Introduction
Umbilicaria subpolyphylla Oxner was described from the Donetsk Region in southern Ukraine. As its name suggests, the species is closely related to U. polyphylla (L.) Baumg. and is distinguished by a thick, uneven and wrinkled thallus, light greyish to brownish in colour, sometimes covered with pruina (Oxner Reference Oxner1968) which contrasts with the thin, smooth and even, brown to black-brown thallus of U. polyphylla. For a long time the only known locality of this species was from granite outcrops in the nature reserve “Kamyani Mohyly” within the Steppe zone of Ukraine. The taxonomic rank of U. subpolyphylla has remained uncertain, as it could be considered an ecological variation or subspecies of Umbilicaria polyphylla.
Umbilicaria iberica Sancho & Krzewicka was described recently from Spain (Krzewicka et al. Reference Krzewicka, García, Johansen, Sancho and Martín2009) and subsequently recorded in France (Masson Reference Masson2010). The original description of U. iberica was based on morphological, anatomical and molecular phylogenetic data. The authors provided detailed information on how the new species was distinguishable from U. polyphylla but they were apparently unaware of the species U. subpolyphylla. According to the description and photograph provided by Krzewicka et al. (Reference Krzewicka, García, Johansen, Sancho and Martín2009), both U. iberica and U. subpolyphylla may be distinguished from U. polyphylla by the same features: a monophyllous thallus and dull, weakly wrinkled grey-brown to dark brown upper surface with an elevated, areolate and pruinose centre. Krzewicka et al. (Reference Krzewicka, García, Johansen, Sancho and Martín2009) mentioned additional diagnostic characters for U. iberica which were not emphasized by Oxner (Reference Oxner1968), viz. a medulla of the ‘U. havaasii’ type (non ‘U. deusta’ type) and actinodisc (non gyrodisc) apothecia.
We hypothesized that Umbilicaria subpolyphylla and U. iberica were conspecific. The main goal of the study was, therefore, to review the Umbilicaria subpolyphylla species concept and its geographical distribution based on phenotypic observations as well as phylogenetic analysis of nrITS and mtLSU data from specimens with a wide geographical range.
Materials and Methods
Sampling
The core material for this study was collected by the authors and deposited in herbaria ALTB and KW. Additionally, specimens were studied from the herbaria CHR, H, GZU, KRAM, KW, LE, M and OSC, including the type specimens of Umbilicaria subpolyphylla and U. iberica.
For phylogenetic analyses, the holotype of Umbilicaria iberica was included. Because the holotype of U. subpolyphylla was too old for sequencing, samples were collected in locus classicus. In addition, two populations of U. subpolyphylla were sampled from the Crimean Peninsula and two from the East Pyrenees. One specimen of Umbilicaria cf. subpolyphylla from New Zealand (CHR) was also included. Sequences of U. polyphylla from Europe, Asia, North America and New Zealand were obtained and supplemented with sequences from GenBank (Table 1). We failed to obtain a PCR product from the specimens of U. polyphylla from Chile.
Table 1. Sample information and corresponding GenBank Accession numbers for species of Umbilicaria used in the phylogenetic analyses in this study. GenBank Accession numbers of new sequences are in bold. Numbers after species names refer to the samples used in the concatenated nrITS+mtLSU dataset.

Morphology and anatomy
Morphological observations were made using a dissecting microscope. Cross-sections were cut by hand with a razor blade and observed in water mounts. Methods outlined by Valladares & Sancho (Reference Valladares and Sancho1995) were used to examine medullary structure and to name the different medullary types found during the study. Measurements are presented as follows: (smallest value recorded–) (x − SD) − x − (x. + SD) (–largest value recorded), where x is the (arithmetic) sample mean, and SD the sample standard deviation. The two extreme values are given to the nearest 0·5 μm and the sample mean to the nearest 0·1 μm.
Chemical analyses
Secondary products were analyzed by applying standard thin-layer chromatography (TLC) techniques (Culberson & Kristinsson Reference Culberson and Kristinsson1970) using solvents A, B and C.
For the high performance liquid chromatography (HPLC) analysis, air-dried lichen material was placed into a 1·5 ml vial and extracted with reagent grade methanol (at proportions of 10 mg per 1 ml respectively) for 3–5 days in darkness at room temperature, filtered through a syringe PTFE membrane filter of 13 mm diam. and porosity 0·45 μm, and then stored at −20 °C. The Agilent 1100 HPLC system was equipped with a gradient quaternary pump, vacuum degasser, autosampler, column thermostat and diode-array UV-VIS detector. Extracts were separated using the column ZORBAX Eclipse Plus C18, narrow bore RR 2·1 × 150 mm, 3·5 μm, thermostated at 30 °С in 2-eluent gradient mode: eluent A, water with 0·5% orthophosphoric acid H3PO4; eluent B, methanol with 0·5% H3PO4 (all solvents and reagents HPLC gradient grade). Flow rate was 1 ml min−1 and the elution mode was programmed with the following events, assuming linear change between them: 0 min – 0% B in A, 2 min – 0% B in A, 10 min – 30% B in A, 30 min – 50% B in A, 45 min – 100% B in A, 30 min – 100% B in A. Then the column was equilibrated with the initial eluent for 20 min before the next analysis run. Injection volume was usually 5 μl but could be varied between 1–10 μl depending on the lichen substance content which is known to range between 0·1–5% of air-dried thalli. Signal detection was registered at 224, 240, 254, 270 and 320 nm. Peak identification was performed on both retention times of standard lichen substances and UV spectral data. In such conditions, for each specimen the content of each lichen substance was evaluated with a 10-point scale based on peak heights at 224 nm and 1000 mAU detector signal range (most sensitive and not specific) as follows: heights of components in the range 500–1000 mAV were considered as major (5–10 points), 300–500 mAV as medium (3–5 points), 30–300 mAV as minor (1–3 points) and less than 30 mAV as traces (1 point). The most abundant components with a peak height much exceeding 1000 mAV were sometimes assigned 11 points.
