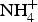Introduction
Barium-rich feldspars are rare in nature. Barium is typically a minor constituent of common silicate minerals, but it can be enriched significantly in some contexts, especially in potassic rock-forming minerals such as K-feldspar and muscovite (e.g. Chabu and Boulègue, Reference Chabu and Boulegue1992; Raith et al., Reference Raith, Devaraju and Spiering2014; Henry et al., Reference Henry, Will and Mueller2015). Ba-rich K-feldspars are typically associated with manganese–ferromanganese deposits (Viswanathan and Kielhorn, Reference Viswanathan and Kielhorn1983; McSwiggen et al., Reference McSwiggen, Morey and Cleland1994), stratabound Ba-rich sulfide deposits (Fortey and Beddoe–Stephens, Reference Fortey and Beddoestephens1982; Chabu and Boulègue, Reference Chabu and Boulegue1992), baryte deposits (Moro et al., Reference Moro, Cembranos and Fernandez2001; Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013), Ba-rich sedimentary or metasedimentary rocks (Reinecke, Reference Reinecke1982; Jakobsen et al., Reference Jakobsen1990; Deer et al., Reference Deer, Howie and Zussman2001; Essene et al., Reference Essene, Claflin, Giorgetti, Mata, Peacor, Arkai and Rathmell2005; Raith et al., Reference Raith, Devaraju and Spiering2014), and other Ba-rich rocks such as mafic xenoliths affected by Ba metasomatism (Henry et al., Reference Henry, Will and Mueller2015). The formation processes of Ba-rich K-feldspars commonly involve hydrothermal activity and low- to-medium-grade metamorphism.
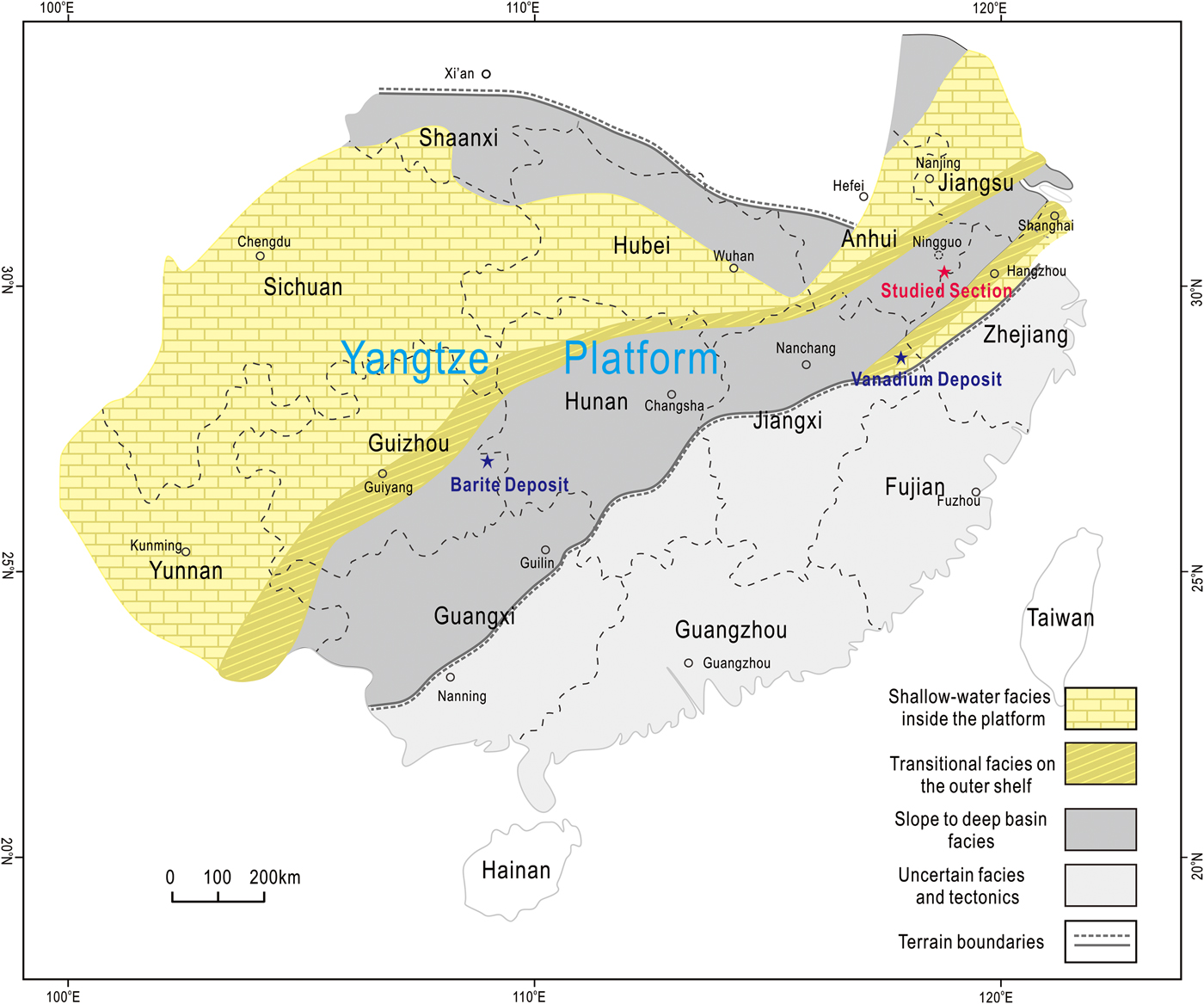
In the lower Cambrian of South China, Ba-rich K-feldspars have been reported from the baryte deposits of Guizhou Province and the vanadium deposits of Jiangxi Province (Fig. 1) (Long et al., Reference Long, Long, Zhong, Zhuang and Liu1994; Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013). In the baryte deposits, Ba-rich K-feldspars occur either as cores surrounded by pyrite grains or as euhedral crystals in contact with quartz or baryte (Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013). In the vanadium deposits, most of the Ba-rich K-feldspars occur