Introduction
Seagrasses are widely distributed from tropical to arctic regions (Short et al., Reference Short, Carruthers, Dennison and Waycott2007) and form extensive meadows termed seagrass beds. Seagrass beds are one of the most productive systems in coastal areas (Duarte & Chiscano, Reference Duarte and Chiscano1999) and provide habitats for a wide variety of animals from small invertebrates to large vertebrates including commercially important fishes (Horinouchi & Sano, Reference Horinouchi and Sano2000; Nakamura et al., Reference Nakamura, Horinouchi, Nakai and Sano2003). In particular, small herbivorous invertebrates such as gastropods and amphipods inhabit seagrass beds in high densities. They are major prey items for crabs and fishes thus providing a trophic link between plants and carnivorous animals in seagrass beds (Duffy, Reference Duffy2006; Douglass et al., Reference Douglass, Duffy and Bruno2008; Fukuoka & Yamada, Reference Fukuoka and Yamada2015; Yamada et al., Reference Yamada, Hayakawa, Nakamoto, Kawamura and Kon2016a).
In seagrass beds, food value for herbivorous invertebrates differs among macrophyte types. For example, seagrasses are considered to have a lower food value than seaweeds for many herbivorous invertebrates because seagrasses have tough, cellulose and lignin-defended cells (Zapata & McMillan, Reference Zapata and McMillan1979; Unabia, Reference Unabia2011). Therefore, seagrasses have been considered to be rarely consumed by herbivorous invertebrates (Nienhuis & Van Ierland, Reference Nienhuis and Van Ierland1978; Van Montfrans et al., Reference Van Montfrans, Orth and Vay1982; Jernakoff et al., Reference Jernakoff, Brearley and Nielsen1996). However, various invertebrates have been reported recently to feed on and assimilate seagrasses (Valentine & Heck, Reference Valentine and Heck1999; Kharlamenko et al., Reference Kharlamenko, Kiyashko, Imbs and Vyshkvartzev2001; Nakaoka, Reference Nakaoka2005), while there are a few invertebrates which specialize in seagrasses (Brearley & Walker, Reference Brearley and Walker1995; Unabia, Reference Unabia2011). Whether herbivorous invertebrates feed on seagrasses may relate not only to the feeding organs or digestive ability of each invertebrate species but also to the availability of each food source.
In tropical and subtropical areas, seagrass beds are formed on sandy bottoms in back reef moats as well as in reef lagoons. Seaweeds can grow inside tropical and subtropical seagrass beds (Heijs, Reference Heijs1985; Lewis, Reference Lewis1987; Davis & Fourqurean, Reference Davis and Fourqurean2001) because hard substrata such as coral rocks and coral gravel are patchily concentrated on sandy bottoms in back reef moats. Abundance of seaweeds fluctuates seasonally, usually increasing toward spring and decreasing to summer (Tsai et al., Reference Tsai, Wong, Chang, Hwang, Dai, Yu, Shyu, Sheu and Lee2004; Tytlyanov et al., Reference Tytlyanov, Titlyanova, Huang and Li2014). By contrast, abundances of tropical and subtropical seagrass species such as Thalassia hemprichii and Cymodocea rotundata are relatively constant throughout the year (Agawin et al., Reference Agawin, Duarte, Fortes, Uri and Vermaat2001; Paula et al., Reference Paula, Ecosta, Martins and Gove2001). Consequently, food sources for herbivorous invertebrates are expected to vary temporally in those seagrass beds in response to seasonal fluctuations of seaweeds, which are likely to be one of the main food sources for inhabiting herbivorous invertebrates (Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008; Douglass et al., Reference Douglass, Duffy and Canuel2011). However, few studies have addressed temporal variations in the food sources for herbivorous invertebrates in seagrass beds.
