Introduction
Oceanic islands are part of many of the biodiversity hotspots (Myers et al., Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000) as they host relatively high endemism and threatened flora (Caujapé-Castells et al., Reference Caujapé-Castells, Tye, Crawford, Santos-Guerra, Sakai, Beaver, Lobin, Florens, Moura, Jardim, Gómes and Kueffer2010). Insular plants are more prone to habitat destruction, fragmentation and impacts of alien invasive species (Brooks et al., Reference Brooks, Mittermeier, Mittermeier, Da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield and Magin2002; Bruna et al., Reference Bruna, Fiske and Trager2009; Baider and Florens, Reference Baider and Florens2011). Consequently, plant conservation on islands has mainly focused on understanding their biology and ecological interactions to inform management to prevent extinction (e.g. Brooks et al., Reference Brooks, Mittermeier, Mittermeier, Da Fonseca, Rylands, Konstant, Flick, Pilgrim, Oldfield and Magin2002; Kaiser-Bunbury et al., Reference Kaiser-Bunbury, Traveset and Hansen2010; Silva et al., Reference Silva, Dias, Sardos, Azevedo, Schaefer and Moura2015; Downey and Richardson, Reference Downey and Richardson2016; Bissessur et al., Reference Bissessur, Baider and Florens2017). More recently, with the growing interest in crop wild relatives (CWR) (Maxted et al., Reference Maxted, Ford-Lloyd, Jury, Kell and Scholten2006), oceanic islands are being targeted for studies on CWR, as they may contain unique genetic diversity of value for crop improvement (Kell et al., Reference Kell, Knüpffer, Jury, Ford-Lloyd, Maxted, Maxted, Ford-Lloyd, Kell, Iriondo, Dulloo and Turok2008).
CWR, which include crop progenitors, are inherently related to cultivated plants such as food and fodder crops, and species with medicinal, ornamental and forestry attributes (Maxted et al., Reference Maxted, Ford-Lloyd, Jury, Kell and Scholten2006). Populations of these wild species contain potential beneficial traits such as pest or disease resistance, tolerance to drought or heat stress, and greater yield, that can be bred into crops to address the varying environmental and market demands (Vincent et al., Reference Vincent, Wiersema, Kell, Fielder, Dobbie, Castañeda-Álvarez, Guarino, Eastwood, León and Maxted2013), and are therefore economically important for food security and environmental sustainability (Heywood et al., Reference Heywood, Casas, Ford-Lloyd, Kell and Maxted2007). Unfortunately, CWR, like other wild species, are vulnerable to an increasing range of threats (Maxted et al., Reference Maxted, Ford-Lloyd, Kell, Maxted, Ford-Lloyd, Kell, Iriondo, Dulloo and Turok2008; Maxted and Kell, Reference Maxted and Kell2009), including habitat degradation and fragmentation, unsustainable farming practices, urbanization, tourism development, invasive alien species, genetic pollution and climate change (Jarvis et al., Reference Jarvis, Lane and Hijmans2008; Bilz et al., Reference Bilz, Kell, Maxted and Lansdown2011; Ford-Lloyd et al., Reference Ford-Lloyd, Schmidt, Armstrong, Barazani, Engels, Hadas, Hammer, Kell, Kang and Khoshbakht2011; Kell et al., Reference Kell, Maxted, Bilz, Maxted, Dulloo, Ford-Lloyd, Frese, Iriondo and Pinheiro de Carvalho2012). Here, we use the islands of Mauritius and Rodrigues as examples to identify and prioritize their CWR and propose conservation measures, especially since both islands have long documented botanical histories and are among the islands with the most human-impacted biodiversity (Rijsdijk et al., Reference Rijsdijk, Zinke, de Louw, Hume, van der Plicht, Hooghiemstra, Meijer, Vonhof, Porch, Florens, Baider, van Geel, Brinkkemper, Vernimmen and Janoo2011; Florens, Reference Florens, Sodhi, Gibson and Raven2013; Norder et al., Reference Norder, Seijmonsbergen, Rughooputh, Van Loon, Tatayah, Kamminga and Rijsdijk2017).
