Introduction
Ticks and associated tick-borne parasitic diseases are a major constraint to the health and productivity of livestock and indirectly affecting the livelihood of their owners particularly in developing countries of the world (Dahiya et al., Reference Dahiya, Salar, Mandal, Kumar, Tripathi, Pal and Kumar2018). Equine piroplasmosis (EP) is an important tick-borne (haemoprotozoan) disease of equids (including horses, donkeys and zebras) caused by Babesia caballi and Theileria equi. This disease is widespread in most subtropical and tropical countries of the world where competent tick vectors are present. Ixodid ticks belonging to the genera Rhipicephalus, Amblyomma, Dermacentor, Hyalomma and Haemaphysalis are known vectors for these pathogens (Scoles and Ueti, Reference Scoles and Ueti2015). However, the above-mentioned pathogens can also be transmitted via blood transfusion, contaminated syringes and clinical instruments (Short et al., Reference Short, Clark, Harvey, Wenzlow, Hawkins, Allred, Knowles, Corn, Grause, Hennager and Kitchen2012), although the latter is of minor epidemiological significance (Onyiche et al., Reference Onyiche, Suganuma, Igarashi, Yokoyama, Xuan and Thekisoe2019).
EP is associated with morbidity and mortality in acute cases with impact on the equine industry. Clinical signs of EP in acute form include fever, inappetence, pale mucous membrane, malaise and increased heart and respiratory rates, while in chronic form of infection, the clinical signs include transient fever, mild anaemia, enlarged spleen, weight loss and reduced work efficiency (Zobba et al., Reference Zobba, Ardu, Niccolini, Chessa, Manna, Cocco and Parpaglia2008). Theileria equi infection is responsible for severe clinical disease compared with B. caballi. Nevertheless, clinical signs and disease severity vary considerably between regions (Wise et al., Reference Wise, Kappmeyer, Mealey and Knowles2013). Recovered equines with T. equi infection remain long life carriers, whereas B. caballi infection is self-limiting and infected animals clear the infection within 4 years (Rothschild, Reference Rothschild2013).
EP has been categorized by the OIE as a notifiable disease due to the socioeconomic impact of the disease as it leads to restrictions in the international movement of horses (OIE, 2014). This has led many countries which are EP free to put in place stringent measures to prevent introduction into their territories. Knowledge of the presence of a disease agent and the epidemiological factors that are responsible for transmission or perpetuation within a particular geographical space is vital in the design of effective control measures. Epidemiological factors/variables associated with the occurrence of EP can vary across different geographical areas, altitude, management system and areas of origin of the equids (Santos et al., Reference Santos, Roier, Santos, Pires, Vilela, Moraes, Almeida, Baldani, Machado and Massard2011).
Nigeria, a country in West Africa, has huge livestock population, especially in its northern region due to abundance of savannah type vegetation and absence of tsetse flies. Horses are kept in small flocks ranging from 5 to 30 in numbers and are used for polo sport and during traditional ceremonies. On the other hand, donkeys are kept in small flocks ranging from 2 to 5 in numbers and are used as working animals in the transportation of goods as they are relatively cheaper (Hassan et al., Reference Hassan, Steenstra and Udo2013). However, limited information is available on the status of EP in equids in Nigeria. Only recently, the first molecular evidence of equine piroplasms was reported in Nigeria equids (Idoko et al., Reference Idoko, Tirosh-Levy, Leszkowicz Mazuz, Mohammed, Sikiti Garba, Wesley and Steinman2020). Prior to this recent report, all the studies conducted so far on the prevalence of EP in Nigeria have been largely based on microscopic examination of blood smears and serological tests (Sanusi et al., Reference Sanusi, Ahmed, Tahir, Mai, Kalla and Shuaibu2014; Turaki et al., Reference Turaki, Kumsha, Biu and Bokko2014; Oladipo et al., Reference Oladipo, Adekunle, Okuneye, Adebayo, Adeoye and Banjoko2015; Mshelia et al., Reference Mshelia, Sambo, Adamu, Edeh and Onoja2016).
Serological tests have been widely adopted in recent times in epidemiological surveys of apicomplexan protozoan parasites. However, cross-reactivity of closely related species has been widely reported due to lesser specificity (Mans et al., Reference Mans, Pienaar and Latif2015). Therefore, further optimization for precision is required. On the other hand, molecular and bioinformatics techniques including gene sequencing have improved our knowledge and understanding of the genetic relatedness between different organisms (Chan and Ragan, Reference Chan and Ragan2013). Therefore, the aim of this study was to (i) investigate the prevalence of B. caballi and T. equi in equines (horses and donkeys) in North-Western Nigeria using a combination of microscopy and molecular techniques; (ii) to identify the possible risk factors associated with the occurrence of these pathogens in horses and donkeys; (iii) to determine the tick species which are potentially involved in the transmission of these piroplasms to equids in the study areas by detecting B. caballi and T. equi DNA in the ticks.
Materials and methods
Study area
The North-West region is a semi-arid zone and is the largest region in Nigeria with a human population of 35 786 944 (NPC, 2006). The current estimates put the population heads of horses and donkeys in the country as 206 212 and 936 832, respectively (FAO, 2016). This region has a savannah type of vegetation. The temperature ranges from 18 to 45°C with a mean of 27°C. The raining season usually begins in May through to October with a mean annual rainfall of 508–1016 mm and dry season is from October to April. Samples (blood and ticks) from horses were collected in four states (Kano, Kaduna, Jigawa and Katsina) while donkeys were sampled in three states (Kano, Jigawa and Katsina). Sampling points are presented on the map of Nigeria shown as Fig. 1.