Stable isotopes have often been used to identify the food sources of herbivorous invertebrates in seagrass beds (Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008; Douglass et al., Reference Douglass, Duffy and Canuel2011; Michel et al., Reference Michel, Dauby, Gobert, Graeve, Nyssen, Thelen and Lepoint2015). While gut content analysis reflects the composition of ingested diets just before sampling and many gut contents in herbivorous invertebrates cannot be identified (Douglass et al., Reference Douglass, Duffy and Canuel2011), stable isotopes record the composition of digested and assimilated diets into animal tissue. δ13C is widely used for estimating the origin of food items used in the diet because δ13C values vary among primary producers (Won et al., Reference Won, Kawamura, Onitsuka, Hayakawa, Watanabe, Horii, Takami and Watanabe2007; Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008). δ15N is used to estimate the trophic level of organisms because δ15N values are relatively constant among primary producers and because δ15N is enriched from prey to consumers (Minagawa & Wada, Reference Minagawa and Wada1984). Using these two stable isotopes together, the relative contribution of each food source can be estimated (Jeong et al., Reference Jeong, Suh and Kang2012; Lebreton et al., Reference Lebreton, Richard, Galois, Radenac, Brahmia, Colli, Grouazel, André, Guillou and Blanchard2012; Michel et al., Reference Michel, Dauby, Gobert, Graeve, Nyssen, Thelen and Lepoint2015).
In the present study, we examined the seasonal changes in carbon and nitrogen stable isotope ratios of dominant invertebrates and their possible food sources in Nagura Bay, Ishigaki Island, Japan in order to assess the temporal variability in food sources of the marine herbivorous invertebrates.
Materials and methods
Study site
Samplings of benthic organisms were conducted in back reef moats in Nagura Bay, Ishigaki Island, Okinawa, Japan (24°23′18″N 124°08′17″E; Figure 1). The depth of the study site is ~0.1 m at the spring ebb tide. On sandy bottoms of the back reef moats, seagrasses, mainly Cymodocea rotundata and Thalassia hemprichii, grow all year round, and from winter to spring, small seaweeds such as Tolypiocladia glomerulata and Hydroclathrus tenuis grow on coral rocks and gravel fragments that occur on the sandy bottom. These hard substrata were abundant even inside the seagrass bed, so that seagrass-seaweed mixed beds were formed from winter to spring.

Fig. 1. The study site in Nagura Bay, Ishigaki Island, Okinawa, Japan.
Sample collection and preparation
The samples were collected in spring (April in 2013 and 2015) and summer (July in 2013 and June in 2015). The biomass of seagrasses in April and July 2013 was 15.3 ± 7.8 gDW m−2 (mean ± SD) and 45.7 ± 7.2 gDW m−2, respectively. The biomass of seaweeds in April and July 2013 was 17.1 ± 15.6 gDW m−2 and 0 gDW m−2, respectively. Although water temperature in 2013 and 2015 was not measured, that in April 2014 varied from 21.5 to 27.3°C and from the second half of June to the first half of July 2014 varied from 28.7 to 33.6°C. Among invertebrates, gastropods were abundant throughout the study period so that dominant gastropods as well as dominant seagrasses and seaweeds were collected in 2013. Seagrasses and seaweeds were cut with a pair of scissors at the sheath or stem, respectively, and then collected in a net with coexistent gastropods. In addition, epibenthic gastropods were collected with sand using a scoop. No seaweeds were collected in summer of 2013 as few seaweeds were found at the study site. In addition to gastropods and macrophytes, periphyton on the seagrass Cy. rotundata, bottom surface sediment and surface water were collected in 2015. Bottom surface sediment was collected using a core sampler to ~1 cm depth. In summer 2015, the seaweed To. glomerulata was collected in small patches attached to hard substrate in the seagrass bed. All samples were immediately put into a cool box filled with seawater and seawater ice, and then transported to the Research Center for Subtropical Fisheries, Seikai National Fisheries Research Institute.