DNA extraction, amplification and sequencing
Single thallus parts (100–200 mg) were carefully checked for fungal infections and were thoroughly cleaned of extraneous matter. Total genomic DNA was extracted by grinding lichen thalli in liquid nitrogen in a porcelain mortar according to the CTAB protocol of Cubero et al. (Reference Cubero, Crespo, Fatehi and Bridge1999) with minor modifications or with the DiamondDNA Plant kit (ABT Llc, Russia) following the manufacturer's protocol. Primers and cycling conditions for amplification of all genes are listed in Table 2. Sequences were determined on an ABI Prism® 3700 DNA Analyzer (Applied Biosystems) or a CEQ™ 8800 Genetic Analysis System (Beckman Coulter). The program Geneious 6.0 (Biomatters Ltd., New Zealand) was used for assembling partial and complementary sequences. Consensus sequences were exclusively compiled from double-stranded parts of the sequences.
Table 2. Summary statistics, PCR settings and substitution models used for the different datasets in the phylogenetic analyses of species of Umbilicaria.

Sequence alignment and phylogenetic analyses
All obtained sequences of the Umbilicaria polyphylla aggregate were supplemented with sequences obtained during a comprehensive study of Umbilicariaceae phylogeny (Davydov et al. Reference Davydov, Peršoh and Rambold2017), representing different subgenera with an emphasis on Umbilicaria subg. Umbilicaria; U. pulvinaria (Savicz) Frey was used as an outgroup. GenBank Accession numbers are provided in Table 1, with those of new sequences in bold. The sequences were aligned in Geneious 6.0 (Biomatters Ltd., New Zealand) using the MUSCLE algorithm (Edgar Reference Edgar2004) and visible deviations in position homology were then manually optimized.
Two single gene datasets were assembled for this study: the internal transcribed spacer regions of nuclear ribosomal DNA (ITS) and the large subunit of the mitochondrial ribosomal DNA (mtLSU). Nucleotide diversity was calculated for each dataset using DNASP v5 (Librado & Rozas Reference Librado and Rozas2009). The most likely tree and 1000 rapid bootstrap replicates were calculated using RAxML 8.0.26 (Stamatakis Reference Stamatakis2014) implemented in raxmlGUI software v1.3.1 (Silvestro & Michalak Reference Silvestro and Michalak2012). The optimal substitution model (Table 2) was inferred using PartitionFinder v1.1.1 (Lanfear et al. Reference Lanfear, Calcott, Ho and Guindon2012), initially assuming three independent subsets of the ITS dataset (i.e. ITS1, 5.8S and ITS2). To select models of nucleotide substitution in the mtLSU dataset, we used jModelTest 2.0 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012) using the Akaike Information Criterion for model selection. We used a partitioned analysis in which each locus was defined as a separate partition, the parameters of which were allowed to vary independently under the GTRGAMMA model of evolution as implemented in RAxML.
Bayesian inference with the Markov chain Monte Carlo (BMCMC) method (Larget & Shimon 1999) was performed using MrBayes 3.2.3 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012). We applied the same partition scheme as used for RAxML with the obtained substitution models (Table 2), a variable rate prior and an unconstrained exponential branch-length prior with a mean of 0·13. The mean of the branch-length prior was calculated based on ML tree reconstructions using the procedure described by Ekman & Blaalid (Reference Ekman and Blaalid2011). Three parallel analyses, each with six incrementally heated chains using the default heating factor of 0·2, were run for 40 million generations and every 200th generation was sampled until the average standard deviation (ASD) of split frequencies had dropped to 0·001. Initially we set ASD at 0·01 but the calculation stopped after c. 0·5–0·8 million generations; ASD of 0·005 resulted in c. 3 million generations, therefore the number of sampled trees after burn-in was not enough to calculate the relevant consensus tree. The first 50% of trees was discarded as burn-in and a 50% majority-rule consensus tree was calculated from the remaining trees of the three runs with the sumt command implemented in MrBayes 3.2.3.