Mauritius and Rodrigues harbour a relatively diverse flora (Baider et al., Reference Baider, Florens, Baret, Beaver, Matatiken, Strasberg and Kueffer2010), well documented since the 18th century (Leguat, Reference Leguat1708; Bojer, Reference Bojer1837; Baker, Reference Baker1877; Balfour, Reference Balfour1877), although new species or records are still being found (Le Péchon et al., Reference Le Péchon, Baider, Gigord, Haevermans and Dubuisson2011; Baider and Florens, Reference Baider and Florens2013, Reference Baider and Florens2016; Byng et al., Reference Byng, Florens and Baider2015), as well as previously non-recorded taxa that went extinct at the onset of human colonization (van der Plas et al., Reference van der Plas, de Boer, Hooghiemstra, Florens, Baider and van der Plicht2012; de Boer et al., Reference de Boer, Hooghiemstra, Florens, Baider, Engels, Dakos, Blaauw and Bennett2013). Research on their indigenous flora varies from distribution, ecology, conservation and restoration (e.g. Strahm, Reference Strahm1989, Reference Strahm1993; Hansen and Müller, Reference Hansen and Müller2009; Baider and Florens, Reference Baider and Florens2011; Florens et al., Reference Florens, Baider, Martin and Strasberg2012; Florens and Baider, Reference Florens and Baider2013) to traditional or new uses of medicinal plants (e.g. Mahomoodally and Aumeeruddy, Reference Mahomoodally, Aumeeruddy, Mohamed, Najjaa and Máthé2017; Rummun et al., Reference Rummun, Neergheen-Bhujun, Pynee, Baider and Bahorun2018). In the agricultural sector, the most studied species is the cultivated sugar cane (Saccharum officinarum L.), given its economic importance, especially to develop new varieties adapted to the local conditions, like those with tolerance to pests and diseases and higher sugar content with less fibre (Ramdoyal and Badaloo, Reference Ramdoyal, Badaloo, Engels, Ramanatha Rao, Brown and Jackson2002), but also with high fibre content for energy production (Santchurn et al., Reference Santchurn, Ramdoyal, Badaloo and Labuschagne2014).
While the importance of plant genetic resources (PGR) is recognized by the agricultural sector, until now no systematic study has addressed the CWR diversity of both islands. The lack of inventories of CWR on both Mauritius and Rodrigues is a major constraint for their conservation and potential use. The study thus aimed to create a comprehensive inventory of the native wild relatives for both islands and prioritize them for crop improvement and sustainable use.
Methods
Study sites
The Republic of Mauritius is one of the small island developing states (known as SIDS), located in the Indian Ocean, within the Mascarene archipelago, forming part of the Western Indian Ocean Biodiversity Hotspot (Myers et al., Reference Myers, Mittermeier, Mittermeier, Da Fonseca and Kent2000). Mauritius, centred at 20°15′S, 57°35′E is around 900 km east from Madagascar, whereas Rodrigues is centred at 19°43′S, 63°25′E and about 580 km east of Mauritius. Both are volcanic islands of about 8–10 million years old, with Rodrigues harbouring 47 endemic (31.3%) of its 150 known native angiosperm species, and Mauritius having higher levels of endemism (39%, or 273 species out of 691 recorded native angiosperms) (Baider et al., Reference Baider, Florens, Baret, Beaver, Matatiken, Strasberg and Kueffer2010). However, their floras are among the most endangered worldwide (Hilton-Taylor, Reference Hilton-Taylor2000), mainly because of massive habitat destruction for agricultural and infrastructural development. Virtually no forest remnants survive on Rodrigues, while on Mauritius, since its colonization in 1638, only 4.4% of native habitat survives (Hammond et al., Reference Hammond, Gond, Baider, Florens, Persand and Laurance2015), including 2% comprising of good quality native vegetation fragments dispersed across the island, with the largest continuous area found in the Black River Gorges National Park (Vaughan and Wiehe, Reference Vaughan and Wiehe1937; Safford, Reference Safford1997; Cheke and Hume, Reference Cheke and Hume2008; Florens, Reference Florens, Sodhi, Gibson and Raven2013).