In the laboratory, attached materials on Cy. rotundata blades were scraped with a brush and then sieved with a 200 µm net. The filtrates were filtered again on GF/F filters, which were combusted at 450°C for at least 6 h prior to filtration, and used as the periphyton sample. Surface water through a 200 µm net were filtered on pre-combusted GF/F filters and used as the POM sample. Foot muscles of gastropods were rinsed with ultrapure water (Milli-Q; Millipore) and used as samples. Several individuals of some gastropod species, that is, Cantharidus urbanus, Cerithium sp. and Clithon parvulum in both years and Euplica scripta in spring 2013, were pooled for the measurement because individuals were too small to obtain sufficient material for the stable isotope analysis. In order to reduce any biases of δ13C caused by the lipids in the samples, lipid extraction was conducted for the gastropod samples collected in 2013 according to Folch et al. (Reference Folch, Lees and Sloane Stanley1957). However, lipid extraction was not conducted for the samples collected in 2015 considering the possible influence on δ15N by the lipid extraction (Mateo et al., Reference Mateo, Serrano, Serrano and Michener2008). Instead, the δ13C values of gastropods collected in 2015 were normalized using the regression equation between the C:N ratio and δ13C (Post et al., Reference Post, Layman, Arrington, Takimoto, Quattrochi and Montana2007). This mathematical normalization for δ13C has an equivalent effect to the direct lipid extraction (Post et al., Reference Post, Layman, Arrington, Takimoto, Quattrochi and Montana2007). As lipid extraction may affect δ15N values, only samples collected in 2015 were used in evaluating the trophic levels and estimating the contribution rates of food sources. POM and periphyton samples were dried and fumigated with 12 M HCl for 2 h to remove carbonate. Sieved sediment was rinsed with 1 M HCl until bubbles could not be seen. All samples were dried (60°C), homogenized and stored in a desiccator.
Stable isotope analysis
Approximately 1.5 mg of homogenized plants and about 0.5 mg of homogenized gastropods were packed into tin capsules and then combusted using a flash elemental analyser (Flash 2000, Thermo). Generated gases were sent to an isotope ratio mass spectrometer (DELTA V, Thermo) through a continuous flow interface (Conflo IV, Thermo). δ13C and δ15N was calculated as:
where X = 13C or 15N and R = 13C/12C or 15N/14N, respectively. International standards of Pee Dee Belemnite and atmospheric N2 were used as standards. Glycine was used as a reference material and run every 10 samples. The analytical error of δ13C and δ15N measured by glycine were both 0.24‰ in standard deviation.
Gastropods with δ15N values lower than the averaged δ15N value of macrophytes plus 2.4, the fractionation factor of δ15N (Mittermayr et al., Reference Mittermayr, Fox and Sommer2014), were regarded as herbivores. The food sources of the herbivorous gastropods were estimated using the mixing models in siarmcmcdirichletv4 function of package SIAR (Stable Isotope Analysis in R; Parnell et al., Reference Parnell, Inger, Bearhop and Jackson2010) in R version 3.3.3 (R Development Core Team, 2017). The number of possible food sources for the herbivorous gastropods was too large to estimate the contribution rates of all possible food sources. Therefore, we used isotopic values of Cy. rotundata and Th. hemprichii (seagrass), Hypnea charoides and To. glomerulata (rhodophyte), Hyd. tenuis and Cladosiphon okamuranus (phaeophyte), and Acetabularia ryukyuensis (chlorophyte) for estimating the contribution rates for herbivorous gastropods in spring. In addition, the isotopic values of periphyton were used to estimate the contribution rates for Cant. urbanus, Ce. zonatum, Ce. sp., Cl. parvulum, E. scripta and Petalifera punctulata as these gastropods were abundant on the seagrasses and seaweeds, and the isotopic values of sediment were used for Canarium mutabile as this species was abundant on the sandy bottom. In summer, seagrass, rhodophyte calculated using only To. glomerulata and periphyton (for gastropods other than Cana. mutabile) or sediment (for Cana. mutabile) were used for estimating the contribution rates because no phaeophytes or chlorophytes were found at the study site. The fractionation factors were used as 0.5 ± 0.5 for δ13C and 2.4 ± 1.1 for δ15N, respectively (Mittermayr et al., Reference Mittermayr, Fox and Sommer2014).
