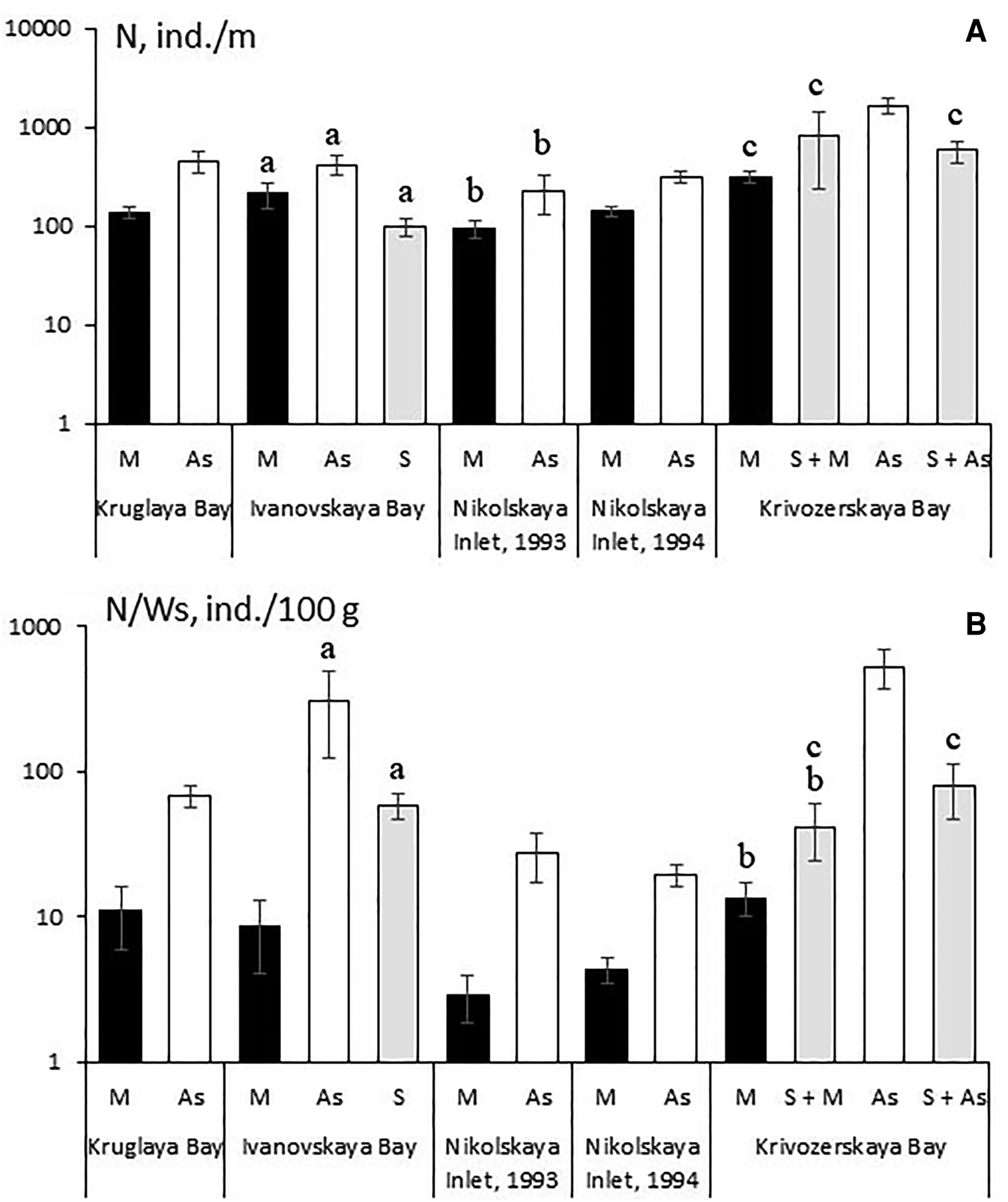
Fig. 7. Density of the vagile fauna per sample (N) (A) and per 100 g of total weight of sedentary and sessile organisms (N/Ws) (B) in the fouling communities that were combined by different engineering species. Abbreviations as in Figure 4. The same letters indicate the values that do not have statistically significant differences.