For combining the ITS and mtLSU datasets, both alignments were trimmed to include only those specimens for which we had information on both markers. We tested trimmed ITS and mtLSU datasets for topological incongruence by studying single gene maximum likelihood consensus trees (not shown) from separate RAxML analyses. There were no well-supported (PP ≥ 0·7) incongruences therefore we concatenated the datasets into a combined dataset. As both phylograms were similar regarding well-supported clades and lacking conflicts, all sequences were combined into one matrix consisting of 1246 sites, 238 of which were variable and used for RAxML and Bayesian analyses. The optimal substitution model was inferred initially assuming four independent subsets, ITS1, 5.8S, ITS2 and mtLSU, using PartitionFinder.
Phylogenetic trees were visualized in FigTree v1.4.1 (http://tree.bio.ed.ac.uk/software/figtree/). Microsoft PowerPoint® was used for artwork.
Results
The phylogenetic study
For the phylogenetic analyses, we used new ITS nrDNA sequences from fresh material and those retrieved from GenBank representing a wide geographical range. To test the monophyly of species, mtLSU was used in addition to ITS, both as a single gene matrix and in a combined dataset. Summary statistics are provided in Table 2. The mtLSU region is more conserved in comparison to ITS but is still useful for studying variability at the species level of lichenized ascomycetes (Printzen Reference Printzen2002). The ITS phylogram (Fig. 1) contained four well-supported lineages for Umbilicaria polyphylla and two for U. subpolyphylla, but the backbone was unsupported. The phylogram based on the more conservative mtLSU marker segregated identical sequences of U. subpolyphylla and slightly variable U. polyphylla (Fig. 2). A concatenated ITS and mtLSU sequence dataset provided phylograms with high support for most of the clades (Fig. 3). The sequence of the holotype of Umbilicaria iberica, as well as sequences of U. cf. subpolyphylla from New Zealand, are clustered within sequences of U. subpolyphylla from the locus classicus in a well-supported clade (MrBayes 1.0 PP; RAxML 100% BS). Umbilicaria subpolyphylla s. lat. (including U. iberica) and U. polyphylla cluster as sister clades. Three constant residues in the ITS sequences and five residues in the mtLSU differentiate Umbilicaria polyphylla and U. subpolyphylla. The ITS + mtLSU phylogram reflects the topology of the ITS tree regarding smaller clades but supports monophyly of species, as in the phylogram based only on mtLSU with more limited sampling. All phylograms demonstrate the higher intraspecific genetic diversity of Umbilicaria polyphylla than U. subpolyphylla.

Fig. 1. Phylogenetic relationships amongst Umbilicaria species, based on a maximum likelihood analysis of ITS1-5.8S-ITS2. The topology was produced from the RAxML analysis. The reliability of each branch was tested by ML and Bayesian methods. Numbers at tree nodes indicate bootstrap values of ML (left) and BMCMC posterior probabilities (right). Thicker branches indicate when the BMCMC posterior probability is ≥ 0·95 or the bootstrap value of ML is ≥ 70%. GenBank Accession numbers and sample information are given in Table 1 and new sequences are marked in bold. The basal branches of Umbilicaria are shown on Fig. 3. Branch lengths represent the estimated number of substitutions per site assuming the respective models of substitution. Exceptions are the branches with a black dot, which were shortened to reduce the overall figure size. Abbreviation: l. c. = locus classicus.

Fig. 2. Phylogenetic relationships amongst Umbilicaria species, based on a maximum likelihood analysis of mitochondrial LSU. The topology was produced from the RAxML analysis. The reliability of each branch was tested by ML and Bayesian methods. Numbers at tree nodes indicate bootstrap values of ML (left) and BMCMC posterior probabilities (right). Thicker branches indicate when the BMCMC posterior probability is ≥ 0·95 or the bootstrap value of ML is ≥ 70%. GenBank Accession numbers and sample information are given in Table 1 and new sequences are marked in bold. The basal branches of Umbilicaria are shown on Fig. 3. Branch lengths represent the estimated number of substitutions per site assuming the respective models of substitution. Exceptions are the branches with a black dot, which were shortened to reduce the overall figure size. Abbreviation: l. c. = locus classicus.

Fig. 3. Phylogenetic relationships amongst Umbilicaria species, based on a maximum likelihood analysis of the concatenated nrITS+mtLSU dataset. The topology was produced from the RAxML analysis. The reliability of each branch was tested by ML and Bayesian methods. Numbers at tree nodes indicate bootstrap values of ML (left) and BMCMC posterior probabilities (right). Thicker branches indicate when the BMCMC posterior probability is ≥ 0·95 or the bootstrap value of ML is ≥ 70%. GenBank Accession numbers and sample information are given in Table 1. Branch lengths represent the estimated number of substitutions per site assuming the respective models of substitution.
Both the ITS and ITS + mtLSU phylograms show two well-supported lineages within the monophyletic Umbilicaria subpolyphylla. The first clade combines specimens from Spain and Ukraine, the second from the Crimean Peninsula, France and New Zealand.