as a major constituent of the host rocks, while some occur as clusters of lamellar crystals in amygdaloidal geodes (Long et al., Reference Long, Long, Zhong, Zhuang and Liu1994). The Ba-rich K-feldspars in the baryte and vanadium deposits were identified as hyalophane, and are regarded as important evidence for the hydrothermal origin of the deposits, yet their origin has not been adequately discussed (Long et al., Reference Long, Long, Zhong, Zhuang and Liu1994; Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013). Although Ba-rich feldspars in the vanadium deposits were identified as hyalophane by whole-rock X-ray diffraction, it is noteworthy that they have non-stoichiometric compositions characterized by abnormally high contents of SiO2, based on the limited compositional data provided in those earlier studies. However, because no further investigations were undertaken, the formation mechanisms of the Ba-rich K-feldspars in the vanadium deposits, as well as the cause of their non-stoichiometry, remains unclear.
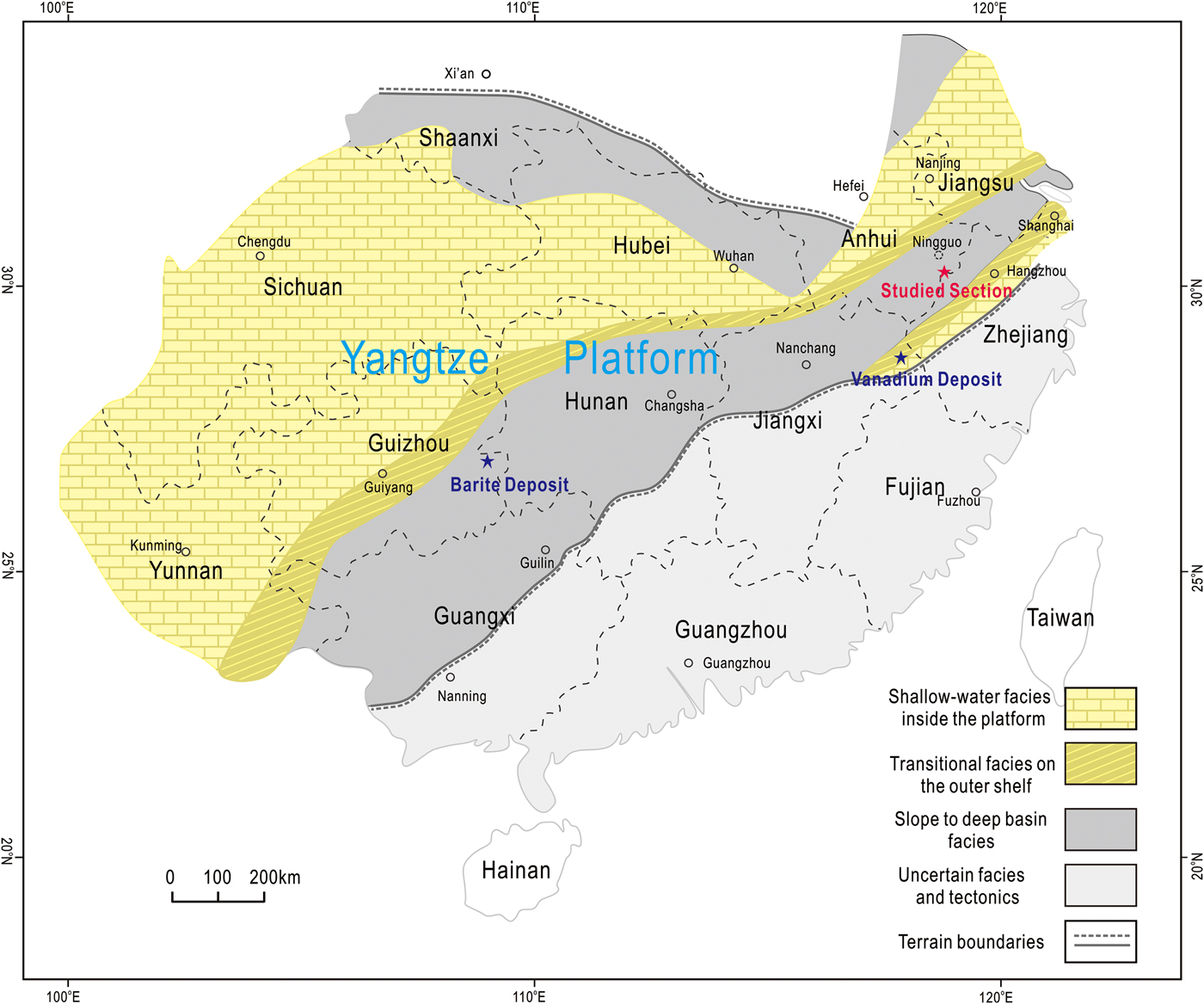
Fig. 1. Simplified palaeogeographic map of the Yangtze Platform in early Cambrian, modified after Jiang et al. (Reference Jiang, Yang, Ling, Chen, Feng, Zhao and Ni2007) and Chen et al. (Reference Chen, Wang, Qing, Yan and Li2009). The location of the section studied is labelled in red, while locations of the baryte and vanadium deposits are also shown in blue (Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013).
Recently, Ba-bearing silicates were found in drill cores of the lower Cambrian Hetang Formation in Anhui Province (Fig. 1). They were distributed across the whole formation, exhibiting complex occurrence patterns and unusual compositional features, thus providing an excellent opportunity for further investigations of the characteristics and formation processes of Ba-bearing silicates in early Cambrian rocks of South China. The compositions of the Ba-rich silicates were analysed by electron microproy (EMP), scanning electron microscope (SEM) and Raman and infrared (IR) spectroscopy. The results demonstrate that the Ba-bearing silicates include cymrite and Ba-bearing K-feldspars with non-stoichiometric compositions. In this paper, we systematically characterize the occurrences and compositions of these Ba-bearing silicates, discuss the causes of the non-stoichiometry in the Ba-bearing feldspars, and establish the possible genesis of all these Ba-bearing silicates.