Fig. 1. Map of Nigeria showing the study areas where samples were collected from horses and donkeys. (Map was created using ArcGIS version 10.6 by ESRI, Redlands, CA, USA.)
Study design and sampling locations
In this study, a cross-sectional study design was used. Non-probability sampling, combining both convenient and snowball sampling techniques, was employed. Samples were collected from May to September 2017. The population of equines (horses and donkeys) is not evenly distributed within these study areas, and therefore, representative samples were collected in these areas. A total of 468 samples were collected from 281 horses (Kano = 113, Jigawa = 67, Katsina = 54 and Kaduna = 47) and 187 donkeys (Kano = 57; Jigawa = 70 and Katsina = 60). Within each study area, samples were collected from different sampling points. We visited stables and individual owned horses for sampling after obtaining owner's consent. Samples were also collected from working donkeys at known points where their owners congregate with their animals and clients come around to hire them for their services.
Blood and tick sample collection
About 5 mL of whole blood was collected from the jugular vein of horses and donkeys after which the collected whole blood was transferred into labelled EDTA coated tubes. All animals were apparently healthy during sampling. All collected blood samples were transported to the laboratory on ice pack within 4 h after collection. In the laboratory, 125 μL of blood was dispensed on the marked spot within the Classic FTA card (Whatman® GE Healthcare, UK). All cards were labelled, air dried and stored at room temperature for further analysis.
The skin on both sides in horses and donkeys covering known predilection sites for ticks (the perineum region, abdomen, thigh, ear, and neck) was carefully examined for the presence of ticks. Ticks were collected using forceps into labelled tubes plugged with cotton wool for each animal. The labelled tubes contained information of the identity of the animals including their location.
Epidemiological questionnaire
To assess the possible risk factors associated with exposure to tick-borne apicomplexan parasites (B. caballi and T. equi) in equids, a closed-ended, semi-structured questionnaire was used to collect information of each horse and donkey on individual and ecological factors that might influence positivity to EP. Some of the factors investigated by the questionnaire were organized as follows: satisfactory husbandry practices which include (i) appropriate husbandry practices for the horses, (ii) farm with adequate infrastructure and (iii) presence of sanitary control and constant veterinary assistance; unsatisfactory husbandry practices include (i) farm with inadequate infrastructure and (ii) farm lacking systematic sanitary control, and when veterinary assistance was present, it was sporadic or used only in cases of emergency; presence or absence of ticks on animals; horse bred in close contact with other mammals (if cattle and horse shared the same pasture or were located close in proximity) or without contact with cattle; breeding systems: intensive (confined system: animals had no access to outside areas), semi-confined/extensive system (limited access or total access to pasture areas) or extensive (complete access to the environment) (Santos et al., Reference Santos, Roier, Santos, Pires, Vilela, Moraes, Almeida, Baldani, Machado and Massard2011); activities of the horses (sport exhibition; sports and ceremony only, ceremony and recreation only, work/walk and reproduction); acaricide used (carbamate, cypermethrin, diazinon, abomec, kerosine); frequency of acaricide use (monthly, quarterly and occasionally); gender (male or female); age (<6; 6–12; over 12 years); breed definition and location. Due to the differences in the management systems between horses and donkeys in the study areas, all the variables listed above were collected for horses, while for the donkeys, we collected age, gender, breed, location, presence of ticks and altitude only. Data were collected using Open Data Kit (ODK), a digital application system for Android devices. We uploaded the questionnaire on the online platform (https://odk.ona.io/). Subsequently, we downloaded the offline version on a mobile phone and available offline for usage without mobile network. The captured data were first locally stored on the mobile phone. The collected data were later transferred to the cloud at the end of the day's using Internet service for safe retrieval. On completion of the study, the data were downloaded from the server as a Microsoft Excel worksheet file.
Microscopy for the detection of piroplasms
Thin blood smear was prepared using a drop of blood on a grease-free glass slide. After air drying and fixing with absolute methanol, glass slides were stained with 10% Giemsa stain in the laboratory. All slides were observed using immersion oil at 100 × objective for the presence of B. caballi and T. equi using a light microscope (Olympus®, Japan) (Bose et al., Reference Böse, Jorgensen, Dalgliesh, Friedhoff and De Vos1995).
Morphological identification of ticks
All tick samples collected from infested animals were identified to species level based on standard keys using a stereomicroscope (Olympus®) (Walker et al., Reference Walker, Bouattour, Camicas, Estrada-Pena, Horak, Latif, Pegram and Preston2003; Apanaskevich and Horak, Reference Apanaskevich and Horak2005). Species identification and gender of the ticks were recorded. All tick specimens were preserved in 70% ethanol and kept at 4°C.
DNA extraction from blood dried on FTA cards
Genomic DNA was extracted from Whatman FTATM cards using 5% Chelex 100 resin following the manufacturer's instructions. Briefly, about 6 mm punch of the dried blood spot was removed from the card. The disc was macerated using 20 G needle in a 2 mL microcentrifuge tube. Thereafter, 1000 μL of double distilled water (DDW) was added and incubated at room temperature for 10 min. After incubation, the supernatant was removed, and the step was repeated. The tubes were centrifuged for 3 min at 20 000 g. The supernatant was removed after which 200 μL of the freshly prepared 5% Chelex was added using a large-bore pipette tip. The samples were incubated at 56°C for 20 min after which they were briefly vortexed for 15 s and further incubated for 8 min at 100°C. Samples were again centrifuged for 3 min at 20 000 g. The supernatants containing DNA were transferred to a new 1.5 mL microcentrifuge tubes and stored at −20°C until used for downstream analysis.
