INTRODUCTION
The relatively cold and humid climatic conditions in the northern boreal domain are favorable to organic matter accumulation over mineral soil (paludification), particularly in flat and poorly drained environments (Payette, Reference Payette2001; Charman, Reference Charman2002; Korhola et al., Reference Korhola, Ruppel, Seppa, Väliranta, Virtanen and Weckström2010). Paludification is responsible for the development of vast peatland ecosystems on clayey substrates in regions such as southern Finland (Korhola, Reference Korhola1995), proglacial Lake Agassiz basin (Heinselman, Reference Heinselman1970; Siegel, Reference Siegel1983), and the Clay Belt and James Bay area in Québec and Ontario (Taylor et al., Reference Taylor, Carleton and Adams1987; Klinger and Short, Reference Klinger and Short1996; Fenton et al., Reference Fenton, Lecomte, Légaré and Bergeron2005; Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007). Paludification has also been documented on other glacial and fluvioglacial deposits in maritime regions of eastern Canada (Payette et al., Reference Payette, Garneau, Delwaide and Schaffhauser2012; Magnan and Garneau, Reference Magnan and Garneau2014; Schaffhauser et al., Reference Schaffhauser, Payette, Garneau and Robert2016), in continental western Canada (Bauer et al., Reference Bauer, Gignac and Vitt2003; Bauer and Vitt, Reference Bauer and Vitt2011), in Alaska (Heilman, Reference Heilman1966; Jones and Yu, Reference Jones and Yu2010; Loisel et al., Reference Loisel, Yu, Parsekian, Nolan and Slater2013), and in boreal western Siberia (Glebov and Korzukhin, Reference Glebov and Korzukhin1992; Sheng et al., Reference Sheng, Smith, MacDonald, Kremenetski, Frey, Velichko, Lee, Beilman and Dubinin2004).
Paludification is the most common process of peatland formation in boreal regions (Sjörs, Reference Sjörs1983; Payette, Reference Payette2001; Charman, Reference Charman2002; Ruppel et al., Reference Ruppel, Väliranta, Virtanen and Korhola2013), characterized by a progressive accumulation of thick organic layers (>40 cm) on mineral substrates, without an initial aquatic phase (Payette, Reference Payette2001). The paleoecological conditions associated with the onset of peat accumulation from paludification have not been sufficiently documented at the stand scale in the boreal biome of northeastern North America. Paludification is influenced by a combination of autogenic (topography and mineral substrate) and allogenic (fire and climate) factors. The invasion of sphagna and the accumulation of a thick organic layer lead to a lowering in soil temperatures (Van Cleve et al., Reference Van Cleve, Dyrness, Viereck, Fox, Chapin and Oechel1983), a decrease in soil nutrient availability (Prescott et al., Reference Prescott, Maynard and Laiho2000), a rise in water table (Taylor et al., Reference Taylor, Carleton and Adams1987; Fenton and Bergeron, Reference Fenton and Bergeron2006; Magnan et al., Reference Magnan, Le Stum-Boivin, Garneau, Grondin, Fenton and Bergeron2018), and a decline in forest productivity (Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007). At the landscape scale, the paludification process and subsequent widespread peatland formation can also cause a reduction of fire recurrence (Cyr et al., Reference Cyr, Bergeron, Gauthier and Larouche2005) and modify forest vegetation composition, particularly a decrease in the abundance of balsam fir (Abies balsamea; Ali et al., Reference Ali, Asselin, Larouche, Bergeron, Carcaillet and Richard2008; Messaoud et al., Reference Messaoud, Asselin, Bergeron and Grondin2014) and jack pine (Pinus banksiana; Payette et al., Reference Payette, Garneau, Delwaide and Schaffhauser2012).
Two main processes of paludification have been documented in boreal regions. The first one described as primary paludification (Sjörs, Reference Sjörs1983; Payette, Reference Payette2001; Charman, Reference Charman2002; Rydin and Jeglum, Reference Rydin and Jeglum2006; Korhola et al., Reference Korhola, Ruppel, Seppa, Väliranta, Virtanen and Weckström2010) refers to an accumulation of peat directly on mineral substrates following land emergence and without an initial forest phase. Primary paludification is the equivalent of “primary mire formation” described in northern Europe (Ruppel et al., Reference Ruppel, Väliranta, Virtanen and Korhola2013) and to “edaphic paludification” reported in studies in the Clay Belt and James Bay lowlands (Fenton et al., Reference Fenton, Lecomte, Légaré and Bergeron2005; Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007; Laamrani et al., Reference Laamrani, Valeria, Fenton, Bergeron and Cheng2014). In the present study, the term paludification is used as opposed to terrestrialization, which means that no infilling of aquatic ecosystems is involved. The second process, called secondary paludification, corresponds to the accumulation and/or lateral expansion of peat in well-drained forest stands (Heinselman, Reference Heinselman1963; Payette, Reference Payette2001; Charman, Reference Charman2002), also described as “successional paludification” in the Clay Belt (Fenton and Bergeron, Reference Fenton and Bergeron2006; Simard et al., Reference Simard, Bernier, Bergeron, Paré and Guérine2009; Laamrani et al., Reference Laamrani, Valeria, Fenton, Bergeron and Cheng2014). This mechanism requires specific humid climate conditions that promote Sphagnum invasion (Korhola, Reference Korhola1995). Secondary paludification is considered reversible because forest stands can regenerate on mineral substrates when the organic layer is eliminated by high-severity fires (Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007; Laamrani et al., Reference Laamrani, Valeria, Fenton, Bergeron and Cheng2014). Severe fires can delay paludification by promoting the regeneration of dense forest stands on mineral soil creating conditions unfavorable for Sphagnum growth (Schaffhauser et al., Reference Schaffhauser, Payette, Garneau and Robert2016). When the organic layer is nearly entirely burned, successional paludification often begins with the colonization of postfire mosses such as Polytrichum spp. (Jasieniuk and Johnson, Reference Jasieniuk and Johnson1980; Viereck, Reference Viereck1983). Heliophyte mosses particularly Sphagnum spp. then establish on moist surfaces before the forest canopy begins to close (Benscoter and Vitt, Reference Benscoter and Vitt2008; Wieder et al., Reference Wieder, Scott, Kamminga, Vile, Vitt, Bone, Xu, Benscoter and Bhatti2009). These bryophyte communities are followed by feather mosses like Pleurozium schreberi and shade-tolerant Sphagnum spp. such as Sphagnum capillifolium and S. russowii (Fenton et al., Reference Fenton, Béland, De Blois and Bergeron2007; Turetsky et al., Reference Turetsky, Bond-Lamberty, Euskirchen, Talbot, Frolking, McGuire and Tuittila2012). Low decomposition rates, rapid growth, and high-density colonies of Sphagnum spp. promote their spreading above the feather mosses carpet (Foster, Reference Foster1984; Fenton et al., Reference Fenton, Béland, De Blois and Bergeron2007, Reference Fenton, Bergeron and Paré2010; Lang et al., Reference Lang, Cornelissen, Klahn, van Logtestijn, Broekman, Schweikert and Aerts2009). Within aging forests, the progressive accumulation of organic layer induces an opening of the canopy and the establishment of peatland species associated with wet and bright conditions such as Sphagnum magellanicum (Fenton and Bergeron, Reference Fenton and Bergeron2006). However, fires that do not completely burn the organic layer and cause tree mortality increase available light on the ground surface, promoting the growth of shade intolerant Sphagnum spp. and a high water table because of reduced evapotranspiration (Chambers, Reference Chambers1997; Fenton et al., Reference Fenton, Lecomte, Légaré and Bergeron2005; Wieder et al., Reference Wieder, Scott, Kamminga, Vile, Vitt, Bone, Xu, Benscoter and Bhatti2009; Schaffhauser et al., Reference Schaffhauser, Payette, Garneau and Robert2016).