Geological setting
During the Ediacaran–Cambrian transition, the Yangtze platform gradually evolved from a rift into a passive continental margin basin under the influence of an extensional tectonic setting (Wang and Li, Reference Wang and Li2003; Chen et al., Reference Chen, Wang, Qing, Yan and Li2009). The sedimentary sequences of the platform can be divided into three distinct facies: shallow water, transitional, and slope-to-deep basin (Fig. 1). Thick carbonate strata represent the shallow-water facies, whereas the slope-to-deep basin facies consists mainly of a series of interbedded black cherts and shales. The transitional facies consists of carbonates and black shales (Zhu, Reference Zhu2004; Jiang et al., Reference Jiang, Yang, Ling, Chen, Feng, Zhao and Ni2007).
The drillcore section of the Hetang Formation investigated in this study was recovered from Well Wanning 2 in Ningguo County, Anhui Province (Fig. 1). The sedimentary sequence is not metamorphosed, and comprises the Xijianshan, Hetang and Dachenling formations (Fig. 2). The top of the Xijianshan Formation is characterized by alternating dolostones and chert. The Xijianshan Formation is overlain by the Hetang Formation, and the boundary between the two can be identified by the disappearance of dolostone and the appearance of mudstone. The Hetang Formation is composed mainly of mudstone, including carbonaceous, siliceous, calcareous and dolomitic varieties. Thin layers of chert and limestone are also observed. The mudstones in the Hetang Formation are rich in organic matter with TOC contents of 2.10–27.13 wt.% (Chang et al., Reference Chang, Hu, Fu, Cao, Wang and Yao2016). The Hetang Formation is overlain by the Dachenling Formation, which consists mainly of limestone. Quartz and calcite veins are common in these strata, indicating strong fluid migration during diagenesis.