Washing and homogenization of ticks
Prior to washing and homogenization, ticks were pooled. Pooling was carried out for ticks of the same species and on the same animal with a maximum of five ticks per pool. Ticks were washed twice using DDW after the removal of ethanol in microcentrifuge tubes as described by Silaghi et al. (Reference Silaghi, Hamel, Thiel, Pfister and Pfeiffer2011). A sterile scissor was used to cut the ticks into small pieces and 200 μL of sterile PBS was added. Ticks were homogenated using Tissue Lyser LT (Qiagen, Hilden, Germany) for 10 min at an oscillation frequency of 30 Hz. The supernatant was removed after centrifugation at 3000 g for 3 min.
Extraction of genomic DNA from tick homogenate
A maximum of 80 μL of the tick homogenate (supernatant) was used for DNA extraction. DNA was extracted using QIAamp DNA mini Kit (Qiagen) following the manufacturer's instruction.
Amplification of B. caballi and T. equi DNA in tick and blood
Species-specific polymerase chain reaction (PCR) was carried out for detecting B. caballi and T. equi DNA in both blood and ticks from equines. For T. equi, the reaction was carried out using primer pairs Bec-UF2 (forward): 5′-TCGAAGACGATCAGATACCGTCG-3′ and Equi-R (reverse): 5′-TGCCTTAAACTTCCTTGCGAT-3′ (Alhassan et al., Reference Alhassan, Pumidonming, Okamura, Hirata, Battsetseg, Fujisaki, Yokoyama and Igarashi2005, fragment size: 392 bp), while for B. caballi, the primer pairs used are Bec-UF2 (forward): 5′-TCGAAGACGATCAGATACCGTCG-3′ and Cab-R (reverse): 5′-CTCGTTCATGATTTAGAATTGCT-3′ (Alhassan et al., Reference Alhassan, Pumidonming, Okamura, Hirata, Battsetseg, Fujisaki, Yokoyama and Igarashi2005, fragment size: 540 bp). The reactions were performed in a total volume of 25 μL consisting of 12.5 μL of Amplitaq Gold® 360 master mix, 1 μL of each primer (10 μ m each of both forward and reverse primer), 5.5 μL Nuclease-Free Water and 5 μL template DNA using a proFlex thermocycler (Applied Biosystem, California, USA, Supplied by Thermo Fisher Scientific) with the following cycling conditions: initial denaturation at 95°C for 2 min, followed by 35 cycles of 95°C for 30 s, 60°C for 30 s and 72°C for 60 s and a final extension at 72°C for 7 min. Every reaction set had a positive and negative control (molecular grade water). DNA samples prepared from in vitro cultures of B. caballi and T. equi were used as positive controls.
Gel electrophoresis and sequencing
All PCR products were separated on a 1% agarose gel stained with ethidium bromide and visualized under UV trans-illuminator (Gene Genius Imaging System, Syngene, UK). Amplicons were sent for sequencing in the forward direction [Inqaba Biotechnical (Pty) Ltd., Pretoria, South Africa]. Nucleotide searches were carried out to identify highly similar sequences on the NCBI database using BLAST(n).
Statistical analysis
Data obtained from the prevalence study from horses and donkeys were analysed using SPSS version 20.0. We used the PCR results and a linear model to identify variables associated with B caballi and T. equi infections. Univariable regression model was built to estimate the risk factors associated with positivity to T. equi and B. cabalii in both horses and donkeys to a variety of independent variables. The output from the univariable analysis was used in the multivariable model with P value set at <0.25. The multivariable model was constructed only for infection with T. equi and not for B. caballi due to few numbers of positives. Stepwise approach was used in the building of the multivariable model creating a main effects model with a significance level set at α = 0.10. Changes in the coefficient (cofounding) were examined in the model for the remaining variables that were significant after expulsion of the cofounding variable. The fitness of the model was assessed following completion of the multivariable model using the Hosmer–Lemeshow test (Bursac et al., Reference Bursac, Gauss, Williams and Hosmer2008). Confidence intervals (CI: lower and upper) at 95% were calculated for infection rates with piroplasms in ticks. The minimum infection rate (MIR) was accepted and expressed in simple percentages (only one sample was considered as positive, in a pool of adult tick). Statistical significance of differential infection rates in the ticks was analysed using GraphPad Prism version 5.0. Significant level (P < 0.05) was used throughout the analysis.
Results
Microscopic detection of equine piroplasms in blood
Microscopic examination of Giemsa stained blood films from 468 equid samples revealed the presence of B. caballi and T. equi. In total, 43 equids were positive with an overall prevalence of 9.2%. The prevalence was higher in the donkeys (25/187; 13.4%) as compared to horses (18/281; 6.4%). Based on parasite species and host, the prevalence of T. equi was higher in both horses and donkeys (4.9 and 11.8%) compared with B. caballi (1.4 and 1.6%), respectively.