Umbilicaria polyphylla sequences in the ITS phylogram (Fig. 1) clustered into four clades: the first combined specimens from Canada, Poland, Great Britain, Finland and New Zealand; the second from Siberia (Altai Mts); the third from Spain, Finland and the Crimean Peninsula; the fourth from Norway. Such grouping appears not to be correlated with geographical distance. Two sequences of Umbilicaria subpolyphylla from Crimea clustered in different clades (clades 1 and 2); similarly, sequences of U. polyphylla from lowland Finland did not cluster together (see clades 3 and 5). There are two clades with specimens from both hemispheres (clades 2 and 3), one in Umbilicaria polyphylla and one in U. subpolyphylla.
Morphology, anatomy and secondary chemistry
Original descriptions of Umbilicaria subpolyphylla and U. iberica (Oxner Reference Oxner1968; Krzewicka et al. Reference Krzewicka, García, Johansen, Sancho and Martín2009) were based on material from one locality each. Morphological circumscription and diagnostic characters were re-evaluated based on collections from a wider geographical range (Table 3).
Table 3. Principal morphological and anatomical differences between Umbilicaria polyphylla and U. subpolyphylla.

Marginal and partly central sections of the lower surface of Umbilicaria subpolyphylla lacking thalloconidia are smooth and brownish grey. Two morphotypes can be distinguished for U. polyphylla based on variations in the lower surface: one is similar to that mentioned above, with the marginal and/or central part lacking thalloconidia and lighter in colour, and the other with such parts being entirely black and areolate, similar to U. cinerascens (Arnold) Frey.
The holotype of Umbilicaria subpolyphylla (Fig. 4) includes c. 25 thalli and some thallus fragments. It is rather uniform in morphology and corresponds to the original description. Oxner (Reference Oxner1968) did not investigate the thalloconidia of U. subpolyphylla. According to our study of the holotype, thalloconidia are 2–4- to 6–12-celled, (11·5–)13·7–17·2–20·6(–31·0) × (8·5–)12·0–14·3–16·7(–24·5) μm in size (n = 50).
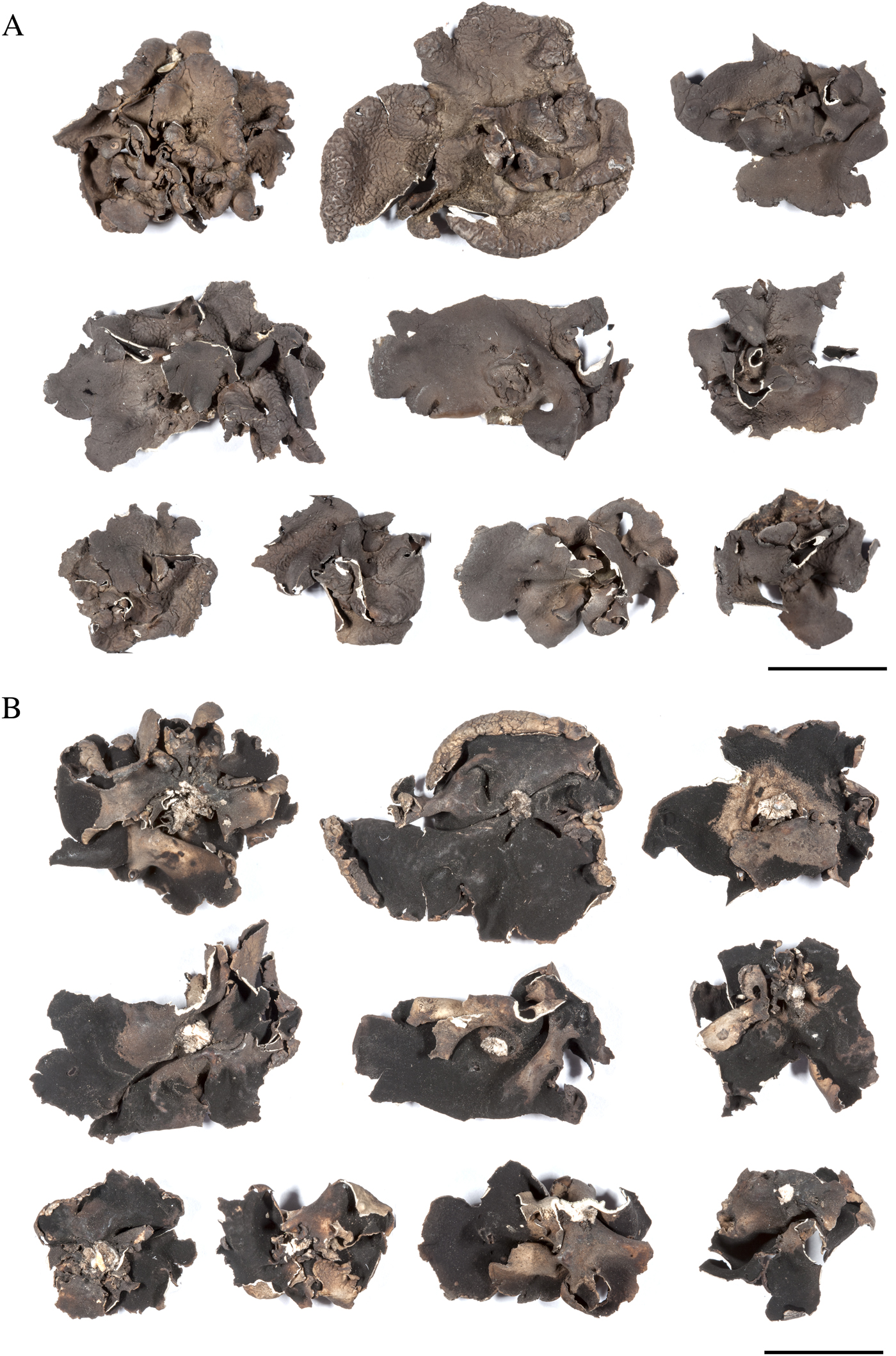
Fig. 4. Umbilicaria subpolyphylla Oxner. Fragments of the holotype (KW L21651). A, upper surface; B, lower surface. Scales = 1 cm. In colour online.