On Mauritius, most of the remnants indigenous forests are found within protected areas (comprising of 4.7% of its land area, Baret et al., Reference Baret, Baider, Kueffer, Foxcroft, Lagabrielle, Foxcroft, Pysěk, Richardson and Genovesi2013), but these are often heavily invaded by alien plants, especially the shade-tolerant strawberry guava, Psidium cattleyanum Sabine (Florens et al., Reference Florens, Baider, Martin, Seegoolam, Zmanay and Strasberg2016), and alien mammals, such as rats (Rattus rattus (Linnaeus, 1758) and R. norvegicus (Berkenhout, 1769)), wild boars (Sus scrofa Linnaeus, 1758) and macaques (Macaca fascicularis Raffles, 1821), among others. Alien species also threaten the remaining native biodiversity of Rodrigues (Strahm, Reference Strahm1989, Reference Strahm1993), an island with almost no formal protected areas (0.6% of its land surface, Baret et al., Reference Baret, Baider, Kueffer, Foxcroft, Lagabrielle, Foxcroft, Pysěk, Richardson and Genovesi2013).
With around 43% of the land area of Mauritius (ca. 806 km2 of its 1864 km2), under agriculture and most under sugarcane, this sector represents the third main source of revenue in the country (Statistics Mauritius, 2015). Agricultural production is even more important on Rodrigues (ca. 20 km2 of its 108 km2), since 38% of its local employment comes from the agricultural and forestry sector (Republic of Mauritius, 2017). Agriculture in Rodrigues is characterized mainly by the production of staple food, including red beans, lime, chillies, maize, cassava, bread-fruits, potatoes and sweet potatoes (Perrine, Reference Perrine2016). Other important food crops include onions, garlic, cabbage, tomatoes and creepers, including chayote, zucchini, cucumber and pumpkins. The production of coffee has also started on a pilot basis since 2015 (Republic of Mauritius, 2017).
Creation of the national CWR checklist
The national CWR checklist was generated through a process of data harmonization and cross-checking of the native flora of Mauritius and Rodrigues with the Mansfeld's World Database of Agricultural and Horticultural Crop (Hanelt and IPK, Reference Hanelt2001). Only native genera having globally and locally cultivated taxa were compiled for each island. Family classification followed APG IV (Angiosperm Phylogeny Group, 2016), and for species (or infraspecies taxa), the nomenclature used followed Flore des Mascareignes (Bosser et al., Reference Bosser, Cadet, Guého and Marais1976-onwards), unless when superseded by newer literature. New species or new records published were also added to the checklist (e.g. Roberts et al., Reference Roberts, Florens, Baider and Bosser2004; Delmail, Reference Delmail2009; Le Péchon et al., Reference Le Péchon, Baider, Gigord, Haevermans and Dubuisson2011; Baider et al., Reference Baider, Florens, Rakotoarivelo, Bosser and Pailler2012; Baider and Florens, Reference Baider and Florens2013; Byng et al., Reference Byng, Florens and Baider2015; Fournel et al., Reference Fournel, Micheneau and Baider2015).
The next step was to add accepted scientific names (family included) with their respective authority, occurrence status, global distribution, economic use category of related crop(s), the economic value of the related crop (based on global and local market values), the genetic potential of the CWR in crop improvement, taxonomic relationship to the crop and threat assessment (see online Supplementary Table S1 for more details). One given taxon could be listed in more than one economic use category.
Usually there is less information available on the market value of native species related to cultivated plants for medicinal, ornamental, aromatic and forestry purposes, thus the list was later restricted to the food-related category only, which is in general better documented. The prioritization process of the food-related species was done using four key criteria: (1) economic value of the related crop, (2) utilization potential as a gene donor, (3) IUCN Red List conservation status and (4) occurrence status (online Supplementary Table S1). Each criterion was assigned a score varying from 0 to 10 (online Supplementary Table S2), depending on their importance to derive total scores for each species (Given and Norton, Reference Given and Norton1993; Magos Brehm et al., Reference Magos Brehm, Maxted, Martins-Loução and Ford-Lloyd2010), resulting in a ranked list of species. The total score number was an additive index, calculated by adding the values of the four parameters. Entry threshold of a species to the calculation started with economic parameter with the others added afterwards. Therefore, the highest possible score for a species was 35, when all parameters were attributed the highest score. This method has been successfully applied to a wide range of taxa of plants in studies ranking priority species for conservation globally (e.g. Sapir et al., Reference Sapir, Shmida and Fragman2003; Magos Brehm et al., Reference Magos Brehm, Maxted, Ford-Lloyd and Martins-Loução2008, Reference Magos Brehm, Maxted, Martins-Loução and Ford-Lloyd2010).