Statistical analysis
The results of estimated contribution rates in SIAR in 2015 indicate that herbivorous gastropods changed their food sources from seaweeds to seagrasses in 2015. The dietary change from seaweeds to seagrasses in the same way in 2015 would result in an increase in δ13C values of herbivorous gastropods because δ13C values of seaweeds were lower than seagrasses in 2013. Therefore, we examined whether the seasonal variation of δ13C values of the herbivorous gastropods was larger than the seasonal variation of the basal food sources in 2013 by testing the effect of the interaction between trophic position (basal food sources vs herbivorous gastropods) and season (spring vs summer). We first constructed the LMM with δ13C values of the basal food sources and the herbivorous gastropods as response variables, and season, trophic position, and the interaction between season and trophic position were included as predictor variables. Then, the above model was compared with the LMM in which predictor variables include only season and trophic position. Each species was included in the random slope and intercept. Significance of the interaction between season and trophic position was determined by ANOVA using Wald chi-square test with one degree of freedom. All LMMs were analysed using lmer function in R package lme4 (Bates et al., Reference Bates, Maechler, Bolker and Walker2015).
Results
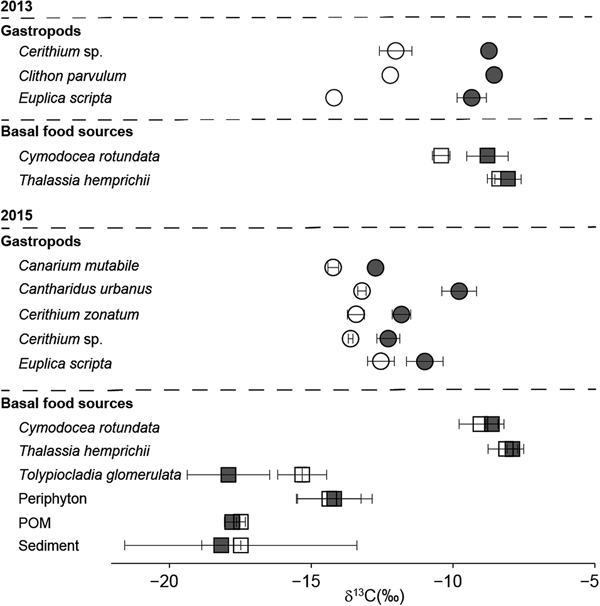
Each basal food source had similar isotopic values between the two seasons (Table 1, Figure 2). The δ13C values of seagrasses, Cymodocea rotundata and Thalassia hemprichii, ranged from −7.9‰ to −10.4‰ and were higher than values of seaweeds, periphyton and sediment (Table 1). The δ13C values of seaweeds varied between −9.6‰ and −18.8‰, although phaeophytes and the chlorophyte were more enriched in 13C than rhodophytes (Table 1). The δ13C values of periphyton ranged from −14.2‰ to −14.4‰ and in between those of seagrasses and seaweeds (Table 1).

Fig. 2. Mean (± SD) δ13C and δ15N values of seagrasses (black squares), seaweeds (grey squares), other basal food sources (white squares) and herbivorous gastropods (white circles) collected in the seagrass bed in Nagura Bay in spring (a) and in summer (b), 2015.
Table 1. Size, δ13C and δ15N values (mean ± SD) of possible food sources and gastropods in spring and summer 2013 and 2015

Size represented by shell height in gastropods except for Clithon parvulum and as shell width in Clithon parvulum. The number of analysed samples are represented by N.
Seven species of gastropods, Canarium mutabile, Cantharidus urbanus, Cerithium zonatum, Ce. sp., Clithon parvulum, Euplica scripta and Petalifera punctulata, were considered as herbivores. The δ13C values of the herbivorous gastropods varied from −8.5‰ to −14.3‰ and differed among the gastropod species (Table 1). All the herbivorous gastropod species in summer were more enriched in 13C than those in spring (Table 1, Figure 3).

Fig. 3. Comparison of δ13C values (mean ± SD) for basal food sources (squares) and herbivorous gastropods (circles) between spring (white) and summer (grey) in 2013 and 2015.