The patterns revealed for the population density of the vagile species (N) were made more pronounced with the use of specific population density (N/Ws) (Figure 7B). In all samples, the specific density of the vagile species was highest in the populations of the ascidian Styela rustica, and lowest in the communities dominated by mussels. This index in the community of Halichondria panicea + Halisarca dujardini from Ivanovskaya Bay was higher than in the mussel community and had no statistically significant differences from the ascidian community. In Krivozerskaya Bay, the fouling communities formed by the sponge (Halichondria panicea + Styela rustica and Halichondria panicea + Mytilus edulis) had lower values than the ascidian community, but showed no statistically significant differences from each other. Specific population density of the vagile species for the communities of Halichondria panicea + Mytilus edulis and those of Mytilus edulis were statistically indistinct.
To summarize, in all the parameters examined in this study the ‘biological capacity’ of the population of Mytilus edulis was usually not higher and in most cases was lower than the ‘biological capacity’ of both the populations of Styela rustica and those dominated by sponges. Somewhat exceptional for the mussel community was only the biomass of the vagile fauna, which in Ivanovskaya Bay was slightly higher than in the community of Halichondria panicea + Halisarca dujardini and in Krivozerskaya Bay was higher than in the community of Styela rustica. The mussel community, however, was nowhere superior in specific biomass of the vagile species.
Discussion
The distinctive feature of the biological model used in this study is that in the fouling assemblages as well as in the epibenthic communities of the hard substrates the attached organisms tend to create the environment rather than modify it. They create the structures that serve as the environment for other organisms and, according to the existing terminology, act as autogenic, rather than allogenic engineering species, which modify the existing physical environment and biota (Bouma et al., Reference Bouma, Olenin, Reise and Ysebaert2009). In our opinion, the integral and the most accurate estimate of the ‘efficiency’, which is best suited specifically for autogenic engineering species, is the species diversity and richness of the associated organisms, i.e. the biota, whose existence is supported by the engineering species. However, we have intentionally limited our scope to the analysis of the associated vagile fauna. These animals are without doubt consumers of the environment produced by engineering species. Furthermore, vagile animals are quick in colonizing the fouling communities and in the White Sea they usually form a sufficiently rich fauna by the end of the first year of exposure (Khalaman, Reference Khalaman1989).
By contrast, the high richness and species diversity of the associated sedentary and sessile species cannot always be interpreted solely as a result of favourable living conditions created by an engineering species. When considering these species, a distinction should be made between space competitors and epibionts. The epibionts use mussels and other engineering species as a substrate required for their attachment and further survival. In this case, the environment essential to the existence of these forms is indeed created by the engineering species. However, according to the existing studies, for instance, the mussels living in the White Sea begin to become actively overgrown by other organisms only when they reach the age of 3 or 4 years and/or shell length of 40–50 mm (Khalaman, Reference Khalaman1989; Naumov, Reference Naumov2006). A similar relationship between the associated organisms and the size/age of mussels was also noted for other seas (Tsuchiya & Nishihira, Reference Tsuchiya and Nishihira1985, Reference Tsuchiya and Nishihira1986). Therefore, the factor of the substrate exposure period and the age/size of the engineering species can have a significant influence on the results of comparative studies evaluating the ‘efficiency’ of these species.
Space competitors are the organisms that compete for the base substrate. The presence of individuals of other sedentary or sessile organisms in the population of an engineering species can in fact represent an unfinished competition, rather than an effect of the engineering species, whose existence is a factor increasing species diversity. However, the boundary between space competitors and epibionts is not always distinct. Some species can play both roles. For instance, at the early succession stages of the White Sea fouling communities the ascidian Molgula citrina Alder & Hancock acts as a dominant species living on the base substrate (Khalaman et al., Reference Khalaman, Komendantov, Malavenda and Mikhaylova2016), but when the populations of mussels or the ascidian Styela rustica are developed over the course of succession and displace Molgula citrina from the base substrate, this species, according to our observations, begins to live exclusively as an epibiont.