Once the priority CWR were identified, we collated occurrence data for each species, based on published literature including Flores des Mascareignes, IUCN Red List, reports (e.g. Page and D'Argent, Reference Page and D'Argent1997), journal articles (e.g. Dulloo et al., Reference Dulloo, Maxted, Newbury, Florens and Ford Lloyd1999) and expert knowledge. Localities were geo-referenced using Google Earth Pro v. 7.3.1.4507, wherever the coordinates were missing, then mapped using Q-GIS v.2.18.7, and superimposed to the protected areas limits, to determine local hotspots for CWR in existing protected areas.
Results
The CWR checklist generated for Mauritius comprised of 528 species (97 families, 234 genera), accounting for 76% of the species of the Mauritian angiosperm flora, while for Rodrigues, it contained 142 species (59 families, 112 genera, including species believed extinct), or 95% of the flora of Rodrigues. However, only 43 species in the checklist for Mauritius (or 6%) and 10 species for Rodrigues (or 7%) were identified to be related to globally cultivated species at the genus level (Table 1). Most species in both checklists were related to cultivated crops with medicinal attributes and ornamental use, while others were related to food and fodder (Table 1). In the latter category, those with higher taxa richness were Myrtaceae, Moraceae, Poaceae and Rubiaceae, which comprised ca. 10% of all species included in the inventory. More than half of the recorded species had a wide geographic distribution, including 60% of native angiosperms of Mauritius and 72% of Rodrigues. The two islands shared 68 species in all. One-quarter (N = 131) and one-fifth (N = 28) of the species in the checklists of food CWR of Mauritius and Rodrigues, respectively, were single island endemics, with a further 15 and 6% of endemic species (not single island endemics) occurring in both Mauritius and Rodrigues respectively. A total of 29% of the species were considered as probably native. A total of 13.5% of the species in these checklists, if assessed, would be placed in the category of Critically Endangered (CR) and 37% in Data Deficient (DD).
Table 1. Number of species per use category of selected families of native crop wild relatives of Mauritius and Rodrigues*

Percentage represented by each category out of total number of species in each family is provided.
* Some species might be represented in more than one ‘Use category’.
Within the most economically important food crop genera (Table 2), only 28 species were prioritized as related to major agricultural food cultivated crops (27 on Mauritius, 10 on Rodrigues, with nine of them present on both islands). Of those, in terms of the most important global and national priority crops, 21 species were selected, 52% (N = 11) of which were strict island endemics or Mascarene endemics (Table 3). Of these species, three endemic Coffea species may be of interest for coffee breeding and production. Some species of local importance were included, for example, Dictyosperma album (Bory) H. Wendl. & Drude, which is locally cultivated for its edible palm heart. The CWR most easily used in breeding programmes are those in Gene Pool (GP) 1b or Taxon Group (TG) 1b, which are the closest wild relatives to the crop (Maxted et al., Reference Maxted, Ford-Lloyd, Jury, Kell and Scholten2006). However, the ones identified in the Republic of Mauritius were mostly related at the secondary or tertiary GP or TG level. The exception was the wild populations of palm heart which are primary wild relatives and they may be collected from the wild and brought into cultivation to increase diversity in cultivated populations, in case the species is globally exploited for consumption. None of the priority species have yet been characterized for their potential for crop improvement.
Table 2. Economically important food crop genera and numbers of Mauritian and Rodriguan native CWR species related. Information of the species’ global distribution: endemic to Mauritius (MAU), endemic to Rodrigues (ROD), endemic to the Mascarenes including Réunion (REU), endemic to the Western Indian Ocean islands (WIOI, which include Seychelles, Madagascar, Mayotte, Comoros) or beyond (Native)

* Locally produced in the Mascarenes.
Table 3. The priority CWR species of Mauritius and Rodrigues with their utilization potential, distribution and Red List status

The distribution of the species is denoted by MAS – Endemic to Mascarenes, MAU – Endemic to Mauritius, N – Native, PN – Probably Native, ROD – Endemic to Rodrigues. The Red List categories follow IUCN Red List Categories and Criteria version 3.1 (2001), but status for each island is given when it differs from the species global assessment. The data on utilization potential were derived from Vincent et al. (Reference Vincent, Wiersema, Kell, Fielder, Dobbie, Castañeda-Álvarez, Guarino, Eastwood, León and Maxted2013).
* Locally produced in the Mascarenes.