In the Clay Belt, the two paludification processes can coexist within the same forest stand (Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007) as paludified environments coalesce when peat thickness increases (Foster and Fritz, Reference Foster and Fritz1987; Lindsay et al., Reference Lindsay, Charman, Everingham, O’Reilly, Palmer, Rowell and Stroud1988). Based on the knowledge of these two successional processes, our study aims at understanding the spatial and temporal dynamics of paludification in distinct geomorphological contexts in the Québec black spruce (Picea mariana)–moss domain using a paleoecological approach. The comparison of paludification dynamics along three toposequences contributes to a better understanding of initial vegetation successions in order to discriminate the factors affecting peat initiation. More specifically, the objectives of this study were to (1) evaluate the relative importance of autogenic (topography and mineral substrate) and allogenic (fire and climate) factors triggering paludification processes and (2) determine how these factors influence temporal and spatial paludification dynamics within the black spruce–moss domain.
METHODS
Study regions and sites
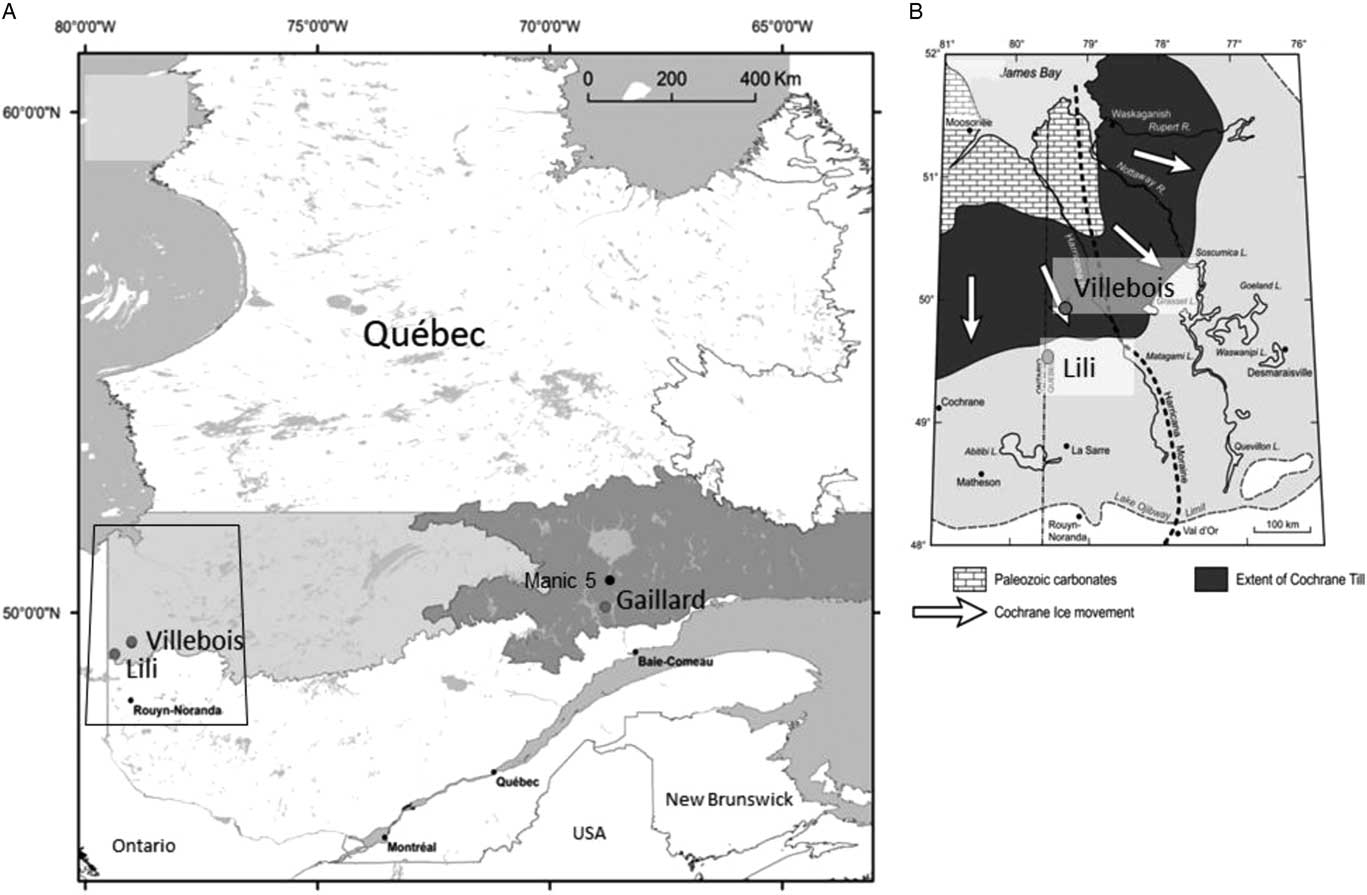
Basal peat cores (first 50 cm accumulated over the mineral substrate) were collected along three topographic sequences (transects) each located within a distinct geomorphological context within the black spruce–moss bioclimatic domain (Fig. 1A). Two sites were chosen in the western boreal forest of Québec, respectively on the Cochrane till (Villebois) and on clayey substrate of Lake Ojibway (Lili), and a third one in eastern boreal Québec (Gaillard). The choice of these three sites was made to improve understanding of the factors that have affected peat initiation through time within the black spruce–moss bioclimatic domain. The two transects Lili and Villebois are located in the western part of the black spruce–moss domain, near the village of Val-Paradis. The climate is humid continental (Peel et al., Reference Peel, Finlayson and McMahon2007) with a mean annual temperature of 0.1°C and a mean annual precipitation of 910 mm (closest meteorological station, Val-Saint-Gilles; Environment Canada, 2016).
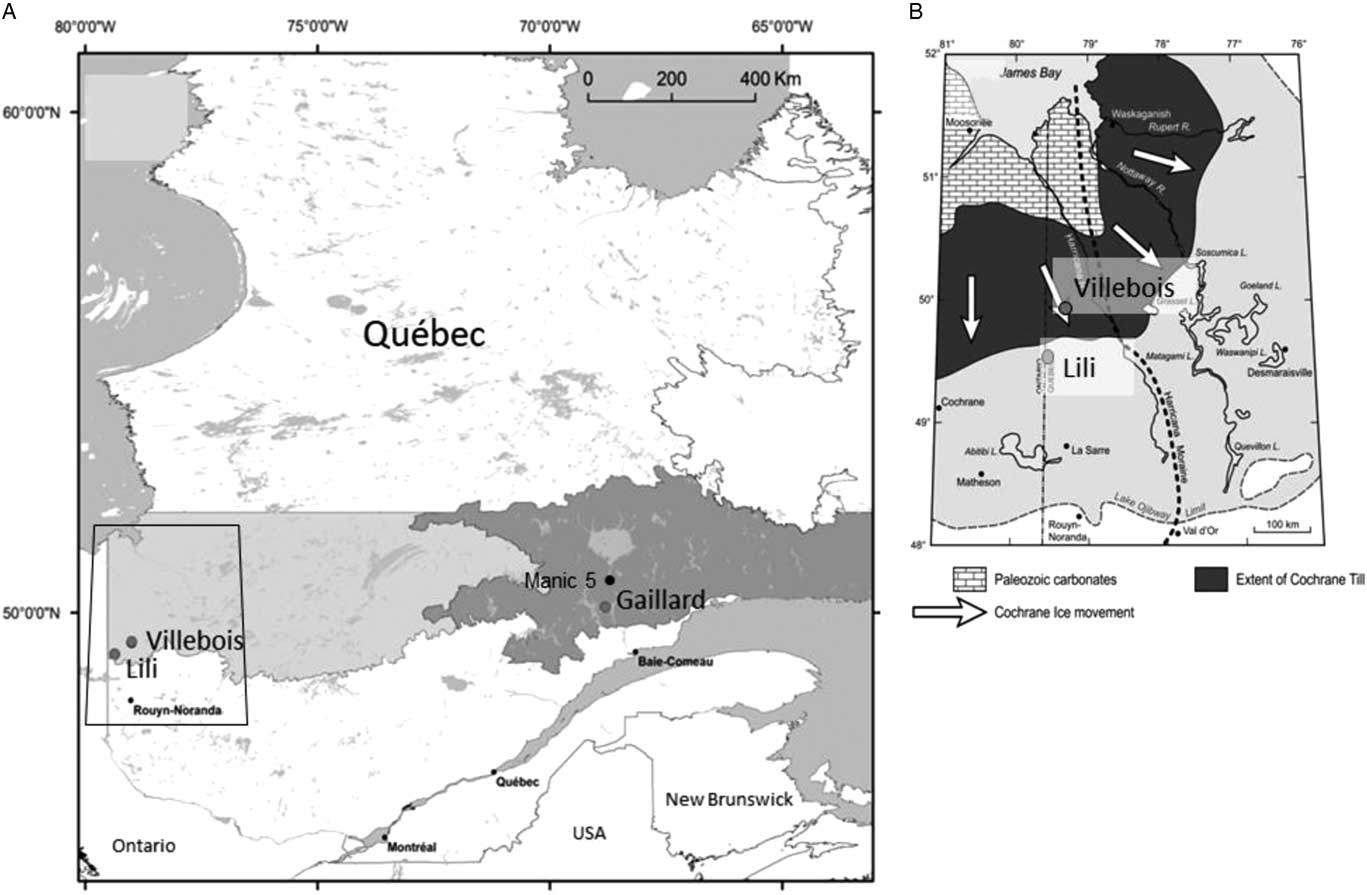
Figure 1. (A) The three study site locations within the black spruce–moss bioclimatic domain (east and west) (Saucier et al., Reference Saucier, Robitaille and Grondin2009). (B) The geomorphological context of the two studied sites in the Clay Belt region (modified from Roy et al., Reference Roy, Veillette, Daubois and Ménard2015).