Molecular detection of T. equi in the blood of equines
Theileria equi infections were demonstrated in horses and donkeys by amplifying 392 bp fragment of 18S rRNA. The overall prevalence for T. equi in horses in all sampled areas of Nigeria was 39.9% (112/281) while that in the donkeys was 41.2% (77/187). BLASTn query of the sequences obtained from selected PCR amplicons showed 96.9–99.8% homology with T. equi 18S rRNA sequence from horses in Israel (GenBank accession no: MK392054.1 and MK392059.1). The T. equi sequences generated in the present study have been registered with the NCBI GenBank with the following accession numbers: MN620483, MN620483, MN620484, MN620485, MN620486 and MN620487.
Risk factors analysis in relation to T. equi
Horses
A total of 281 horses were tested of which 112 were positive with an overall prevalence of 39.9% (Table 1). The multivariable logistic regression analysis showed statistically significant (P < 0.05) influence on risk factors such as location, breed, activities of horses, husbandry practices, contact with other mammals and management system on the prevalence of T. equi in horses (Table 2). Based on location, horses in Jigawa state were 3.89 times (OR = 3.890, 95% CI 1.849–8.219, P = 0.001) more likely to be infected while those in Kaduna state are 0.234 less prone to infection (OR = 0.234, 95% CI 0.108–0.506) (Table 2). The odds of a positive result were almost 4-fold (OR = 3.872, 95% CI 1.752–8.558) greater in horses involved in reproduction and work (Table 2). Concerning management system, horses on extensive system were found to be more positive to infection compared to those on intensive management (51.4 vs 29.6%) in the univariable analysis (Table 1). The multivariable analysis also confirmed that horses on intensive management were 2.5-fold less prone to infection (OR = 2.513, 95% CI 1.201–5.261, P = 0.019) (Table 2). According to breed, Talon are 0.307 times less prone to infection as compared to other breeds comprising of Argentine and Thoroughbred. Furthermore, horses with satisfactory husbandry practices were 0.489 times less prone (OR = 0.489, 95% CI 0.301–0.796, P = 0.005) to get infection. Additionally, horses with no contact with other mammals are 0.278 times less prone to infection (OR = 0.278, 95% CI 0.119–0.649, P = 0.004). Other risk factors which were not statistically significant in the multivariable regression analysis include gender, age and frequency of acaricide use (Table 2).
Table 1. Results of the univariable analysis associated with Theileria equi and Babesia caballi PCR positivity in horses in North-Western, Nigeria

Table 2. Multivariable logistic analysis of risk factors associated with Theileria equi PCR positivity in horses in North-Western Nigeria

a Reference category.
Donkeys
A total of 187 horses were tested of which 77 were positive with an overall prevalence of 41.2% (Table 3). The multivariable logistic regression analysis showed statistically significant (P < 0.05) influence on risk factors such as location and breed on the prevalence of T. equi in donkeys (Table 3). Based on location, horses in Jigawa state were 3.20 times (OR = 3.200, 95% CI 1.525–6.716, P = 0.002) more likely to be infected (Table 4). The odds of positivity were 11-fold greater in Jangora breed (OR = 11.330, 95% CI 1.779–72.200, P = 0.010). Other risk factors which were not statistically significant in the multivariable risk factors are age, presence of ticks and altitude.
Table 3. Results of the univariable analysis associated with Theileria equi and Babesia caballi PCR positivity in donkeys in North-Western, Nigeria

Table 4. Multivariable logistic analysis of risk factors associated with Theileria equi in donkeys in North-Western Nigeria
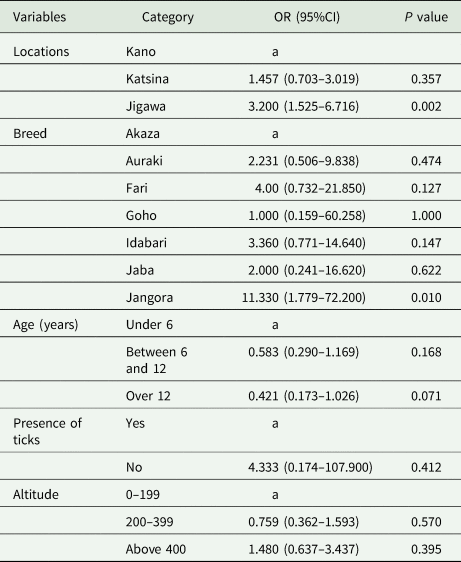
a Reference category.
Molecular detection of B. caballi in the blood of equines
Babesia caballi-infected animals were detected by amplifying a 450 bp fragment of rhoptry associated protein-1 (rap-1) gene from DNA samples. The overall prevalence of B. caballi was 1.4% (4/287) in the horses and 1.6% (3/187) in the donkeys. The sequences obtained from the positive PCR amplicons showed 99.0–100.0% homology with B. caballi sequences from a horse in Israel (GenBank accession no: MK346860.1). The B. caballi sequences generated in the present study have been registered with the GenBank with the following accession numbers: MT126819, MT126820, MT126821 and MT126822.
Risk factors analysis in relation to B. caballi
Horses
Due to low infection rate, the multivariable regression analysis was not carried out. In the univariable analysis, risk factors such as location and acaricide used were statistically significant (P < 0.001). Based on location, B. caballi was detected only in Kano with a prevalence of 3.5% (4/113) while no positive sample was found from other sampled locations (Kaduna, Katsina and Jigawa) (Table 1). Horses in which Kerosene was used as possible acaricide had higher prevalence (33.3%) to B. caballi compared with those placed on other types of acaricide such as carbamate, cypermethrin and diazinon (Table 1).