About 25% of the prioritized species are threatened (CR, EN or VU, according to the IUCN Red List Categories and Criteria, IUCN, 2001), all having at least one occurrence within protected areas. For Mauritius, 13 occurred within sites where invasive species are controlled (e.g. weeding of alien plants). For Rodrigues, 23 occurrences of all prioritized CWR were found within arboreta, Nature Reserves or private land managed for conservation purposes, with 27 others found in areas with no formal protection or not undergoing conservation management. The main threat in Mauritius to these CWR is the deleterious effects of invasive alien species, especially strawberry guava, P. cattleyanum, through competition, and by introduced animals such as rats and macaques that destroy large numbers of flowers, fruits and seeds of native species (e.g. Baider and Florens, Reference Baider, Florens, Laurance and Peres2006; Monty et al., Reference Monty, Florens and Baider2013; Florens, Reference Florens2015). Other additional specific threats for some species as Elaeocarpus bojeri R.E. Vaughan include vegetation clearing (three of the ten known plants were recently cut to provide a viewpoint). In Rodrigues, soil erosion and overgrazing by cattle pose serious threats, besides impacts of invasive alien plants, especially Vachellia nilotica (L.) P.J.H. Hurther & Mabb. (Fabaceae).
The distribution of the priority species of both islands were grouped by the number of species co-occurring per site (Figs. 1–3). In Mauritius, Mondrain had six species coexisting, hence high valued for conservation of CWR. There were 24 sites that harboured at least three different species, 17–100% of the sites found within the Black River Gorges National Park, thus receiving some degree of protection like Pétrin, Florin or Brise Fer, where active conservation management (weed and pest control) is ongoing for decades. In Rodrigues, most of the species occurred in areas with no legal protection. On this island, there are only two mainland Nature Reserves (Grande Montagne and Anse Quitor), where most of the active conservation management is also ongoing for decades.
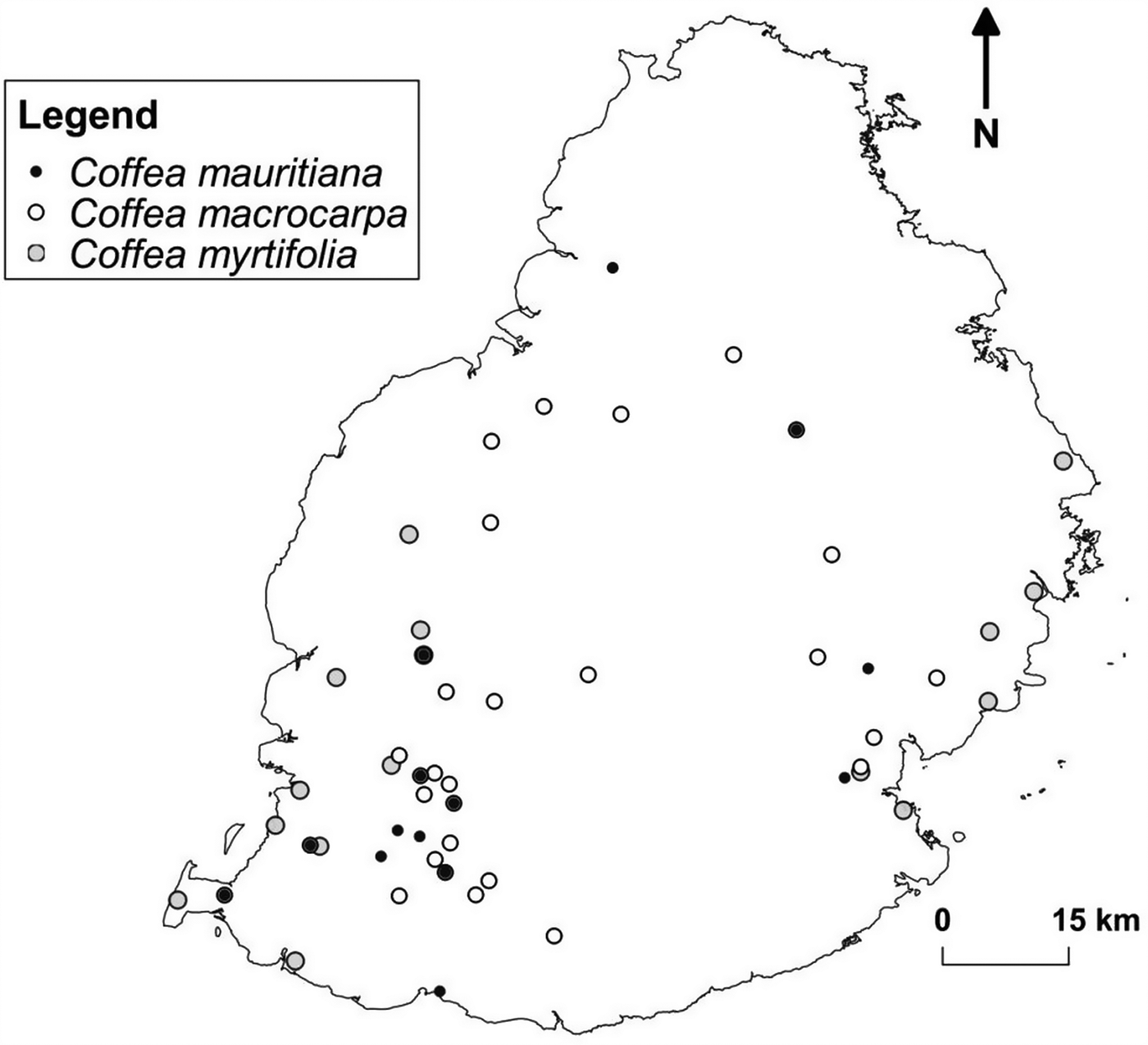
Fig. 1. Known distribution of the three Coffea species in Mauritius. Some sites had more than one species co-occurring.

Fig. 2. The 21 food crop priority CWR species in Mauritius, grouped in areas comprising of one, two or three species in a same site. The Black River Gorges National Park is outlined.

Fig. 3. Distribution of the 10 food crop priority CWR species in Rodrigues.
Discussion