The Lili site (49°17′21″N, 79°36′91″W) located in the Clay Belt, just south of the James Bay lowlands (Fig. 1B), is characterized by a flat to undulated topography (slope <3%) with a clayey substrate from proglacial lake Ojibway ca. 8200 cal yr BP (Roy et al., Reference Roy, Dell’Oste, Veillette, de Vernal, Hélie and Parent2011). The regional upland forest vegetation is dominated by black spruce [Picea mariana (Mill.) Britton, Sterns et Poggenb.] and jack pine (Pinus banksiana Lamb.) with mosses such as Pleurozium schreberi (Willd. ex Brid.) Mitt., Ptilium crista-castrensis (Hedw.) De Not., and Sphagnum capillifolium (Ehrh.) Hedw. in the understory. Trembling aspen (Populus tremuloides Michx.) forest with Alnus crispa (Aiton) Pursh and Alnus rugosa (DuRoi) Spreng. are also well represented (Asselin et al., Reference Asselin, Grondin, Lavoie and Fréchette2016). Fire interval was 135 yr between AD 1850 and 1920 and since has increased to ca. 400 yr (Bergeron et al., Reference Bergeron, Gauthier, Flannigan and Kafka2004).
The Villebois site (49°42′09″N, 79°00′03″W) is located 40 km northeast of the Lili site on the flat-lying terrain corresponding to the Cochrane till (Fig. 1B) composed of clayey and compact fine-grained sediments resulting from a late-glacial ice readvance into Lake Ojibway ca. 8200 cal yr BP (Roy et al., Reference Roy, Dell’Oste, Veillette, de Vernal, Hélie and Parent2011). This region presents a flat topography and is covered by extensive peatlands. The vegetation cover of forested peatlands is largely dominated by Picea mariana and Sphagnum communities. Jack pine is less common and limited on sandy soils (Jules et al., Reference Jules, Asselin, Bergeron and Ali2018). Fire interval for this region is very long (>450 yr), in part because of the large expanses of peatlands limiting fire ignition and propagation (Cyr et al., Reference Cyr, Bergeron, Gauthier and Larouche2005).
The Gaillard site (50°11′32″N, 68°80′83″W) is located in the St.-Lawrence North Shore region (Fig. 1A) characterized by Precambrian rocks from the Grenville Province covered by glacial and fluvioglacial sandy deposits originating from the Laurentian Ice Sheet retreat (Vincent, Reference Vincent1989). Slopes are more accentuated (slope >3%) than in the western Clay Belt region. The mean annual temperature is 1.6°C, and the mean annual precipitation is 870 mm (meteorological station, Labrieville; Environment Canada, 2016). The study site is located at the transition among the eastern (maritime) and western (continental) black spruce–moss subdomains (Saucier et al., Reference Saucier, Robitaille and Grondin2009), near lac Gaillard between the city of Baie-Comeau and the Manic 5 hydropower dam (Fig. 1A). The regional vegetation is mainly composed of black spruce and balsam fir [Abies balsamea (L.) Mill.]. Jack pine sporadically found on sandy terraces is at its eastern limit of distribution (Grondin et al., Reference Grondin, Noël and Hotte2007). Regional fire interval has been estimated at 270 yr between AD 1800 and 2000 (Bouchard et al., Reference Bouchard, Pothier and Gauthier2008).
Field measurements and sample processing
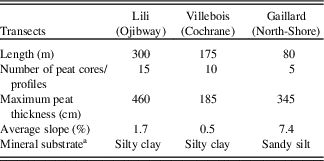
The three topographic sequences were documented along transects of different lengths: Lili, L.0 m–L.300 m; Villebois, V.0 m–V.175 m; and Gaillard, G.0 m–G.80 m. For each of these transects, mineral topography underneath the peat deposit and peat thickness were obtained at 5-m intervals by measuring relative surface altitudes with a ZIP LEVEL pro 2000 altimeter and manual probing using an Oakfield soil sampler. Water table depths were also measured along the transects at 25-m intervals from soil profiles dug 24 hours before measurements to allow the water table level to stabilize.
Along each transect, basal peat sections were sampled and included at least 5 cm of the underlying mineral sediment. A total of 12 basal peat sections of 50 cm long were retrieved and analyzed at intervals of 20, 25, or 50 m along the transect using a 50-cm-long small Russian corer (4.5-cm diameter; modified from Jowsey, Reference Jowsey1966). Also, 18 thick forest humus profiles (<40 cm) were extracted in the weakly paludified even-aged forest stand with a Box corer (10-cm diameter and 120 cm long; Jeglum et al., Reference Jeglum, Rothwell, Berry and Smith1992). The Lili transect covered a distance of 300 m, and three peat basal sections of 50 cm were collected at 50 m intervals between 150 and 250 m (respectively cores L.150 m, L.200 m, and L.250 m). In addition, in the even-aged black spruce and jack pine forest at L.0 m, 12 forest humus profiles <30 cm were retrieved randomly in a 50-m2 quadrat in order to characterize the most recent postfire bryophyte succession. Villebois transect covered a distance of 175 m, and seven 50-cm peat basal sections were collected every 25 m along this transect. In addition, three forest humus profiles were sampled randomly in a 20 m2 quadrat from the AD 1760 postfire forest (V.0 m). Gaillard transect covered a distance of 80 m, and two peat basal sections were extracted at 40 and 60 m. In addition, three forest humus profiles were collected randomly in a 10-m2 quadrat in the even-aged black spruce and sparse jack pine forest (G.0 m) originating from the most recent fire.
In order to characterize the composition of the present-day vegetation along the three transects, surface vegetation inventories were made at each sampling site within 100 m2 plots. Diameter at breast height of trees >1 cm was also systematically measured in continuous 20-m2 plots (4 × 5 m) along the transects.
Laboratory analysis
Macrofossil analysis
In the laboratory, peat basal sections were cut into 1-cm-thick slices. Subsamples of 1 cm3 were used to quantify the organic matter content and to identify the transition between the mineral and the organic layers using loss-on-ignition at 550°C during 3.5 hours (modified from Dean, Reference Dean1974). Past vegetation successional sequences were reconstructed using detailed plant macrofossil analyses (2 or 4 cm intervals; 4 cm3 peat samples) following the protocol of Mauquoy et al. (Reference Mauquoy, Hughes and van Geel2010). Samples were slightly heated in a 5% KOH solution to extract macrofossils from the organic matrix and then rinsed with distilled water through a 212-μm sieve. Sieved material was placed on a gridded petri dish and analyzed under a stereomicroscope (magnification: 10–40×). Relative abundance of each main peat type (Sphagnum spp., non-Sphagnum mosses, lichens, Cyperaceae, ligneous fragments, roots, angiosperm leaves, and Equisetum stems) was estimated visually as percent volume of the total sample. Macrofossil remains (conifer needles, seeds, Cenococcum sclerotia, charcoal fragments) were counted. Moss leaves were identified to species or genus level or to the section level for Sphagnum spp. (Acutifolia, Cuspidata, and Sphagnum) using a light microscope (magnification: 40–100×). References used for plant identification were Lévesque et al. (Reference Lévesque, Dinel and Larouche1998) and Laine et al. (Reference Laine, Harju, Timonen, Laine, Tuittila, Minkkinen and Vasander2009), as well as the plant macrofossil reference collection at the laboratory of continental paleoecology at Geotop-UQAM (Garneau, Reference Garneau1995). Taxonomy for vascular plants follows Brouillet et al. (Reference Brouillet, Coursol, Meades, Favreau, Anions, Bélisle and Desmet2018) and bryophyte taxonomy is based on Faubert (Reference Faubert2013). Macrofossil diagrams were generated with C2 software version 1.7.6 (Juggins, Reference Juggins2014; Supplementary Figs. 1 to 3).