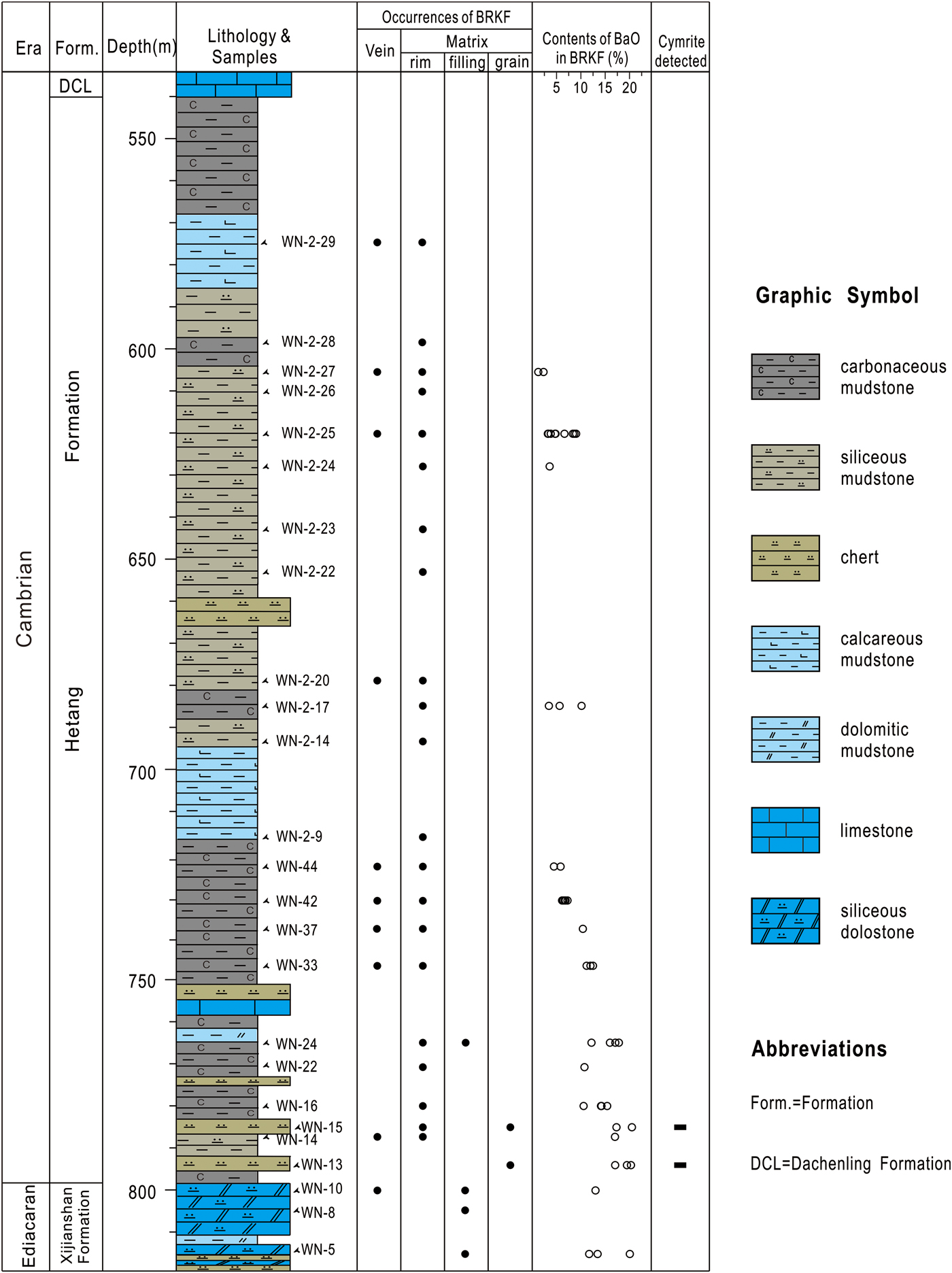
Fig. 2. Lithological column of the studied drillcore. The locations of representative samples, the occurrences of Ba-rich K-feldspar (BRKF), and the BaO contents of the Ba-rich K-feldspar are also shown.