The bulk of the vagile fauna in all the fouling communities studied consisted of the same species (Amphitrite cirrata, Asterias rubens L., Corophium bonelli, Harmothoe imbricata, Lepidonotus squamatus (L.), Neoamphitrite figulus, Nereimyra punctata (Müller), Nereis pelagica). The assemblages of the vagile fauna, which were associated with an engineering species, had differences that were primarily determined by the quantitative parameters of these vagile organisms.
The brittle star Ophiopholis aculeata (L.) is worth mentioning as one of the few species that showed a consistent propensity toward a single engineering species. It lived primarily on those fouling communities, in which the sponge Halichondria panicea was present. There are indications in the literature that sponges often serve as habitats for brittle stars. In particular, the brittle star Ophiactis savignyi (Müller & Troschel) is the main endobiotic species in the sponge Zygomycale parishii (Bowerbank) (Duarte & Nalesso, Reference Duarte and Nalesso1996). It is possible that the sponge Halichondria panicea provides the most suitable shelter for Ophiopholis aculeata, the use of which is characteristic of this species of brittle stars (Drolet et al., Reference Drolet, Himmelman and Rochette2004).
The nature of differences between the assemblages of vagile species and the quantitative characteristics of vagile species depended on the location and time of sampling. The influence of local conditions on the development of epibenthic communities and on the associated fauna has been suggested earlier (Costello & Myers, Reference Costello and Myers1987; Lintas & Seed, Reference Lintas and Seed1994; Duarte & Nalesso, Reference Duarte and Nalesso1996; Svane & Setyobudiandi, Reference Svane and Setyobudiandi1996; Adami et al., Reference Adami, Tablado and López Gappa2004; Abdo, Reference Abdo2007; Khaitov et al., Reference Khaitov, Artemyeva, Gornykh, Zhizhina and Yakovis2007; Sepúlveda et al., Reference Sepúlveda, Camus and Moreno2016; Simpson et al., Reference Simpson, Smale, McDonald and Wernberg2017). The results of our study strongly support these previous findings. In our opinion, one of the reasons for variability of the associated fauna were interannual fluctuations in abundance of mass species of polychaetes, which are known for the White Sea (Khalaman and Naumov, Reference Khalaman and Naumov2009; Naumov et al., Reference Naumov, Khalaman and Fokin2009; Khalaman, Reference Khalaman2013). However, regardless of the extent of fluctuations and their underlying causes, the assemblages of the associated fauna that belonged to different engineering species were usually different. It can therefore be concluded that the first of the hypotheses tested in this study was confirmed.
The second of the proposed hypotheses was not confirmed. Species richness and diversity in the assemblages of the mussel-associated vagile species was not higher than in similar assemblages associated with ascidians or sponges and in many instances were even lower. The only exception were the samples collected from Krivozerskaya Bay, in which species diversity of the mussel-associated vagile fauna was higher than that of the ascidian-associated vagile fauna. As mentioned above, this is explained by a high population density of Crassicorophium bonellii in the ascidian community, which caused a decrease in species evenness and consequently the species diversity index. According to the data reported in the literature (Uryupova et al., Reference Uryupova, Spiridonov and Zhadan2012) and our own observations, the mass populations of C. bonellii are associated with algae, and the fouling communities especially rich in algae are those formed by Styela rustica (Khalaman, Reference Khalaman2001a). The efficiency of the mussel population in terms of the biomass and number of individuals of the vagile animals inhabiting the mussel aggregations also proved to be significantly lower than the efficiency of the ascidian and sponge populations.
To date, only a few studies have conducted a comparative analysis on efficiency of different engineering species. Our results, however, are in agreement with some findings reported for other aquatic areas. For instance, Prado & Castilla (Reference Prado and Castilla2006) have reported that species richness of the fauna living along the Chilean coast in dense populations of the mussel Perumytilus purpuratus is the same as that of the fauna associated with another bioengineer, the tunicate Pyura praeputialis (Heller). The associated fauna in the populations of the invasive mussel Mytilus galloprovincialis living off the coast of Australia is less rich than that found on the mats of local coralline algae (Chapman et al., Reference Chapman, People and Blockley2005).