Charcoal analyses
Charcoals were identified botanically; the depth of the charred layer was evaluated, and the fire event was dated. Charcoal fragments were first extracted from 1-cm3 subsamples previously heated in a 10% KOH solution and rinsed through a 0.5-mm sieve with distilled water. Charcoal fragments were counted in two size fractions (0.5–2 mm and >2 mm) at contiguous 1 cm intervals for each core. The thickness of the residual organic layer underneath the charred horizon was measured in order to determine if the fire events had consumed it partially or completely. The fire severity was determined by evaluating the thickness of the residual organic matter after a fire (Nguyen-Xuan et al., Reference Nguyen-Xuan, Bergeron, Simard, Fyles and David2000). High-severity fires are characterized by a charcoal layer located directly on the mineral substrate or fewer than 5-cm-thick residual organic layer, whereas low-severity fires produce a charcoal layer on a variable thickness of residual organic matter (Lecomte et al., Reference Lecomte, Simard, Fenton and Bergeron2006). Charcoal fragments in the first charred horizon above the mineral sediment were identified to the species level when possible (Thinon, Reference Thinon1978, Reference Thinon1992). If charcoals were too abundant, a maximum of 100 fragments were retained. Botanical identification of these charcoal fragments was based on charred modern wood species reference collection from the Institut des Sciences de l’Évolution de Montpellier (Université de Montpellier) and the wood anatomy atlas of Schweingruber (Reference Schweingruber1990). Wood anatomy was observed under a light microscope (magnification: 40–100×). Botanical criteria do not easily differentiate Picea spp. from Larix spp. (Talon, Reference Talon1997); consequently, these two genera were grouped.
Granulometry analyses
Granulometry analyses of basal mineral sediments were realized by Laser Particle Sizer (ANALYSETTE 22 NanoTec) at the Department of Geography, Université du Québec à Montréal. Grain size was measured following the wet dispersion method (ultrasonic of 90 W) on 100 grains varying from 0.01 to 2100 μm in size. For each sample, measurements were replicated three times (Supplementary Table 2). Each mineral soil under the basal peat section was analyzed to evaluate the soil texture and identify the dominant mineral substrate composition along the three toposequences.
Chronologies
A total of 20 radiocarbon dates (14C) were obtained from selected terrestrial plant macrofossils (Sphagnum spp. stems, ericaceous leaves, Carex spp. seeds, and conifer needles) extracted directly at the organic/mineral contact or charcoal fragments contained in the first charred layer. Samples were prepared at the Radiochronology Laboratory of the Centre d’études nordiques (Université Laval, Québec) and sent for accelerator mass spectrometry (AMS) radiocarbon dating at Keck-Carbon Cycle AMS Laboratory (University of California at Irvine, USA). Results were calibrated using calibration data set IntCal13 calibration curve (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey and Buck2013) implemented in CALIB software version 7.1 (Stuiver et al., Reference Stuiver, Reimer and Reimer2017). All dates are expressed in calendar years before present (cal yr BP: before AD 1950) except for the most recent dates that are presented in years AD.
The age of the most recent fire was evaluated from the forest currently undergoing paludification (respectively profiles L.0 m, V.0 m, and G.0 m) using dendrochronological analysis. Cross sections of tree trunks were cut at the root collar, and growth rings were counted in the Laboratoire de dendrochronologie de la direction de la recherche forestière du Ministère des Forêts, de la Faune et des Parcs (MFFP). Five trees per site were analyzed, and the oldest age obtained was attributed to the last fire.
RESULTS
Characteristics of the three toposequences
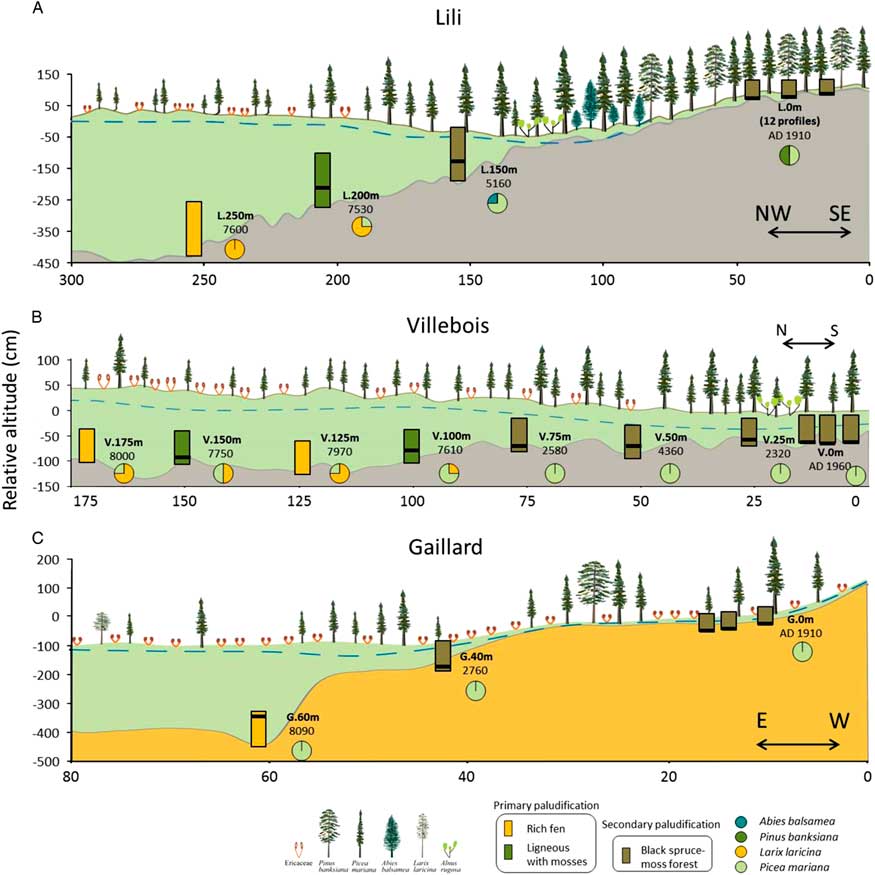
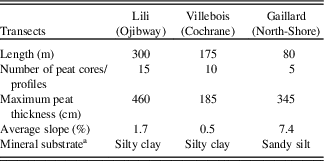
Lili (0–300 m) is dominated by silty clay substrate, slight slopes (1% to 2%) (Fig. 2, Table 1; Supplementary Tables 1 and 2), and organic layer thickness varying between 30 and 460 cm. Surface vegetation at the end (0 m) of the transect is characterized by an even-aged black spruce and jack pine forest where trees are relatively tall (∼15 m). At about 100 m along the transect, topography seems to promote the presence of Alnus rugosa and balsam fir. From 200 m, the forested peatland gives way to an open black spruce peatland with heliophyte Sphagnum species (e.g. Sphagnum magellanicum) and ericaceous shrub species (mainly Rhododendron canadense).

Figure 2. Description of the basal peat section along the three studied transects (A–C). Rectangles illustrate the initial process of paludification. Black lines show the position of the first charcoal layer above the mineral within the profiles, and circles refer to the forest composition based on the macrofossil data. Blue dashed lines represent the water table. Basal dates are represented in cal yr BP, except for the most recent fires, which are in years AD. Mineral substrates correspond to silty clay (gray) and sandy silt (brown). The x- and y-axes do not have the same values for the three transects. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 1. Transect descriptions.
a No granulometric variations along transects.