The mixing models in SIAR showed that the contribution rates of seagrass for all herbivorous gastropod species increased from spring to summer in 2015 (Figure 4). The mean contribution rates of seagrass in spring ranged from 12% in Ce. zonatum to 16% in E. scripta, and those in summer ranged from 32% in P. punctulata to 60% in Cant. urbanus. In contrast, those of rhodophyte slightly decreased from spring to summer although there was a large overlap in the posterior distribution. The mean contribution rates of phaeophyte in spring for herbivorous gastropods ranged from 10% in Ce. zonatum to 15% in E. scripta and Cant. urbanus. Those of chlorophyte were from 11% in Ce. zonatum to 18% in E. scripta. The contribution rates of respective food sources were also different among gastropod species (Figure 4). For example, the contribution rate of seagrass for Cant. urbanus was higher than for the other herbivores in summer although the between-species-difference was less clear in comparison to the between-season-difference. The increase of δ13C from spring to summer was larger in herbivorous gastropods than those of the basal food sources in 2013 according to the results of LMMs (season × trophic position, χ2 = 8.15, P < 0.01).

Fig. 4. Contribution rates of food sources for each herbivorous gastropod species in spring and summer of 2015. Black, grey and white boxes represent 50, 75 and 95% credibility intervals, respectively. Cm, Canarium mutabile; Cu, Cantharidus urbanus; Cz, Cerithium zonatum; Cs, Ce. sp.; Cp, Clithon parvulum; Es, Euplica scripta; Pp, Petalifera punctulata.
Discussion
The mixing models in SIAR showed that the total contribution rates of seaweeds, that is rhodophyte, phaeophyte and chlorophyte, for all herbivorous gastropod species decreased from spring to summer in 2015; in contrast, those of seagrasses increased (Figure 4). This was supported by the results of LMMs which showed that the seasonal variations in δ13C of the herbivorous gastropods were larger than those of the basal food sources in 2013, indicating that the food sources of the herbivorous gastropods changed seasonally. Many herbivorous gastropods do not depend on any particular food source (Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008; Doropoulos et al., Reference Doropoulos, Hyndes, Lavery and Tuya2009) and this ability of generalistic food use would be responsible for the temporal dietary change in the herbivorous gastropods.
There is a time lag for animal isotopic values to reflect the diet isotopic value. However, the turnover rate generally depends on the metabolic rate and growth of the organisms, and thus body mass and water temperature (McIntyre & Flecker, Reference McIntyre and Flecker2006; Thomas & Crowther, Reference Thomas and Crowther2015). Therefore, the half-life time of the small gastropods in subtropical areas is much shorter than one season, which is 3 months (Thomas & Crowther, Reference Thomas and Crowther2015; Vander Zanden et al., Reference Vander Zanden, Clayton, Moody, Solomon and Weidel2015), and the stable isotopes of the herbivorous gastropods in this study must have reflected the isotopic values of relatively recent food sources. This supported the result of this study that the increase in isotopic values of the herbivorous gastropods from spring to summer was caused by the change in food sources.
Seasonal changes in food sources of the herbivorous gastropods corresponded with the change in food availability. In Nagura Bay, abundance of seagrasses such as Cymodocea rotundata and Thalassia hemprichii did not change much through the year. In contrast, as in the case of other areas of Indo-Pacific tropical or subtropical regions (Agawin et al., Reference Agawin, Duarte, Fortes, Uri and Vermaat2001; Paula et al., Reference Paula, Ecosta, Martins and Gove2001; Tsai et al., Reference Tsai, Wong, Chang, Hwang, Dai, Yu, Shyu, Sheu and Lee2004; Titlyanov et al., Reference Titlyanov, Titlyanova, Li, Hansen and Huang2014; Tytlyanov et al., Reference Tytlyanov, Titlyanova, Huang and Li2014), abundance of seaweeds such as Tolypiocladia glomerulata and Hydroclathrus tenuis fluctuated seasonally. As seaweeds are superior to seagrasses in food value (Nienhuis & Van Ierland, Reference Nienhuis and Van Ierland1978; Van Montfrans et al., Reference Van Montfrans, Orth and Vay1982; Jernakoff et al., Reference Jernakoff, Brearley and Nielsen1996), contribution rates of seaweeds are expected to be high when the abundance of seaweeds is high, which was consistent with the results of this study.