To initiate any sustainable use and conservation programme of CWR, there is a need for baseline inventory (Maxted et al., Reference Maxted, Ford-Lloyd, Jury, Kell and Scholten2006, Reference Maxted, Scholten, Codd and Ford-Lloyd2007; Heywood et al., Reference Heywood, Casas, Ford-Lloyd, Kell and Maxted2007). This has been achieved by creating the checklist of CWR for Mauritius and Rodrigues. As experienced by other countries like Venezuela or China (e.g. Berlingeri-González and Crespo, Reference Berlingeri-González and Crespo2012; Kell et al., Reference Kell, Qin, Chen, Ford-Lloyd, Wei, Kang and Maxted2015), the development of the CWR inventory can be time consuming as necessary information is not always readily available, and expert knowledge needs to be tapped on. Nonetheless, once the checklist is created, updates based on new information (from species distribution to crop management) are easy, motivating stakeholders involved to collaborate as well (Magos Brehm et al., Reference Magos Brehm, Maxted, Martins-Loução and Ford-Lloyd2010). The Mauritius and Rodrigues CWR checklists were databased using a standard format to make it easily available online (such as the Harlan and de Wet CWR website (https://www.cwrdiversity.org/checklist/)), once the NBSAP is formally approved (Republic of Mauritius, 2016). Using global standards also facilitates updates and the exchange of information within the PGR or broader research community.
Despite the relatively high native plant diversity and endemism of Mauritius and Rodrigues, among oceanic islands (Kier et al., Reference Kier, Kreft, Lee, Jetz, Ibisch, Nowicki, Mutke and Barthlott2009; Baider et al., Reference Baider, Florens, Baret, Beaver, Matatiken, Strasberg and Kueffer2010), only 28 species were related to food crops. As expected, these islands are relatively CWR-poor partially because of their small geographical size, isolation and lower genetic variation compared with mainland areas (Frankham, Reference Frankham1997), but possibly also because most crops were developed in continental areas, and by default, their progenitors and wild relatives are also expected to be more species rich there. Although the CWR of Mauritius and Rodrigues may not be of immediate interest to plant breeders, they could be of value in the future and efforts to characterize material would therefore be expected to be worthwhile. For example, the three Coffea species have long seen to be of potential use for breeding programmes for two main reasons. Coffee is one of the most popular beverage worldwide (Heckman et al., Reference Heckman, Weil, Mejia and Gonzalez2010), with C. arabica L. predicted to have up to 90% reduction of land with suitable bioclimatic conditions for its cultivation in the next 60 years due to climate change (Davis et al., Reference Davis, Gole, Baena and Moat2012). Secondly, there is also a demand for decaffeinated coffee (Castañeda-Álvarez et al., Reference Castañeda-Álvarez, Khoury, Achicanoy, Bernau, Dempewolf, Eastwood, Guarino, Harker, Jarvis and Maxted2016). The three Coffea of the Mascarenes may be potential candidates for eventual use as they might have climate change-resistant traits and also due to their low caffeine content (Dulloo et al., Reference Dulloo, Guarino, Engelmann, Maxted, Newbury, Attere and Ford-Lloyd1998; Hamon et al., Reference Hamon, Rakotomalala, Akaffou, Razafinarivo, Couturon, Guyot, Crouzillat, Hamon, de Kochko and Preedy2015).