Villebois (0–175 m) is underlain by silty clay substrate and a flat topography (slope <1%) (Fig. 2, Table 1; Supplementary Tables 1 and 2) with an organic layer thickness ranging from 25 to 185 cm. Surface vegetation varies from an even-aged black spruce forest with shade-tolerant bryophytes (e.g. Sphagnum wulfianum) (0 m) to a forested peatland (75 m) and an semi-open forested peatland with ericaceous shrubs at its northern extremity (175 m).
Gaillard (0–80 m) located in the St.-Lawrence North Shore region is characterized by sandy silt deposits on average slopes of 5% to 9% (Fig. 2, Table 1; Supplementary Tables 1 and 2) with peat thickness varying from 40 to 300 cm. The central part (60 m) of the peatland is an open bog with small black spruces (∼8 cm diameter) and tamaracks with heliophyte Sphagnum species (e.g. Sphagnum magellanicum).
The granulometric analyses on the basal sediments show that the soil texture does not vary significantly along each toposequences (Supplementary Table 2).
Vegetation dynamics related to the paludification process
The plant macrofossils and the basal radiocarbon ages allow us to distinguish the timing of peat inception and the type of paludification processes (primary or secondary) along the three toposequences (Table 2, Fig. 2). In Lili and Gaillard, characterized by a deeper topographic depression, initial rich fen environments were dominated by Larix laricina (Lili), Picea mariana, brown mosses (Aulacomnium palustre, Calliergon spp., Drepanocladus spp., and Paludella squarrosa), and Cyperaceae (Fig. 2, Table 3). Peat initiation started around 8090 (Gaillard) and 7600 (Lili) cal yr BP without a forest phase (Table 3, Fig. 2) on soil textures composed of silty clay (Lili, Villebois) and sand (Gaillard) (Supplementary Table 2). The age of paludification for these rich fens is the oldest obtained in the two sites and corresponds to primary paludification. Slightly higher on Lili toposequence (L.200 m), the initial vegetation assemblage mainly composed of Picea mariana, Larix laricina, Tomentypnum nitens, and Sphagnum sect. Acutifolia also corresponds to primary paludification but with a higher abundance of ligneous plants. At Villebois, we observed the same overall vegetation dynamics influenced by topography and drainage from wet treeless minerotrophic fens (8000 cal yr BP) to dry forested plant communities. At this site, the influence of small-scale (<50 cm: horizontal and vertical) mineral microtopographic depressions on the initial ecological conditions is detected. The macrofossil data show an alternation of wet rich fens and drier microhabitats dominated by ligneous remains and mosses (Fig. 2) and a time intervals of approximately 200–400 yr between the initiation of these two microhabitats (Table 3).
Table 2. Ages from 14C radiocarbon and dendrochronology. (F) indicates a date associated with a fire event.
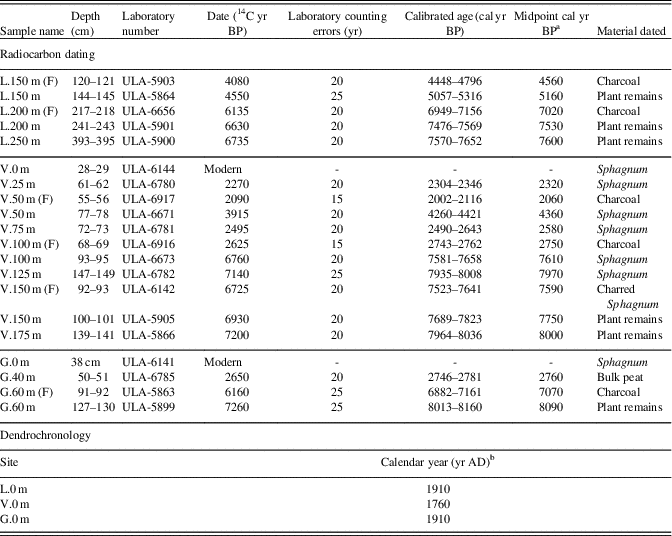
a Midpoint cal yr BP calculated by IntCal13 (Reimer et al., Reference Reimer, Bard, Bayliss, Beck, Blackwell, Ramsey and Buck2013) is rounded to the nearest decade.
b Calendar year from dendrochronology is rounded to the nearest decade.
Table 3. Description of the initial vegetation composition at each sampling site. All dates are in cal yr BP except as indicated otherwise.
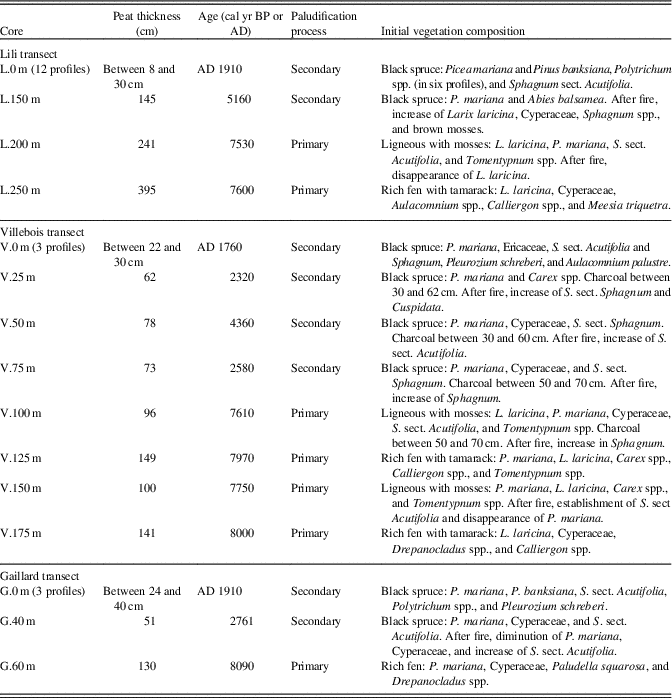
Higher on the three toposequences, peat initiation started between 5160 and 2300 cal yr BP (Table 2, Fig. 2) with initial vegetation mainly composed of Picea mariana, Abies balsamea, (L.150 m), Ericaceae, Cyperaceae, and some bryophytes (Polytrichum, S. sect. Acutifolia, and Pleurozium schreberi) (Table 3). These sections were paludified through a secondary process following fire. In the relatively well-drained forests of the three sites (L.0 m, V.0 m, and G.0 m), peat started to accumulate following the last fire (between AD 1760 and 1910) that consumed entirely the organic layer (Fig. 2, Table 3). These three forest stands are at an early stage of secondary paludification with an organic horizon thickness <40 cm.
The recent bryophyte successions associated with the secondary paludification process following the last fire that consumed entirely the organic horizons are shown in Figure 3. These data thus represent a modern analogue of the former vegetation successions at an early stage of forest paludification. The replication of 12 short profiles at L.0 m allowed us to validate the succession representativeness for each dominant bryophyte species at the surface. Macrofossil analyses show that when sphagna were the first to establish after fire, they remained dominant until today. Sphagnum spp. increased after fire, whereas the abundance of forest mosses remained low except for cores P1 to P4, which record a high abundance of feather mosses, an absence of Sphagnum spp. resulting in a thin organic layer accumulation (Fig. 3). In Villebois, the most recent secondary paludification process has started with a high abundance of Sphagnum spp.; however, P1 shows a shift from Sphagnum spp. to forest mosses. At Gaillard, Sphagnum spp. abundance varies further during vegetation succession. For example, at C2 and P1 the forest mosses, which were abundant after fire, were successively replaced by Sphagnum spp. (Fig. 3).