Donkeys
The multivariable regression analysis was not carried out for donkeys as well due to very low number of positive animals for the different variables. No significant difference (P > 0.05) was observed across all the variables in the risk factors in the univariable analysis (Table 3). Based on location, the prevalence was highest in Kano with 8.8% compared with 1.7% in Katsina. All 70 donkeys screened in Jigawa state tested negative (Table 3). The Goho breed had the highest prevalence of 8.3% compared with 2.9% recorded for the Idabari. The B. caballi prevalence in donkeys increased with altitude, and the highest prevalence was found in altitude above 400 m above sea levels (Table 3).
Molecular detection of equine piroplasms in ticks
A total of 538 ticks infesting equines were collected and morphologically identified to species level. Of these ticks, 535 were from 105 horses and three were from a donkey. Altogether, six species were found including Rhipicephalus e. evertsi, Hyalomma dromedarii, H. rufipes, H. truncatum, R. decolaratus and R. sanguineus (Table 5). These ticks were pooled based on species and host and a total of 201 pools were screened for the detection of B. caballi and T. equi.
Table 5. Minimum infection rate for Babesia caballi and Theileria equi in tick pools from North-Western Nigeria

n = Total number of ticks in the entire pool.
Both B. caballi and T. equi DNA was successfully detected in tick pools using species-specific PCR primers. For T. equi, of the 201 tick pools tested, the overall MIR was 57.2% (108/201) (Table 5). Based on location, Kano state had the highest MIR of 64.3% followed by Katsina (MIR 59.9%), Kaduna (MIR 48.1%) and lastly Jigawa (MIR 38.7%). No significant difference (P > 0.05) was observed across study locations (Table 5). Theileria equi DNA was detected in three tick species namely R. e. evertsi, H. dromedarii and H. truncatum. Out of 176 tick pools of R. e. evertsi, 102 were positive with MIR of 61.6%. Only one out of two tick pools of H. truncatum was positive with MIR of 50.0% and lastly H. dromedarii had MIR of 23.7% (Table 5). BLASTn query of 392 bp sequences obtained in the present study had 96.9–99.8% identity scores with sequences on the NCBI database (Genbank accession no: MK346860.1 and MG052902.1).
For B. caballi, 201 tick pools were tested with an overall MIR of 6.9% (Table 5). Only R. e. evertsi tick pools were positive for the presence of B. caballi DNA with MIR of 7.6% (12/176) (Table 5). Based on location, the highest infection rate was observed in Kano state with eight positive pools comprising of 26 ticks with MIR of 10.7%. This was followed by Kaduna state (MIR 9.1%) and lastly Katsina state with MIR of 2.8% (Table 5). No significant difference (P > 0.05) was observed across the four study locations (Table 5). BLASTn query of the sequences obtained from the PCR amplicons confirmed the identity of the pathogen as they shared 100% identity score with a previously registered B. caballi gene sequence (Genbank accession no: MK392060.1). Co-detection of B. caballi and T. equi was observed only in R. e. evertsi ticks from Kano and Katsina states with MIR of 1.4% (Table 5).
Discussion
We used a large sample size (n = 468) and molecular tools to carry out a robust epidemiological assessment on the prevalence and distribution of the causative agents of EP in horses and donkeys in North-Western Nigeria. EP has been reported previously in other parts of Africa including South Africa (Gummow et al., Reference Gummow, De Wet and De Waal1996; Bhoora et al., Reference Bhoora, Collins, Schnittger, Troskie, Marumo, Labuschagne, Smith, Dalton and Mbizeni2020), Sudan (Salim et al., Reference Salim, Hassan, Bakheit, Alhassan, Igarashi, Karanis and Abdelrahman2008), Egypt (Mahmoud et al., Reference Mahmoud, El-Ezz, Abdel-Shafy, Nassar, El Namaky, Khalil, Knowles, Kappmeyer, Silva and Suarez2016), Kenya (Oduori et al., Reference Oduori, Onyango, Kimari and MacLeod2015) and Nigeria (Turaki et al., Reference Turaki, Kumsha, Biu and Bokko2014).
General prevalence in horses
In the horses, the overall prevalence of equine piroplasms was 41.3% using molecular methods and 6.4% using microscopy. The high prevalence in horses due to T. equi reported in this study is comparable to the prevalence of 39–42% reported in Romania (Gallusova et al., Reference Gallusová, Qablan, D’Amico, Oborník, Petrželková, Mihalca and Modrý2014), Mongolia (Boldbaatar et al., Reference Boldbaatar, Xuan, Battsetseg, Igarashi, Battur, Batsukh and Fujisaki2005) and Italy (Del Pino et al., Reference Del Pino, Roberto, Vincenzo, Francesca, Antonella, Luca, Francesco and Teresa2016). Previous studies conducted in Africa have reported higher prevalence above 50.0% (Motloang et al., Reference Motloang, Thekisoe, Alhassan, Bakheit, Motheo, Masangane and Mbati2008; Salim et al., Reference Salim, Hassan, Bakheit, Alhassan, Igarashi, Karanis and Abdelrahman2008). In Nigeria, the prevalence of equine piroplasms obtained in this study was higher than that reported recently by Idoko et al. (2020). The differences could be due to the variation in the sample sizes and study locations. Nevertheless, our result is comparable to the prevalence of 39.2% reported by Turaki et al. (Reference Turaki, Kumsha, Biu and Bokko2014) using Giemsa-stained blood smears in North-Eastern Nigeria comprising of Borno, Gombe and Taraba states.