The reason why the mussel fouling communities are not as rich in associated fauna as the communities of ascidians or sponges, is in our opinion rooted in the structure of mussel patches, which are very densely packed according to certain geometric laws (Lezin, Reference Lezin2007). The mussels, therefore, make a very efficient use of the substrate. According to our unpublished observations, with the sizes of mussels Mytilus edulis and ascidians Styela rustica in longstanding White Sea fouling communities being roughly equal, the ascidians have populations with a density which is one order of magnitude lower than those of mussels. This paucity of free space together with a dense network of byssus threads can actually be the cause that prevents the development of the associated fauna in the mussel aggregations. This hypothesis, however, needs further verification. In the case of sponges an extra space is provided by their well-developed irrigation system: as noted by a number of authors it may serve as a habitat for an abundant associated fauna, especially that of polychaetes and amphipods (Serejo, Reference Serejo1998; Çinar et al., Reference Çinar, Katagan, Ergen and Sezgin2002; Neves & Omena, Reference Neves and Omena2003; Abdo, Reference Abdo2007). It is probably for this reason that despite a solid cover that the sponge Halichondria panicea forms over the mussel or ascidian populations these communities are comparable or even exceed those of mussels in abundance of the associated fauna.
The effect of mussels on the associated fauna in the benthic communities can be explained, in particular, by their sedimentation activity (Dittmann, Reference Dittmann1990; Norling & Kautsky, Reference Norling and Kautsky2007). The artificial substrates used in the White Sea for mussel cultivation (strips of Capron netting) tend to accumulate sediment. The intensity of this biodeposition, however, is lower than the accumulation that may have been provided by the organisms inhabiting this substrate. Most of the faeces and pseudofaeces produced both by mussels and other filter feeders are not retained on the substrates suspended in the water column, but fall to the sea bottom (Sukhotin, Reference Sukhotin1992; Ivanov et al., Reference Ivanov, Smagina, Chivilev and Kruglikov2013). Unfortunately, we do not have any data that would allow for an accurate comparison of mussels, ascidians and sponges in terms of their biodeposition. According to our own observations and experience in processing the samples, the artificial substrates inhabited by mussels tend to be significantly muddier than those covered with ascidians or sponges after the same period of exposure in the sea. The pumping rate of Mytilus edulis is known to exceed that of Styela rustica (Lezin et al., Reference Lezin, Agat'eva and Khalaman2006). Since the biomass and the population density of ascidians in the fouling communities are lower than in the communities of ascidians (Figures 2 and 3), the sedimentation intensity should be lower in the settlements of ascidians than in those of mussels, which is consistent with our observations. However, the question as to the extent to which the structure of the associated fauna is determined by biodeposition in the White Sea mussel fouling communities remains unanswered.
Mussels significantly change the structure of the benthic communities and can increase the overall abundance and species diversity of the associated fauna (Günther, Reference Günther1996; Borthagaray & Carranza, Reference Borthagaray and Carranza2007; Koivisto & Westerbom, Reference Koivisto and Westerbom2010; McLeod et al., Reference McLeod, Parsons, Morrison, Van Dijken and Taylor2014). This conclusion is also true for the White Sea (Khaitov et al., Reference Khaitov, Artemyeva, Gornykh, Zhizhina and Yakovis2007; Khaitov & Brovkina, Reference Khaitov and Brovkina2014). Our results have shown, however, that in the White Sea fouling communities the ‘efficiency’ of Mytilus edulis in maintaining the abundance and diversity of the associated fauna is lower than that of other engineering species: Styela rustica and Halichondria panicea. In our opinion, this result can be interpreted in a wider sense, specifically that there are epibenthic species that are more ‘efficient’ as engineering species than mussels. However, the final resolution of this question would have to come from additional comparative studies of the communities formed by mussels, ascidians and sponges in other biotopes, in which these species can serve not only as autogenic, but also as allogenic ecosystem engineers.
Financial support
This work was supported by the Russian Foundation of Basic Research (grant number 18-04-00062a) and the State Task (Reg. number АААА-А19-119022690122-5).