A number of species were identified for local use such as the palms Dictyosperma album, Acanthophoenix rubra (Bory) H.Wendl. and the screwpine, Pandanus utilis Bory, primarily due to their local economic importance. Palm hearts are considered as a delicacy, and a lucrative business within the tourism sector as palm hearts’ dishes are marketed as part of the traditional cuisine of the islands. Wild populations of these palms could potentially be used not only to introduce new diversity to cultivated populations on the islands but could be of high value for crop improvement in case cultivation spreads to other countries, as even species with few surviving individuals on Mauritius have shown to hold relatively high genetic diversity (Bone, Reference Bone2009). Improved cultivars could boost interest in these species for eventual regional or global production. In this specific case, there is the possibility that the cultivated individuals of D. album have a larger genetic diversity than the 30 or so remaining in the wild in Mauritius, therefore the crop may be useful to boost genetic diversity in the wild population.
The present study highlights the paucity of information on the potential of CWR as trait donors for crop improvement – a global issue limiting the use of CWR (Maxted et al., Reference Maxted, Ford-Lloyd, Kell, Maxted, Ford-Lloyd, Kell, Iriondo, Dulloo and Turok2008, Reference Maxted, Kell, Ford-Lloyd, Dulloo and Toledo2012, Reference Maxted, Avagyan, Frese, Iriondo, Magos Brehm, Singer and Kell2015, Reference Maxted, Amri, Castañeda-Álvarez, Dias, Dulloo, Fielder, Maxted, Ford-Lloyd and Dulloo2016; Maxted and Kell, Reference Maxted and Kell2009; McCouch et al., Reference McCouch, Baute, Bradeen, Bramel, Bretting and Buckler2013; Dempewolf et al., Reference Dempewolf, Eastwood, Guarino, Khoury, Müller and Toll2014; Kell et al., Reference Kell, Ford-Lloyd, Magos Brehm, Iriondo and Maxted2017), and thus impacting their perceived value for conservation action. The 21 prioritized CWR species here are either closely related to the priority crops/crop groups or more distantly related. However, to distinguish whether this relatedness would actually benefit breeding programmes, it is necessary to investigate their potential use. Consequently, the database must be supplemented with genetic studies characterising each prioritized CWR to improve understanding of the structure of the genetic variation of the species. So far, the only full eco-geographic study for the region was done for the Coffea (Dulloo et al., Reference Dulloo, Maxted, Newbury, Florens and Ford Lloyd1999). Similar studies should be encouraged, at least, for the local crop priority species.
CWR species prioritization is a dynamic process and largely depends on the method and scientific information available at the specific point in time (Magos Brehm et al., Reference Magos Brehm, Maxted, Martins-Loução and Ford-Lloyd2010). Therefore, re-evaluation should be done regularly. In this study, around 62% of the priority species would be threatened with extinction according to the IUCN Red List categories and criteria. Many of the species are represented within protected areas on Mauritius, and nearly half of the CWR species of Rodrigues. However, despite presence in protected areas, their long-term survival in situ is not guaranteed due to the ongoing threats (Caujapé-Castells et al., Reference Caujapé-Castells, Tye, Crawford, Santos-Guerra, Sakai, Beaver, Lobin, Florens, Moura, Jardim, Gómes and Kueffer2010; Florens et al., Reference Florens, Baider, Martin, Seegoolam, Zmanay and Strasberg2016, Reference Florens, Baider, Seegoolam, Zmanay and Strasberg2017a). Consequently, there is a need to improve in situ conservation, through methods like use of in situ biocontrol for invasive alien plants, some of which have proved effective (e.g. Dumoulin, Reference Dumoulin2017; Florens et al., Reference Florens, Bissessur, Bunsy and Ramdonee2017b). Ex situ facilities, as seed banks or living collections in botanical gardens or arboreta (Castañeda-Álvarez et al., Reference Castañeda-Álvarez, Khoury, Achicanoy, Bernau, Dempewolf, Eastwood, Guarino, Harker, Jarvis and Maxted2016), could serve as tools for eventual sustainable use of species. For instance, in Rodrigues, the Critically Endangered endemic species A. lomatophylloides Balf.f. is being propagated in the ex situ facility of the Forestry Service at Solitude (and also in botanical gardens abroad). This species, used topically against muscular pain and to increase menstrual flow (Gurib-Fakim and Guého, Reference Gurib-Fakim and Guého1994), has only a small surviving population comprising of the eight mature plants situated at Grande Montagne.