For B. caballi infection, the prevalence was low at 1.4% and comparable to that obtained in other regions of Africa (Ros-Garcia et al., Reference Ros-García, M'ghirbi, Hurtado and Bouattour2013; Salim et al., Reference Salim, Bakheit, Kamau and Sugimoto2013). Absence of B. caballi infection in horses was reported recently in Nigeria (Idoko et al., Reference Idoko, Tirosh-Levy, Leszkowicz Mazuz, Mohammed, Sikiti Garba, Wesley and Steinman2020). The use of small sample size in the latter study might be responsible for their observation. Despite the large sample size used in our study, we detected only seven positive samples. Generally, numerous studies have reported higher prevalence of T. equi compared to B. caballi in epidemiological studies conducted in several parts of the world including Africa (Gummow et al., Reference Gummow, De Wet and De Waal1996; Motloang et al., Reference Motloang, Thekisoe, Alhassan, Bakheit, Motheo, Masangane and Mbati2008; Kouam et al., Reference Kouam, Kantzoura, Gajadhar, Theis, Papadopoulos and Theodoropoulos2010). Babesia caballi-infected animals usually clear the infection after 1–4 years in the absence of re-infection while T. equi-infected animals remain lifelong carriers (Brüning, Reference Brüning1996; Ruegg et al., Reference Rüegg, Torgerson, Deplazes and Mathis2007). These animals serve as a source of infection to ticks which subsequently transmit the infection to naïve and susceptible uninfected animals. Another possible reason for the differences in the prevalence between the two pathogens could be due to the differences in vector distribution (Salim et al., Reference Salim, Hassan, Bakheit, Alhassan, Igarashi, Karanis and Abdelrahman2008). Mixed infection of the two piroplasms has been reported in different studies and is unconnected with the presence of the tick vectors responsible for the transmission of both pathogens within the same geographical area.
Diagnosis of EP can be achieved by either the use of direct or indirect methods (Abedi et al., Reference Abedi, Razmi, Seifi and Naghibi2015). Recently, combination of both methods is increasingly been adopted. The gold standard for piroplasm's diagnosis is microscopy but poor sensitivity during low parasitaemia limits its use (Böse et al., Reference Böse, Jorgensen, Dalgliesh, Friedhoff and De Vos1995). PCR is a more sensitive test for the diagnosis of infection with piroplasms. Therefore, PCR was able to detect more infections compared with microscopy in our study. In other studies, piroplasms were not detected in blood smears but the use of PCR and serology on same samples proved otherwise (Abutarbush et al., Reference Abutarbush, Alqawasmeh, Mukbel and Al-Majali2012; Munkhjargal et al., Reference Munkhjargal, Sivakumar, Battsetseg, Nyamjargal, Aboulaila, Purevtseren, Bayarsaikhan, Byambaa, Terkawi, Yokoyama and Igarashi2013). Generally, differences in prevalence to EP could also be due to differences in diagnostic methods employed in the surveys (Onyiche et al., Reference Onyiche, Suganuma, Igarashi, Yokoyama, Xuan and Thekisoe2019).
We observed that the prevalence was higher in the donkeys compared with the horses. This agrees with previous reports from other studies (Garcia-Bocanegra et al., Reference García-Bocanegra, Arenas-Montes, Hernández, Adaszek, Carbonero, Almería, Jaén-Téllez, Gutiérrez-Palomino and Arenas2013; Qablan et al., Reference Qablan, Obornik, Petrželková, Sloboda, Shudiefat, Hořín, Lukeš and Modrý2013). This observation could be attributed to the differences in the management systems in which these two species of equids are kept and the possible role of donkeys as reservoirs.
General prevalence in donkeys
The overall prevalence of EP in donkeys in Nigeria in this study was 42.8% using PCR and 13.4% using microscopy. The high prevalence of 41.2% in donkeys due to T. equi reported in this study is comparable to that reported in Italy (Garcia-Bocanegra et al., Reference García-Bocanegra, Arenas-Montes, Hernández, Adaszek, Carbonero, Almería, Jaén-Téllez, Gutiérrez-Palomino and Arenas2013), Spain (Piantedosi et al., Reference Piantedosi, D'Alessio, Di Loria, Di Prisco, Mariani, Neola, Santoro, Montagnaro, Capelli and Veneziano2014) and recently in Nigeria (Idoko et al., Reference Idoko, Tirosh-Levy, Leszkowicz Mazuz, Mohammed, Sikiti Garba, Wesley and Steinman2020). Within Africa, higher prevalences above 50% have been reported by other authors (Gizachew et al., Reference Gizachew, Schuster, Joseph, Wernery, Georgy, Elizabeth and Wernery2013; Hawkins et al., Reference Hawkins, Kock, McKeever, Gakuya, Musyoki, Chege, Mutinda, Kariuki, Davidson, Low and Skilton2015; Oduori et al., Reference Oduori, Onyango, Kimari and MacLeod2015). For B. caballi infection, the prevalence of 1.6% was low. Other studies from Spain, Kenya and Iran reported absence of infection with B. caballi (Garcia-Bocanegra et al., Reference García-Bocanegra, Arenas-Montes, Hernández, Adaszek, Carbonero, Almería, Jaén-Téllez, Gutiérrez-Palomino and Arenas2013; Abedi et al., Reference Abedi, Razmi, Seifi and Naghibi2015; Oduori et al., Reference Oduori, Onyango, Kimari and MacLeod2015). Mixed infections of both piroplasms as detected by PCR were low with a prevalence of 1.6%. Low rate of mixed infection in this study is due to low B. caballi infection rate and all B. caballi-infected animals were also infected with T. equi. Low prevalence of mixed infection has also been observed in similar studies in Nigeria (Sanusi et al., Reference Sanusi, Ahmed, Tahir, Mai, Kalla and Shuaibu2014), Brazil (Heim et al., Reference Heim, Passos, Ribeiro, Costa-Júnior, Bastos, Cabral, Hirzmann and Pfister2007) and Greece (Kouam et al., Reference Kouam, Kantzoura, Gajadhar, Theis, Papadopoulos and Theodoropoulos2010).