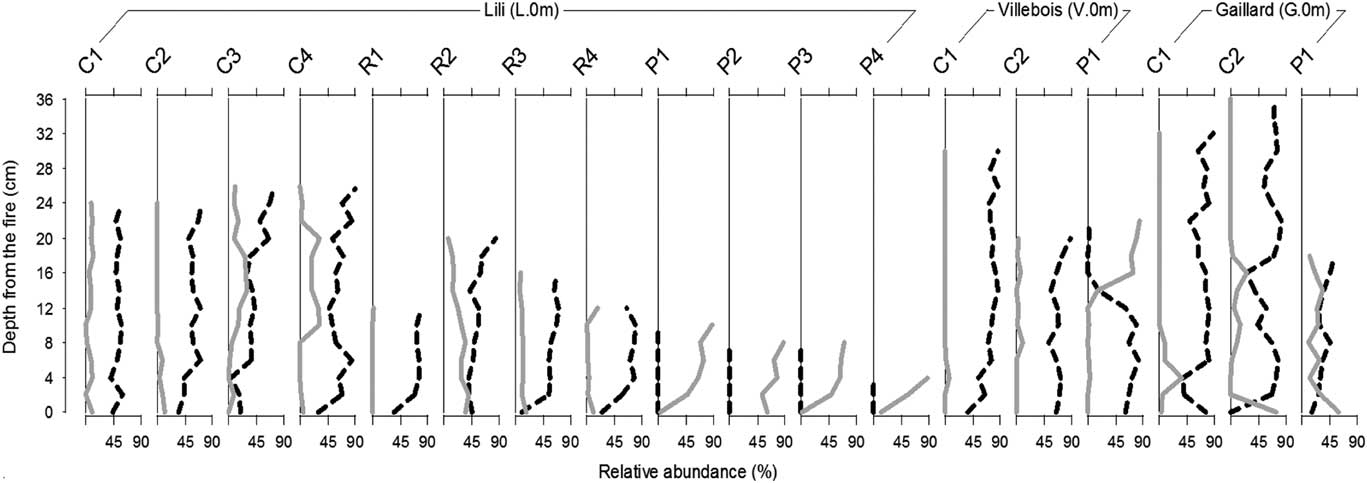
Figure 3. Bryophyte successions following the last fire (approximately 100 yr for Lili and Gaillard and 260 yr for Villebois site) from the 18 profiles in forest humus (L.0 m, V.0 m, and G.0 m) (black: Sphagnum spp.; gray: non-Sphagnum mosses). Profile names represent dominant bryophyte species at the surface: C, Sphagnum capillifolium; R, S. russowii; P, Pleurozium schreberi.
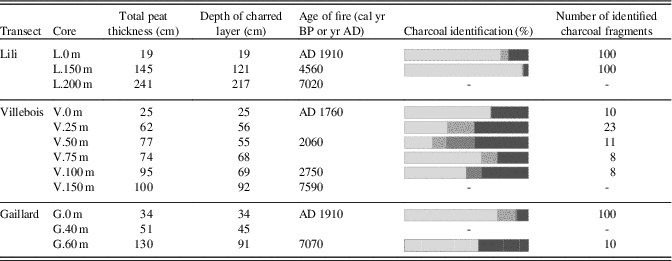
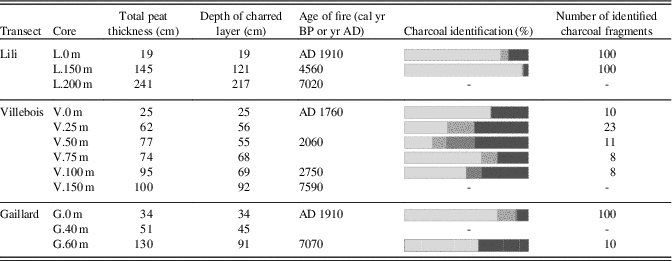
In most of the charred horizons found at the bottom of all the peat profiles, charcoals were mainly found between 5 and 30 cm above the mineral substrate (Table 4) except for the even-aged forests (L.0 m, V.0 m, and G.0 m) where charred layers have been found directly at the mineral contact. The identified charcoal fragments mostly correspond to Picea mariana/Larix laricina species with some Ericaceae in the Villebois transect. Charcoal fragments of Pinus banksiana have been found at L.0 m, V.25 m, V.50 m, V.75 m, and G.0 m (Table 4).
Table 4. Age and botanical identification of charcoal fragments found in the first charred layer above the mineral layer. The dash line means that no analysis was made. All dates are in cal yr BP except as indicated otherwise.

Vegetation dynamics after fire
Some of the peat profiles associated with primary paludification also composed a charcoal layer within the first 50 cm of peat analyzed (between 5 and 30 cm above the mineral substrate) (Fig. 2). Following fire, vegetation composition did not show much change (L.200 m and V.50 m) except an increase in Sphagnum at V.100 m and V.150 m and a decline in black spruce and Larix laricina (tamarack) at V.150 m (Table 3; Supplementary Figs. 1 to 3).
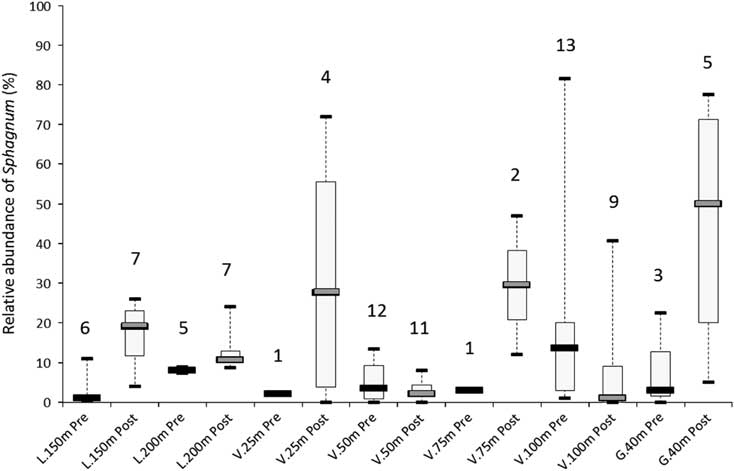
All sites corresponding to secondary paludification have fire horizons in the first 50 cm of the basal peat sections (Fig. 2). These fires have induced significant changes in the vegetation assemblages (Table 3). Above the higher charred layers, corresponding to the last fire event on the basal peat section, macrofossil data show an increase in Sphagnum spp. (S. sect. Acutifolia, Sphagnum, and Cuspidata) (L.150 m, V.25 m, V.75 m, G.40 m), brown mosses (Meesia triquetra), and tamarack (L.150 m), in addition to a diminution of Cyperaceae (G.40 m) and black spruce (V.25 m, V.75 m, and G.40 m) (Table 3). The relative abundance of Sphagnum spp. before and after fire increases from 6% (L.200 m) to 45% (G.40 m). In contrast, V.50 m and V.100 m show a decline in the Sphagnum abundance of 2% and 13%, respectively (Fig. 4), because Sphagnum spp. were already abundant before fire in these sites located in small-scale wetter depressions.

Figure 4. Relative abundance of Sphagnum spp. before and after the first charcoal layer above the mineral soil and within the basal peat sections (Fig. 2). Medians, quartiles, and maximum/minimum values were calculated from Sphagnum spp. abundance below (“Pre,” prefire) and above (“Post,” postfire) the charcoal horizons obtained for the number of macrofossil analyses realized in each section (number on top of each box plot).
DISCUSSION
Influence of autogenic factors (topography and mineral substrate) on vegetation dynamics
The generally flat topography and dominance of fine-textured clayey substrate played a significant role in the widespread development of peatlands in the James Bay lowlands and Clay Belt throughout the Holocene. Peatlands currently cover vast expanses, particularly in the Cochrane till section of the Clay Belt characterized by very poor drainage and where most forest stands are now influenced by secondary paludification dynamics (Saucier et al., Reference Saucier, Robitaille and Grondin2009). Our study showed that the process of forest paludification also occurred in stands from the eastern black spruce–moss bioclimatic domain on fluvioglacial sandy deposits. The mineral substrate texture was not the main factor controlling primary and secondary paludification processes at the scale of the studied transects (Fig. 5) as the granulometric data showed little variations in soil texture along each toposequence (Supplementary Table 2). Peat initiation through primary paludification was primarily controlled by local topography (Fig. 5). Topographic depressions, even shallow (depth ∼ <50 cm to 2 m) and with slight slopes, allowed the of minerotrophic plant communities following glacier retreat in the North Shore ca. 8000 cal yr BP (Vincent, Reference Vincent1989) or drainage of the basin of Lake Ojibway ca. 8200 cal yr BP in western Québec (Roy et al., Reference Roy, Dell’Oste, Veillette, de Vernal, Hélie and Parent2011). In the three studied sites, the initial rich fen stage was rapidly followed by vertical peat accumulation and lateral expansion into the adjacent forests. Furthermore, microtopographic fine-scale depressions (∼ <50 cm) at the Villebois site induced wet conditions in reducing lateral drainage (Tolonen, Reference Tolonen1983; Rydin, Reference Rydin1993; Lavoie et al., Reference Lavoie, Harper, Paré and Bergeron2007; Turetsky et al., Reference Turetsky, Bond-Lamberty, Euskirchen, Talbot, Frolking, McGuire and Tuittila2012), thus favoring a rapid accumulation of hygrophilous bryophytes as observed in the rich fen environments (Calliergon spp. and Drepanocladus spp.) (Table 3, Fig. 2).