It is also important to constantly evaluate success of conservation management (both in situ and ex situ) to ensure long-term survival of species and their potential for use. Use of tools such as diversity and gap analyses also help to identify the ‘Most Appropriate Wild Populations’, to be prioritized given the limited conservation resources (Maxted et al., Reference Kell, Qin, Chen, Ford-Lloyd, Wei, Kang and Maxted2015). In the priority sites, proven cost-effective conservation management should be boosted or initiated (e.g. control of invasive alien species (plants and animals) (Baider and Florens, Reference Baider and Florens2011; Monty et al., Reference Monty, Florens and Baider2013); with research on new or improved methods also being developed. Another step would be the identification of potential sites to establish ‘genetic reserve’ – an in situ designated area for regular monitoring of the genetic diversity and long-term protection and conservation of the CWR natural wild populations (Maxted et al., Reference Maxted, Ford-Lloyd and Hawkes1997), a concept put forward already in the draft of the CWR National Biodiversity Conservation Strategy for both islands (Republic of Mauritius, 2016).
Non-indigenous species were not considered for the inventory, mostly because in both islands remnants of native vegetation are heavily invaded by invasive alien plants (Florens, Reference Florens, Sodhi, Gibson and Raven2013; Florens et al., Reference Florens, Baider, Martin, Seegoolam, Zmanay and Strasberg2016, Reference Florens, Baider, Seegoolam, Zmanay and Strasberg2017a), therefore conserving alien plants in situ might be a risk for conservation of the native CWR.
Conclusion
The creation of the inventory and prioritization process of CWR for Mauritius and Rodrigues are part of an effort to support global food security by providing a baseline for further studies. The production of the checklist is of high importance locally to highlight the need to plan and implement an effective in situ and ex situ conservation strategy for these genetic resources, providing sustainability to eventual use and necessary conservation actions to ensure long-term survival of the CWR and their genetic value. Despite the low proportion of CWR identified and prioritized, they are still important since their potential as gene donors is yet to be determined and they may prove useful for eventual crop improvement. Further studies are needed to determine the relationship between each CWR taxon and its related crop. In particular, the Coffea genus requires in-depth understanding of how their tertiary-level relatedness can be valuable, despite the difficulty in crossing. The three Mascarene Coffea CWR are likely to warrant genetic studies on how local adaptation could eventually be used in one of the most lucrative global crops, which is highly threatened by climate change. It is unlikely that plant breeders can breed climate change-resilient varieties without access to the full range of conserved CWR diversity; hence it is crucial to share the information on international and reputed databases like the Harvard Dataverse (https://dataverse.harvard.edu/). Importantly, to provide sustainability to conservation actions, investigations on the potential use of the prioritized CWR could help halting extinctions, especially in the context of highly threatened species found on oceanic islands.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262118000576
Acknowledgements
The research was carried out within the project ‘In situ conservation and use of crop wild relatives in three ACP countries of the SADC region’ (SADC Crop Wild Relatives project) (http://www.cropwildrelatives.org/sadc-cwr-project/) co-funded by the European Union and implemented through the ACP-EU Co-operation Programme in Science and Technology (S&T) II by the African, Caribbean and Pacific (ACP) Group of States (grant agreement no. FED/2013/330-210). Authors acknowledge technical support from the Commission for Agriculture and Environment (Rodrigues), and of Mrs N. Ramburn, Mr P. Ragen and Dr V. Tatayah who were part of the CWR committee of this project.