The medullary hyphae of both Umbilicaria subpolyphylla and U. polyphylla are radial and made up of long cells. The medulla of U. subpolyphylla is most similar to the ‘U. havaasii’ type (see Valladares & Sancho Reference Valladares and Sancho1995) with loose hyphae, especially in the upper part. The medulla of U. polyphylla belongs to the ‘U. deusta’ type (ibid.) with dense scleroplectenchyma (Fig. 5). The medulla of U. subpolyphylla is looser under the wrinkles of the upper surface and can be locally dense, but generally it is relatively thick and loose parts can be easily found.
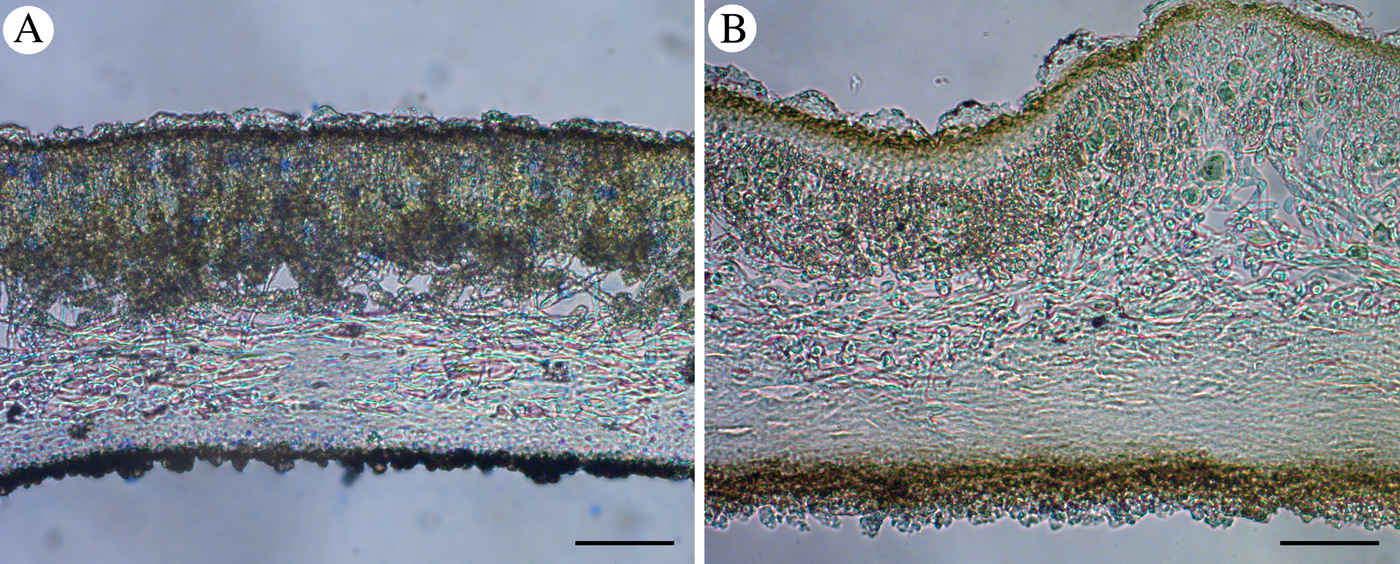
Fig. 5. Vertical sections of Umbilicaria polyphylla and U. subpolyphylla demonstrating the types of medullary structure. A, Umbilicaria polyphylla, medulla of ‘U. deusta’-type; B, U. subpolyphylla, medulla of ‘U. havaasii’-type. Scales = 50 μm. In colour online.
All investigated specimens of Umbilicaria subpolyphylla, except the holotype of U. iberica, lack apothecia. Apothecia of the type of Umbilicaria iberica have a gyrodisc and appear to be overmature.
All specimens examined were studied by TLC and showed the same pattern of spots corresponding to gyrophoric, umbilicaric and lecanoric acids. Selected specimens of Umbilicaria polyphylla and U. subpolyphylla were studied by HPLC. All the specimens have been shown to contain the following lichen substances: gyrophoric and umbilicaric acids as major, lecanoric acid, orsellinic acid and its methyl or ethyl ester as minor (methyl orsellinate or ethyl orsellinate). The latter are probably artefacts arising during extraction (Fig. 6A–C).