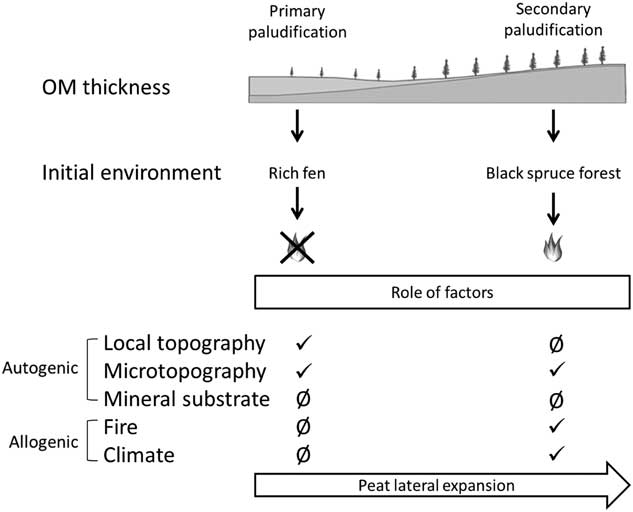
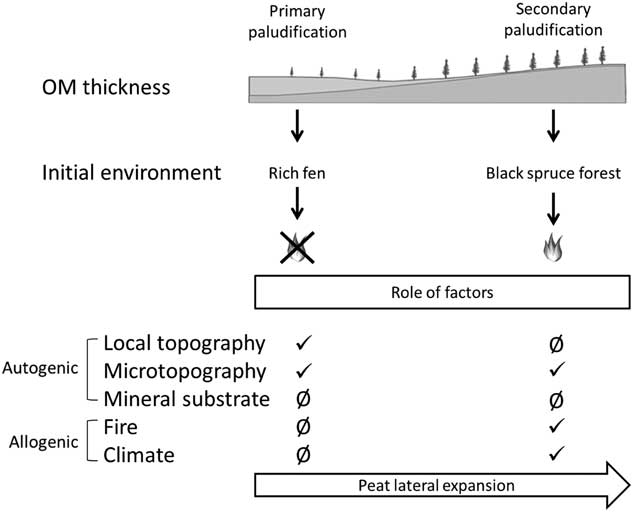
Figure 5. Synthesis of the two paludification processes along a toposequence and role of factors triggering paludification. OM, organic matter.
From ca. 5100–4000 cal yr BP (Fig. 2), lateral peatland expansion took place in adjacent forests (secondary paludification). Microtopography seems to have been an important autogenic factor triggering peat accumulation in the three sites (Fig. 5). Wet conditions in mineral microdepressions promoted the establishment of Sphagnum spp. into former forest communities as recorded at V.0 m, V.50 m, and V.75 m. The establishment of Sphagnum in these poorly drained microtopographic depressions was followed by an expansion of Sphagnum (Table 3) into the entire forest stand through secondary paludification (Fenton et al., Reference Fenton, Béland, De Blois and Bergeron2007).
Influence of allogenic factors (climate and fire) on vegetation dynamics
In the boreal domain, climate throughout the Holocene seems to have been always favorable for peat accumulation by paludification (Korhola et al., Reference Korhola, Ruppel, Seppa, Väliranta, Virtanen and Weckström2010). Our data show that primary paludification began soon after land emergence ca. 8000 and 7500 cal yr BP for the three sites (Table 3, Fig. 2) as observed in many other northern regions (Liu, Reference Liu1990; MacDonald et al., Reference MacDonald, Beilman, Kremennetski, Sheng, Smith and Velichko2006; Yu et al., Reference Yu, Beilman and Jones2009; Korhola et al., Reference Korhola, Ruppel, Seppa, Väliranta, Virtanen and Weckström2010; Ruppel et al., Reference Ruppel, Väliranta, Virtanen and Korhola2013). The relatively warm and moist climate around 8000–6000 cal yr BP (Filion, Reference Filion1987; Viau et al., Reference Viau, Gajewski, Sawada and Fines2006; Ali et al., Reference Ali, Asselin, Larouche, Bergeron, Carcaillet and Richard2008) was favorable to peat accumulation (Korhola, Reference Korhola1994, Reference Korhola1996; Mäkilä, Reference Mäkilä1997; Anderson et al., Reference Anderson, Foster and Motzkin2003; Bauer et al., Reference Bauer, Gignac and Vitt2003; Turunen and Turunen, Reference Turunen and Turunen2003; Ruppel et al., Reference Ruppel, Väliranta, Virtanen and Korhola2013), but the primary paludification process in the studied sites was primarily triggered by local topographic depressions and fine mineral substrate following land emergence.
Since ca. 4200 cal yr BP, a transition toward cooler and wetter climatic conditions over the Northern Hemisphere (Walker et al., Reference Walker, Berkelhammer, Björck, Cwynar, Fisher, Long, Lowe, Newnham, Rasmussen and Weiss2012) has favored peatland expansion into well-drained forest stands (Halsey et al., Reference Halsey, Vitt and Gignac1998; Yu et al., Reference Yu, Beilman and Jones2009; Korhola et al., Reference Korhola, Ruppel, Seppa, Väliranta, Virtanen and Weckström2010). The regional climatic change increased the rates of peat accumulation and reduced the fire frequency (Ali et al., Reference Ali, Blarquez, Girardin, Hély, Tinquaut, El Guellab and Valsecchi2012), which has likely triggered paludification in forests on higher ground ca. 5100 to 2300 cal yr BP in our studied sites (Table 3, Fig. 5).
The initial fens developed from primary paludification have evolved independently of fire (Fig. 5) owing to high water tables and low fuel loads in these peatland ecosystems (Magnan et al., Reference Magnan, Lavoie and Payette2012). An open forest canopy and humid local conditions reduced fire severity and frequency as suggested by the quasi absence of charcoal fragments at the base of our peat profiles (Table 4).
Fires cause a setback of paludification by removing partially or completely the soil organic layers (Fig. 2) (Lecomte et al., 2005, Reference Lecomte, Simard, Fenton and Bergeron2006; Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007, Reference Simard, Bernier, Bergeron, Paré and Guérine2009; Terrier et al., Reference Terrier, de Groot, Girardin and Bergeron2014). Profiles at Villebois have multiple charred layers until the top of the peat basal section (Table 3), suggesting that many fires occurred since peat inception, as observed in other peat deposits of the same region by Ali et al. (Reference Ali, Asselin, Larouche, Bergeron, Carcaillet and Richard2008) and Asselin et al. (Reference Asselin, Grondin, Lavoie and Fréchette2016). Our data also show that with a residual organic layer (∼5 to 30 cm) fire triggered peat accumulation by favoring Sphagnum spp. establishment (Figs. 4 and 5). Tree mortality caused by fire or other disturbances facilitates light penetration on the ground surface and raises water tables as a result of lower evapotranspiration (Fenton and Bergeron, Reference Fenton and Bergeron2011; Schaffhauser et al., Reference Schaffhauser, Payette, Garneau and Robert2016), thus creating favorable conditions for Sphagnum growth and peat accumulation (Fenton et al., Reference Fenton, Lecomte, Légaré and Bergeron2005, Reference Fenton, Béland, De Blois and Bergeron2007; Fenton and Bergeron, Reference Fenton and Bergeron2006; Simard et al., Reference Simard, Lecomte, Bergeron, Bernier and Paré2007; Magnan et al., Reference Magnan, Le Stum-Boivin, Garneau, Grondin, Fenton and Bergeron2018).