Risk factors associated with positivity to equine piroplasms
Horses
In our study, we observed statistically significant differences associated with positive detection of T. equi between study locations. Corroborating our findings, location has been considered as a risk factor to EP previously in different epidemiological studies (Kouam et al., Reference Kouam, Kantzoura, Gajadhar, Theis, Papadopoulos and Theodoropoulos2010; Cortés et al., Reference Cortés, Fernández-García and Martínez-Estéllez2017). Differences in prevalence between locations have been observed in other countries and might be related to several factors including the presence of tick vectors, climatic conditions, host and effectiveness of any control measures (Guidi et al., Reference Guidi, Pradier, Lebert and Leblond2015; Heim et al., Reference Heim, Passos, Ribeiro, Costa-Júnior, Bastos, Cabral, Hirzmann and Pfister2007; Kouam et al., Reference Kouam, Kantzoura, Gajadhar, Theis, Papadopoulos and Theodoropoulos2010). The climatic conditions within our study areas are similar and characterized by high temperature and humidity prior to the raining season (May–October) and a dry/cold season (December–March). Therefore, it is possible that differences in sample size across the four study areas could be responsible for this observation.
The management system in which the horses are raised was shown to be significantly associated with positivity. It is well known that animals kept on extensive or semi-intensive system are at the greatest risk to ticks and tick-borne infection. In the course of grazing, they get in contact with other animals in the pasture as well as questing ticks. This puts the animals at risk to likely exposure to piroplasms compared with those exclusively raised on intensive management system. Furthermore, horses raised under unsatisfactory technical and sanitary management with inadequate housing/infrastructure, veterinary care, grooming and poor sanitation are more prone to infection with EP. Vaccination, fly control and deworming are pointers of decent herd management with resultant low exposure to ticks (Santos et al., Reference Santos, Roier, Santos, Pires, Vilela, Moraes, Almeida, Baldani, Machado and Massard2011). According to Guidi et al. (Reference Guidi, Pradier, Lebert and Leblond2015), they observed in southern France that horses regularly dewormed, with fly control regime present and good husbandry were protected against EP. Additionally, it was also observed in India that irregular grooming or non-grooming practices in horses expose them to high tick infestation and hence the greater the likelihood of infection (Sumbria et al., Reference Sumbria, Singla, Kumar, Sharma, Dahiya and Setia2016).
The activity in which the horses are engaged in plays a significant role in the positivity to T. equi (Kouam et al., Reference Kouam, Kantzoura, Gajadhar, Theis, Papadopoulos and Theodoropoulos2010). In our study, we observed that horses used for reproduction and work showed the highest risk of infection to EP. This is because most of these working horses are kept under poor condition and are exposed to tick vectors thereby increasing their risk of infection (Shkap et al., Reference Shkap, Cohen, Leibovitz, Savitsky, Avni, Giger, Kappmayer and Knowles1998).
Furthermore, contact of horses with other mammals was significantly associated with positivity to EP. Tick vectors are limited in their dispersal, and their abundance depends on the presence of appropriate host (Guidi et al., Reference Guidi, Pradier, Lebert and Leblond2015). The close proximity either through working or living of horses together with cattle has been reported to promulgate the spread of EP (Heuchert et al., Reference Heuchert, de Giulli, De Athaide, Böse and Friedhoff1999; Guidi et al., Reference Guidi, Pradier, Lebert and Leblond2015). Concerning breed, we observed that Talon, a non-native breed, was less prone to EP. This is at variance with a similar study in Bauchi state, Nigeria, where the Arewa breed (native/indigenous horse breed in Nigeria) had the lowest prevalence of EP compared with other non-native breeds (Sanusi et al., Reference Sanusi, Ahmed, Tahir, Mai, Kalla and Shuaibu2014). The reasons for the difference in our findings remain unknown but may be related to genetic predisposition. Therefore, further studies on the genetic predisposition of breeds to EP may be necessary in the future. Other risk factors not statistically significant in the multivariable model were age and gender. Gender has been considered to be protective to EP with male horses less likely to be infected compared to females (Guidi et al., Reference Guidi, Pradier, Lebert and Leblond2015). Similarly, the prevalence was lower in males but higher in the females. This observation corroborates the findings of others as reported in Trinidad, Turkey, Nigeria and France (Asgarali et al., Reference Asgarali, Coombs, Mohammed, Campbell and Caesar2007; Karatepe et al., Reference Karatepe, Karatepe, Çakmak, Karaer and Ergün2009; Sanusi et al., Reference Sanusi, Ahmed, Tahir, Mai, Kalla and Shuaibu2014; Guidi et al., Reference Guidi, Pradier, Lebert and Leblond2015). Hormonal differences between genders have been postulated to be responsible for this observation (Roberts et al., Reference Roberts, Walker and Alexander2001). Furthermore, Alexander and Stimson (Reference Alexander and Stimson1988) were of the opinion that females are more susceptible to protozoan parasites when compared with males. Majority of the horses we sampled were adults above 6 years of age, hence we could not find any association with age. Other studies found no association between infection occurrence and age (Abutarbush et al., Reference Abutarbush, Alqawasmeh, Mukbel and Al-Majali2012; Sanusi et al., Reference Sanusi, Ahmed, Tahir, Mai, Kalla and Shuaibu2014).