The contribution of seagrasses as food sources for herbivorous invertebrates in seagrass beds is controversial. Although early observations led to the generalized view that seagrasses are rarely consumed by herbivorous invertebrates (Nienhuis & Van Ierland, Reference Nienhuis and Van Ierland1978; Van Montfrans et al., Reference Van Montfrans, Orth and Vay1982; Jernakoff et al., Reference Jernakoff, Brearley and Nielsen1996) and some recent studies also reported minimal contribution of seagrasses (Jaschinski et al., Reference Jaschinski, Brepohl and Sommer2008; Douglass et al., Reference Douglass, Duffy and Canuel2011), other studies showed that seagrasses contributed substantially to herbivorous invertebrates as food sources (Kharlamenko et al., Reference Kharlamenko, Kiyashko, Imbs and Vyshkvartzev2001; Vonk et al., Reference Vonk, Christianen and Stapel2008). One cause of the difference in contribution rates of seagrasses for herbivores among the studies must be the difference in feeding organs or digestive ability of inhabiting herbivores. The present study also showed that the contribution rate of seagrass differed between the two seasons even for the same gastropod species (Figure 4). The availability of food sources other than seagrass may be another factor leading to the differences in the contribution rate of seagrasses for herbivores.
The isotopic composition differed among the herbivorous gastropod species, which suggests that the food sources were different among the gastropod species. The mean contribution rate of seagrasses for Cantharidus urbanus was higher than for Cerithium zonatum or Cerithium sp. In addition, the density of Cant. urbanus was higher on seagrasses than on seaweeds, indicating that Cant. urbanus is more associated with seagrasses than the other two gastropod species. However, food partitioning among the gastropod species must be carefully interpreted because there was an overlap in contribution rates due to the small sample size and because the isotopic composition in spring did not largely differ among the gastropod species. In order to elucidate any potential food partitioning, other analyses such as fatty acid analysis or food preference experiments should be done.
In conclusion, the results of this study indicate that food sources of herbivorous gastropods inhabiting the subtropical seagrass bed in Nagura Bay changed seasonally. This seasonal change in food use appears to correspond to the change in seaweed biomass, suggesting that the herbivorous gastropods flexibly change their diets depending on food availability. Because these herbivorous gastropods are major prey items for crabs and fishes (Duffy, Reference Duffy2006; Douglass et al., Reference Douglass, Duffy and Bruno2008; Fukuoka & Yamada, Reference Fukuoka and Yamada2015; Yamada et al., Reference Yamada, Hayakawa, Nakamoto, Kawamura and Kon2016a), the origin of food materials for predators are likely to change temporally. Some herbivorous fishes such as rabbit fish prefer seaweeds to seagrasses (Pillans et al., Reference Pillans, Franklin and Tibbetts2004; Yamada et al., Reference Yamada, Shimabukuro, Hayakawa, Nakamoto, Kawamura and Kon2016b), so the food web in seagrass beds may drastically shift with the change in seaweed biomass. Our results strongly imply the importance of examining macrophyte composition in assessing the food web in seagrass beds.
Author ORCIDs
Kenta Nakamoto 0000-0001-9715-3721.
Acknowledgements
We thank Kouki Fukuoka, Koetsu Kon, Shoji Houki, Akira Hayashi, Kaito Fukuda and Masafumi Kodama for their assistance in the fieldwork, and Toshihiro Miyajima and Nobue Saotome for their assistance for the stable isotope analyses. We also thank two anonymous reviewers for their helpful comments on this manuscript.
Financial support
This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 2529115) and the Coastal Ecosystem Complex Project of the Ocean Resources Use Promotion Technology Development Program, of the Ministry of Education, Culture, Sports, Science and Technology Japan.