In the case of fires that entirely burned the organic layer to the mineral soil such as in the even-aged forests along the three studied transects (L.0 m, V.0 m, and G.0 m), the early bryophyte successions are dominated by Sphagnum spp. instead of feather mosses in 11 of the 18 thick forest humus profiles (Fig. 3). This succession is less frequent at Gaillard site where feather mosses were the first to establish on the mineral soil (Fig. 3). Postfire open canopy and poorly drained microtopographic depressions have induced relatively wet conditions favorable for Sphagnum growth (S. capillifolium and S. russowii). This process was also mentioned for clayey substrate by Lavoie et al. (Reference Lavoie, Paré, Fenton, Groot and Taylor2005, Reference Lavoie, Harper, Paré and Bergeron2007). Nevertheless, Polytrichum spp. was found directly on mineral in nine contemporary profiles (Table 3). This moss is a typical postfire pioneer species in boreal peatlands and forests and has a wide habitat tolerance, particularly to light exposure (Black and Bliss, Reference Black and Bliss1978; Jasieniuk and Johnson, Reference Jasieniuk and Johnson1980; Morneau and Payette, Reference Morneau and Payette1989). The physiology of Sphagnum spp. can also explain their rapid establishment after fire in different conditions because of their capacity to regenerate from buried peat layers (Clymo and Duckett, Reference Clymo and Duckett1986), their low decomposition rate (Lang et al., Reference Lang, Cornelissen, Klahn, van Logtestijn, Broekman, Schweikert and Aerts2009; Fenton et al., Reference Fenton, Bergeron and Paré2010), and their capacity to retain water by capillarity (Clymo and Hayward, Reference Clymo and Hayward1982; Bisbee et al., Reference Bisbee, Gower, Norman and Nordheim2001). Autogenic and allogenic factors inducing wet conditions promote Sphagnum establishment most of the time, suggesting the undeniable paludification trajectory of the boreal forest zone (Fenton et al., Reference Fenton, Béland, De Blois and Bergeron2007).
Forests on low and slightly convex hilltops are more frequently affected by high-severity fires given the better drainage on these sites (Cyr et al., Reference Cyr, Gauthier and Bergeron2007). Our data suggest that the organic layer accumulation at the high end of the three transects (L.0 m, V.0 m, and G.0 m) has constantly been setback by frequent fire events. In the better drained sites (L.0 m, V.0 m, and G.0 m), peat accumulation and lateral peatland expansion has likely been impeded by recurrent fires as suggested by Schaffhauser et al. (Reference Schaffhauser, Payette, Garneau and Robert2016). We can argue that most of the favorable edaphic and topographic sites of the boreal zone have already been more or less paludified and that repeated fires have been a spatial limitation to paludification in the better drained sites (Cyr et al., Reference Cyr, Gauthier and Bergeron2007; Schaffhauser et al., Reference Schaffhauser, Payette, Garneau and Robert2016).
Change in forest vegetation caused by paludification expansion within landscapes
Paludification during the Holocene caused the decrease of some early successional (jack pine) and late-successional (balsam fir) species induced by the expansion of peatland ecosystems into moderately well-drained forests (Ali et al., Reference Ali, Asselin, Larouche, Bergeron, Carcaillet and Richard2008; Payette et al., Reference Payette, Garneau, Delwaide and Schaffhauser2012). The acceleration of peatland expansion and decrease in fire activity from the mid-Holocene onward (Ali et al., Reference Ali, Blarquez, Girardin, Hély, Tinquaut, El Guellab and Valsecchi2012; Blarquez et al., Reference Blarquez, Ali, Girardin, Grondin, Fréchette, Bergeron and Hély2015) disadvantaged jack pine germination and caused a contraction of its northern limit in northeastern Canada (Payette et al., Reference Payette, Garneau, Delwaide and Schaffhauser2012, Reference Payette, Delwaide, Couillard and Pilon2017). This process was also observed at Villebois peatland where jack pine was present before forest paludification occurred around 2500 cal yr BP (Table 4), whereas this species is currently absent from this site and is uncommon on the Cochrane till today (Fréchette et al., in Reference Fréchette, Richard, Grondin, Lavoie and Larouchepress). The decline of balsam fir at the landscape scale (Jules et al., Reference Jules, Asselin, Bergeron and Ali2018) can be explained by the important organic layer accumulation (Gauthier et al., 2000) and the competitive advantage of black spruce (Messaoud et al., Reference Messaoud, Asselin, Bergeron and Grondin2014). As balsam fir is associated with mesic areas, our results have shown that paludification of L.150 m site has led to its migration 50 m higher in the toposequence where soil conditions are drier (Fig. 2). At the landscape scale, balsam fir is now rare in the Cochrane till region and is mainly restricted to shores of lakes and rivers (Jules et al., Reference Jules, Asselin, Bergeron and Ali2018). Because of the vast expanses now covered by peatlands, these two species are restricted spatially suggesting that the paludification process played an important role in the forest composition within the black spruce–moss domain (Fréchette et al., in Reference Fréchette, Richard, Grondin, Lavoie and Larouchepress).
CONCLUSION
In this study, we have documented the spatial and temporal dynamics of paludification within different geomorphological contexts (topography and mineral substrate) to improve knowledge of the autogenic and allogenic factors controlling this process of peatland initiation throughout the Holocene. The original approach using paleoecological reconstructions along topographic sequences shows that peatlands within the three sites initiated through primary paludification in topographic depressions directly after land emergence from initially rich minerotrophic environments. Higher on the toposequences, peatlands formed through secondary paludification in former black spruce forests. Forest paludification was promoted by low-severity fire events and lateral expansion of neighboring peatlands during the cooler late Holocene climatic period. Fires that reduced or eliminated entirely the surface organic layer allowed Sphagnum establishment in microdepressions, thus triggering secondary paludification. The weakly paludified sites are better drained uplands where the organic layers were periodically removed by fires, which restarted forest succession on mineral soil, hence delaying the lateral peatland expansion. The development of large expanses of peatlands in flat and undulated regions through paludification has had major impacts on the Holocene dynamics of boreal ecosystems. This study brings new knowledge that can help support ecosystem-based management in boreal black spruce forests considering that each paludification process requires particular silvicultural treatments (Simard et al., Reference Simard, Bernier, Bergeron, Paré and Guérine2009). It is important to distinguish peatlands that are permanent features in the landscape where paludification is irreversible from other peatlands formed later during the Holocene within forests, where paludification can be reversed. The extent of these two types of paludification is difficult to determine in analyzing the present-day vegetation. We thus show that the paleoecological approach is essential to understand the spatial and temporal dynamics of paludification and its impacts on the vegetation dynamics.
ACKNOWLEDGMENTS
Funding for this project was provided by Mitacs acceleration funding obtained by Professors Yves Bergeron (Université du Québec en Abitibi-Témiscamingue/Université du Québec à Montréal [UQAM]) and Michelle Garneau (UQAM); Ministère des Forêts, de la Faune et des Parcs (MFFP) of Québec; forest industry sector (Barette-Chapais Ltée and Groupe Remabec); and Study Abroad Scholarships (UQAM). We thank Adam Ali and Sarah Ivorra from Institut des Sciences de l’Évolution de Montpellier (University de Montpellier, France) for anthracological analysis training, Veronique Poirier (MFFP) for her help on toposequence design, and Hans Asnong (UQAM) for assistance on granulometric instruments. We thank Danielle Charron from the Centre d’Étude de la Forêt and Pierre Clouâtre (MFFP-Baie Comeau) for their logistic support during the fieldwork. Thanks to Jacques Parent and Jean-Alain Lemieux for providing accommodation at lac Gaillard (North Shore). The paper benefited from discussions with Louis-Martin Pilote, Simon van Bellen, Marc-André Bourgault, Joannie Beaulne, and Steve Pratte. We would like to thank Dale H. Vitt and an anonymous reviewer for providing constructive comments that improved the manuscript.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/qua.2018.101