Donkeys
In the donkeys, positivity to T. equi infection differed significantly with regards to location and breed. Since all the sampling locations lied within the same agro-ecological zone, it is very difficult to ascertain the exact reason for this observation. Animals infected with T. equi are known carriers of the pathogen for life (Brüning, Reference Brüning1996; Ruegg et al., Reference Rüegg, Torgerson, Deplazes and Mathis2007). Furthermore, breed variation in prevalence was observed with the Jangora breed of donkey having the highest prevalence. Genetic predisposition could probably play a role in this observation.
Piroplasms in ticks
Generally, limited epidemiological surveys have been carried out regarding the detection of piroplasms in feeding ticks. Feeding ticks were collected from surveyed animals. Therefore, the detected parasites might have been ingested during blood meal. Thus, the detection of parasites does not identify these ticks as vectors. It is also important to note that all ticks collected from this study were adult ticks.
Rhipicephalus e. evertsi is a known vector responsible for transstadial transmission of B. caballi (De Waal and Potgieter, Reference De Waal and Potgieter1987). DNA of B. caballi was detected only in R. e. evertsi collected from Kano, Kaduna, Katsina and Jigawa. Furthermore, T. equi DNA was detected in R. e. evertsi, H. dromedarii and H. truncatum collected from all study locations. Generally, both H. dromearii and R. e. evertsi are regarded as vectors of T. equi (De Waal and Potgieter, Reference De Waal and Potgieter1987; Ueti and Knowles, Reference Ueti, Knowles, Florin-Christensen and Schnittger2018). The H. dromedarii in horses is regarded as an incidental host especially in areas where camels and cattle are present (Walker et al., Reference Walker, Bouattour, Camicas, Estrada-Pena, Horak, Latif, Pegram and Preston2003). Coincidentally, all the sampling locations in our study are livestock production areas of Nigeria with substantial population of cattle and camels. In a previous study, T. equi DNA was amplified in R. e. evertsi ticks collected from domestic and wild animals in South Africa (Berggoetz et al., Reference Berggoetz, Schmid, Ston, Wyss, Chevillon, Pretorius and Gern2014). We did not amplify any of the piroplasms in R. (B.) decolaratus, but in South Africa, DNA of B. caballi was amplified in R. (B.) decolaratus and this vector has been speculated to be a vector of EP (Berggoetz et al., Reference Berggoetz, Schmid, Ston, Wyss, Chevillon, Pretorius and Gern2014).
Conclusion
We observed a high prevalence of T. equi and low prevalence with B. caballi in both horses and donkeys. The overall prevalence of equine piroplasms was higher in the donkeys compared with horses. Risk factors associated with positivity to T. equi in horses include location, breed, unsatisfactory husbandry practices, contact with other mammal's management system and activities of the horses. Breeds and locations were risk factors to the occurrence of T. equi in the donkeys. Additionally, we report for the first time in Nigeria, the molecular evidence of B. caballi and T. equi in ticks from Nigeria. All positive samples of B. caballi were observed in R. e. evertsi only while that of T. equi was observed in R. e. evertsi, H. dromedarii and H. truncatum. We recommend that further studies be undertaken to ascertain the genetic diversity and haplotypes of the circulating piroplasms within the study areas as well as the probable role of donkey as a reservoir.
Acknowledgements
We thank animal owners for their cooperation. The council of the University of Maiduguri, Nigeria is hereby acknowledged for granting the first author study leave to undertake this study. We thank the assistance of Adamu Aliyu and Gerald Chibuzo Agu for their help during the sample collection. Special thanks to Andries Phukuntsi, Theresa Okute, Evaristus Nwaro, Lehlohonolo Mofokeng and Armstrong imeh Ekong for their useful suggestions during the course of the work. We would like to thank Cornelia Silaghi, Ana Vasic and Cristian Raileanu for their helpful suggestions on the manuscript. Special thanks to the anonymous reviewers who greatly improved the manuscript.
Financial support
The first author was financially supported by the North West University (NWU) Post graduate student bursary. The Unit for Environmental Sciences and Management (NWU) also financially supported the first author scientific travel to Japan through an international travel award. The study was largely made possible by the National Research Foundation (NRF) of South Africa's Incentive Funding for Rated Researchers (GUN94187 and GUN118949) made available to OT. The Grant holder acknowledges that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF-supported research are that of the authors, and that the NRF accepts no liability whatsoever in this regard.
Conflict of interest
No conflict of interest exists among the authors.
Ethical standard
Permission to carry out the study was approved by the faculty ethics committee of the North-West University South Africa with ethics number NWU-01242-19-A9 in line with the guidelines of the committee.