Fig. 6. HPLC profiles and UV spectra of thallus extracts of Umbilicaria spp. A, HPLC profile of U. polyphylla from Crimea (KW 74463); B, HPLC profile of U. subpolyphylla from locus classicus (ALTB-L187); C, HPLC profile of U. subpolyphylla (‘U. iberica’) from eastern Pyrenees (ALTB-L5962); D, UV spectra of thallus extracts; E, relative content of substances extracted from A = U. polyphylla (KW 74463), B = U. subpolyphylla (ALTB-L187) and C = U. subpolyphylla (‘U. iberica’) (ALTB-L5962). Abbreviations of substances: ORC = orsellinic acid, EORC = ethyl orsellinate, LEC = lecanoric acid, UMB = umbilicaric acid, GYR = gyrophoric acid.
Taxonomy
After consideration of anatomical, morphological, chemical and phylogenetic data there is sufficient evidence to synonymise Umbilicaria iberica with U. subpolyphylla.
Umbilicaria subpolyphylla Oxner
Fl. Lich. Ukraini 2(1): 497 (1968); type: RSS Ucraina, ditio Donetzkensis, distr. Wolodarsiensis. In reservato publico Kamjany Mohyly dicto, in saxis graniticus, 1954, A. Oxner (KW L21651— holotype!). Syn. nov. Umbilicaria iberica Sancho & Krzewicka, Lichenologist 41: 644 (2009); type: Spain, El Escorial near Madrid, on a hill above the town, on shaded rocks, 1070 m alt., 17 September 2006, B. Krzewicka 3292 (KRAM L50627—holotype!).
Additional specimens examined (see also specimens listed in Table 1): Bosnia and Herzegovina: Bosnia: Dinaric Alps, Vranica Mt., 43°57′27″N, 17°45′21″E, 1641 m, on rocks, 2017, E. Mašić & S. Barudanović (GZU 000337514).—Russia: Crimea: south seacoast, 10 vii 1910, G. K. Kreyer (LE L6631).
Selected specimens of Umbilicaria polyphylla examined (see also specimens listed in Table 1): USA: Oregon: Hood River County, north end of Parkdale Lava Flow, 45°31′13″N, 121°37′18″W, 554 m, mossy, rough basalt lava flow, 2017, B. McCune 31313 (OSC, ALTB L5655). Montana: Flathead County, Kelsi's Trail, above Middle Folk Flathead River, near Essex, 48°16′52″N, 113°36′59″W, 1300 m, on argillite, 2012, B. McCune 32391 (OSC, ALTB L198).—Chile: XII Region: Isla Grande de Tierra de Fuego, 54°40′32″S, 69°26′25″W, 0–5 m, sobre rocas 2009, S. Pérez-Ortega 1772 (hb. Pérez-Ortega).—Russia: Murmansk Region: Laplandsky Strict Reserve [67°48′N, 31°17′E], c. 140 m, on stones in pine forest, 1973, A. V. Dombrovskaya 81 (KPABG L6253). Republic of Komi: Pechoro-Ilychsky Strict Reserve, 62°51′45″N, 58°52′29″E 483 m, steep rocks, 2006, T. N. Pystina (SYKO, ALTB L6164). Republic of Bashkortostan: Yuzhno-Uralskiy Strict Reserve, 54°10′21″N, 57°41′11″E, 831 m, on quartzite, 2015, A. G. Paukov & L. V. Gagarina (UFU, ALTB L6104). Permsky Krai: Basegi Strict Reserve, 58°56′54″N, 58°29′18″E, 850 m, 1993, A. G. Bezgodov (ALTB L6186).
Discussion
Diagnostic traits
The close relationship between Umbilicaria polyphylla and U. subpolyphylla has already been shown in previous phylogenetic studies using ITS+nuLSU (Krzewicka et al. Reference Krzewicka, García, Johansen, Sancho and Martín2009) and ITS+mtLSU+RPB2 (Davydov et al. Reference Davydov, Peršoh and Rambold2017). Furthermore, both species are similar in morphology and produce identical secondary compounds.
The upper surface of Umbilicaria subpolyphylla is usually dull and in some places whitish, while in U. polyphylla the upper surface often looks glossy. This trait, however, should be used with care because the surface appearance of U. polyphylla can range from entirely glossy to entirely dull and pruinose, but the margins of young parts of the thalli at least remain glossy. Furthermore, specimens of both species could be lighter or darker brown, depending on light conditions. Generally, the upper surface of U. subpolyphylla is dull but young parts of thalli may also remain glossy.
The ‘U. havaasii’-type medulla for Umbilicaria subpolyphylla agrees with the observations of Krzewicka et al. (Reference Krzewicka, García, Johansen, Sancho and Martín2009) for U. iberica. Therefore, this anatomical trait is useful for the identification of the species, along with the morphology of the upper surface.
Many species of the Umbilicariaceae develop thalloconidia, which have been shown to be highly species-specific (Hasenhüttl & Poelt Reference Hasenhüttl and Poelt1978; Hestmark Reference Hestmark1990). Umbilicaria polyphylla, U. iberica and U. subpolyphylla, however, are not separable by thalloconidial characters. According to Hestmark (Reference Hestmark1990), thalloconidia of Umbilicaria polyphylla are usually 6–10-cellular, occasionally more or less. This corresponds with our observation of the type of Umbilicaria subpolyphylla, as well as with the description of U. iberica (Krzewicka et al. Reference Krzewicka, García, Johansen, Sancho and Martín2009). The mean size of thalloconidia of U. polyphylla (16·4 × 15·5 μm, according to Hestmark (Reference Hestmark1990)) and of the types of U. subpolyphylla (17·2 × 14·3 μm) and U. iberica (15·3 × 13·3 μm) are also in the same range. The distribution pattern of thalloconidia is also similar, covering the lower surface completely, often except in the centre of the thallus, or in black patches.
Krzewicka et al. (Reference Krzewicka, García, Johansen, Sancho and Martín2009) described apothecia of U. iberica as actinodisc. However, we consider them as overgrown gyrodisc. Frey (Reference Frey1936) and Henssen (Reference Henssen1970) mentioned that apothecial types might reflect only successional stages of apothecial ontogeny. Gyrodisc-omphalodisc apothecia may lose the disc margin with age; we often observe this in many species of Umbilicaria subg. Umbilicaria. True actinodisc apothecia grow radially from a very early stage of development and lack a disc margin (Henssen Reference Henssen1970).
Both Umbilicaria subpolyphylla and U. polyphylla are characterized by the same secondary chemistry and contain gyrophoric, umbilicaric and lecanoric acids. A compound originally identified from Umbilicaria polyphylla (Narui et al. Reference Narui, Culberson, Culberson, Johnson and Shibata1996, Reference Narui, Sawada, Takatsuki, Okuyama, Culberson, Culberson and Shibata1998) as umbilicaric acid, was present in even higher concentrations than gyrophoric acid (Fig. 6E). As expected, the UV spectrum of umbilicaric acid (Fig. 6D) showed a significant hypsochromic shift of two bands when compared with gyrophoric acid. It is possible that this was another tridepside such as the isomeric compounds hiascic acid (5-hydroxygyrophoric acid) or crustinic acid, a tridepside 5-hydroxylated at the 5″-position of the C-ring and having both para- and meta-depside linkages (Narui et al. Reference Narui, Culberson, Culberson, Johnson and Shibata1996). Both of these compounds have retention times lower than that of gyrophoric acid. However, the UV spectra of hiascic and crustinic acids differ significantly from that of gyrophoric acid, and these compounds have retention times lower than that of umbilicaric acid in HPLC (Seriña et al. Reference Seriña, Arroyo, Manrique and Sancho1996). Furthermore, Narui et al. (Reference Narui, Sawada, Takatsuki, Okuyama, Culberson, Culberson and Shibata1998) analysed extracts of Umbilicaria polyphylla using HPLC-MS; molecular mass determination, mass fragmentation patterns and NMR-spectral analysis confirmed the presence of umbilicaric acid in this species. Thus the second major substance detected in our HPLC analyses was certainly umbilicaric acid which, together with gyrophoric (major) acid and lecanoric (minor) acid, forms its characteristic chemosyndrome (defined as a biogenetically meaningful cohort of major and minor metabolites in a species (Elix et al. Reference Elix, Barbero, Giralt, Lumbsch and McCaffery1995)).
Thus, the most prominent difference between Umbilicaria subpolyphylla and U. polyphylla seems to be the former having mostly thick monophyllous thalli with a dull upper surface and an elevated, slightly wrinkled centre, often covered with white pruina. Such a wrinkled or reticulate ridged pattern at the thallus centre is common for Umbilicaria subgenus Umbilicaria but can be observed on rare occasions in some species for which it is not normally characteristic, such as U. hyperborea (Ach.) Hoffm. (Davydov et al. Reference Davydov, Peršoh and Rambold2017). It is also occasionally observed in Umbilicaria polyphylla but develops poorly and on only a small number of thalli in the population. An additional diagnostic character is the medulla of the ‘U. havaasii’ type. The colour of upper and lower surfaces, as well as thalloconidial and apothecial traits, seem not to be diagnostic for separating U. polyphylla and U. subpolyphylla.
Bipolar distribution pattern
The bipolar element represents a considerable fraction (more than one third) of Antarctic and sub-Antarctic lichen species, and bipolar species are also often present in high-altitude, high-latitude habitats in southern South America and Africa (Garrido-Benavent & Pérez-Ortega Reference Garrido-Benavent and Pérez-Ortega2017). Bipolar taxa represent a considerable proportion, c. 10%, of the New Zealand lichen flora and usually occur in alpine habitats of New Zealand and in boreal (high-altitude, high-latitude) localities in the Northern Hemisphere (Galloway Reference Galloway2007). Some species of Umbilicaria, such as Umbilicaria subglabra (Nyl.) Harm. and U. nylanderiana (Zahlbr.) H. Magn., belong to this group. Here we have provided phylogenetic evidence for the bipolar distribution of Umbilicaria subpolyphylla and U. polyphylla. Thus, Umbilicaria subpolyphylla is recorded here for the first time in the Southern Hemisphere whereas the closely related Umbilicaria polyphylla is known to occur in all continents (Llano Reference Llano1950; Wei & Jiang Reference Wei and Jiang1993; Øvstedal & Lewis Smith Reference Øvstedal and Lewis Smith2001; Galloway Reference Galloway2007; Davydov Reference Davydov, Andreev and Himelbrant2017) and, thus, also has a bipolar distribution.
So far the known distribution area of Umbilicaria subpolyphylla is restricted to Europe and New Zealand. Such a distribution pattern is difficult to explain and the real distribution is probably wider. The local geographical differentiation of species is more obvious in Europe, where Umbilicaria subpolyphylla predominates in Mediterranean and southern Europe (Spain, France, Bosnia, Ukraine, Crimean Peninsula), whereas U. polyphylla mostly occurs in mountains of the subarctic and temperate zones and similar habitats towards the south. However, in New Zealand both species grow in the same region, Otago in the temperate zone. The observed pattern could be an artifact of insufficient sampling and these hypotheses could be tested in future with more extensive sampling. Thus, while we cannot conclude from our data whether the speciation from a common ancestor was sympatric or allopatric, parallel separate speciation in the North and South Hemispheres seems implausible and contradicts our phylogenetic data. Sympatric speciation in one region followed by long-distance dispersal has been supported by molecular data for some species (Fernández-Mendoza & Printzen Reference Fernández-Mendoza and Printzen2013; Garrido-Benavent et al. Reference Garrido-Benavent, de los Ríos, Fernández-Mendoza and Pérez-Ortega2018) and might explain the distribution pattern of U. subpolyphylla and U. polyphylla.
We can only speculate about the mechanisms responsible for transtropical migration. Both species mostly produce thalloconidia and rarely ascospores. Thalloconidia are passively seceded from the lower side of the thallus; these appear to be locally effective in dispersing in rainwater running down the rock beneath the thalli. This vector enables species to be dispersed within the habitat (Hestmark Reference Hestmark1991). Ascospores are smaller and lighter, actively discharged from apothecia and should be more effective in long-distance dispersal by wind (ibid.). Species of Umbilicaria reproducing exclusively by small and wind-dispersed ascospores (e.g. Umbilicaria cylindrica (L.) Del., U. hyperborea (Ach.) Hoffm., U. proboscidea (L.) Schrad. and U. torrefacta (Lightf.) Schrad.) have been shown to be fast and successful colonizers of rocks (Hestmark et al. Reference Hestmark, Skogesal and Skullerud2004), faster than species producing thalloconidia (Hestmark Reference Hestmark1991). Indeed, these four species are common throughout the Northern Hemisphere at high latitudes and altitudes (boreal and alpine vegetation zones) and are the most common Umbilicaria species in the Arctic and subarctic and adjacent territories (Davydov & Zhurbenko Reference Davydov and Zhurbenko2008; Kristinsson et al. Reference Kristinsson, Zhurbenko and Hansen2010; Davydov et al. Reference Davydov, Himelbrant and Stepanchikova2011). These species, except Umbilicaria cylindrica, do not, however, occur in the Southern Hemisphere. This fact is consistent with the mostly latitudinal direction of wind in both hemispheres and suggests a mechanism other than wind for transtropical migration of lichens. Bipolar species of Umbilicaria reproducing mostly by thalloconidia (i.e. U. aprina Nyl., U. africana (Jatta) Krog & Swinscow, U. decussata (Vill.) Zahlbr., U. cinerascens, as well as U. polyphylla and U. subpolyphylla) have a wide disjunctive distribution area. Thus, thalloconidia rather than ascospores may be more effective in long-distance dispersal of Umbilicaria. Thick cell walls might make thalloconidia resistant to adverse environmental conditions for a longer time than thin-walled ascospores. We suggest that migratory birds might play an important role in the long-distance dispersal of thalloconidial species. We do not have evidence of long-distance transtropical migration of Umbilicaria subpolyphylla and U. polyphylla by stepping stones or direct through wind currents or migratory birds, but we cannot exclude long-distance dispersal by migratory birds. For example, at least 20 species of waders from the Asian Arctic or Siberia reached New Zealand as annual migrants (Williams et al. Reference Williams, Gummer, Powlesland, Robertson and Taylor2006). Direct study of dispersal vectors is needed to clarify this topic.
We thank Prof. B. McCune for his valuable comments and for improving the text, and an anonymous reviewer for helpful feedback on an earlier version of this work. We are grateful to Dr Christian Printzen (Frankfurt am Main) for the opportunity to sequence a part of the material in his laboratory, to Dr D. M. Masson (Bordeaux) for providing specimens from France and to the late Dr D. J. Galloway (Otago) for specimens from New Zealand. The curators of the herbaria cited above are thanked for the loan of herbarium specimens. We are also grateful to Prof. John Elix (Canberra) for his valuable advice and for editing the secondary metabolites section of our paper, and to Dr Olga Nadeina (Kassel) for her important comments on phylogenetic analyses. Dr Leena Myllys (H) is thanked for arranging the ITS sequencing of one specimen cited.