Samples and methods
Subsequent to thin section observations and specimen identification, 51 samples were selected for EMP analysis. Twenty-five samples containing Ba-bearing silicates are marked on the lithological column in Fig. 2, and quantitative compositional analyses were carried out on the Ba-bearing silicates in 16 of these samples. The samples cover the entire Hetang Formation and the top of the Xijianshan Formation. Rock types include mudstone, chert and dolostone. The mudstone samples are from throughout the Hetang Formation, while the chert and dolostone samples were collected from the bottom of the Hetang Formation and the top of the Xijianshan Formation, respectively (Fig. 2). Some of the samples were also investigated by SEM for morphological observations of the Ba-silicates. Raman and IR spectra of the Ba-rich K-feldspars were also acquired.
Compositions and back-scattered electron images of the Ba-rich silicates were obtained on polished, carbon-coated thin sections using a JEOL JXA-8100 electron microprobe at the State Key Laboratory for Mineral Deposits Research at Nanjing University, Nanjing, China. The analytical conditions, using three wavelength-dispersive crystal spectrometers, were a 15 kV accelerating voltage, a 20 nA beam current, and an electron spot focused to 1–2 µm. The counting time was 10 s on peak positions, and two times 5 s on background positions. The analytical standards included the following well-characterized natural and synthetic minerals: orthoclase for K and Si, albite for Na and Al, plagioclase for Ca, barium fluoride for Ba, MnTiO3 for Mn, and hornblende for Mg and Fe.
Scanning electron microscope imaging was carried out on carbon-coated thin sections using a JSM-6490 scanning electron microprobe. The working conditions were an acceleration voltage of 20 kV, a temperature of 20°C and an air humidity of <50%.
The Raman spectra of the Ba-rich feldspars were acquired in situ with a Raman spectrometer (LabRAM HR800, Horiba) equipped with a charge coupled device (CCD) detector (1024 × 256 pixels, aircooled to –70°C) at Nanjing University. The conditions used for acquisitions included 532.11 nm (air-cooled, frequency-doubled Nd:YAG) laser excitation, a 50× Olympus objective, and a 1800 groove/mm grating with a spectral resolution of ~1 cm–1. Laser light (9.5 mW) was focused on the Ba-rich feldspar during the spectra measurements. CCD spectra covering a whole wavenumber range of 100–4000 cm–1 were acquired first to configure the Raman signal range of the Ba-rich feldspars. The results indicated that the wavenumber range of the Ba-rich feldspars was ~100–3250 cm–1, and further analyses with a wavenumber range of 100–3250 cm–1 were then carried out. The spectrometer was calibrated with the v 1 band of silicon at 520.2 cm–1 (Parker et al., Reference Parker, Feldman and Ashkin1967). Each spectrum was acquired for 60 s with three acquisitions.
Infrared experiments were performed using a Thermo Nicolet FTIR spectrometer coupled to a Nicolet Centaurμs FTIR microprobe with a reflection accessory at the Key Laboratory of Surficial Geochemistry, Ministry of Education, Nanjing University. The microprobe was equipped with a camera that provided an optical image (~20 µm × 20 µm) of the region sampled by IR. The spectra of analysed areas were recorded with left μscope reflection mode (%R) using a single element mercury cadmium telluride detector. The results were recorded as the average of 128 scans in the spectral range of 4000–650 cm–1 at a resolution of 8 cm–1. Each spectrum was acquired following the withdrawal of a gold-coated glass substrate.
Results and discussion
Occurrences and compositions
Various occurrences of the Ba-bearing silicates were observed by back-scattered electron imaging, including sporadic discrete grains, rims on other minerals, void fillings and the components in veins. There is a close relationship between the occurrences and compositions of the various Ba-bearing silicates, allowing them to be divided into three types: Ba-poor K-feldspar; Ba-rich K-feldspar; and cymrite.
Ba-poor K-feldspar
The Ba-poor K-feldspars occur mainly as anhedral grains that are distributed sporadically in the matrix of the mudstones (Fig. 3). The grain sizes of the Ba-poor K-feldspars in most samples are less than or close to 10 µm (Fig. 3a), but reach ~30 µm in sample WN-16 from the base of the Hetang Formation (Fig. 3b–c).

Fig. 3. Back-scattered electron (BSE) and secondary electron (SE) images showing the occurrences of Ba-poor K-feldspars in mudstones: (a) Ba-poor K-feldspars (BPKF) occur as scattered individual grains partly surrounded by Ba-rich K-feldspar (BRKF) rims, Sample WN-2-28; (b) larger Ba-poor K-feldspar grains (~μm) are observed in Sample WN-16; (c) the Ba-rich K-feldspar rim is discontinuous where the Ba-poor K-feldspar grain is connected to other minerals, e.g. quartz, Sample WN-16.
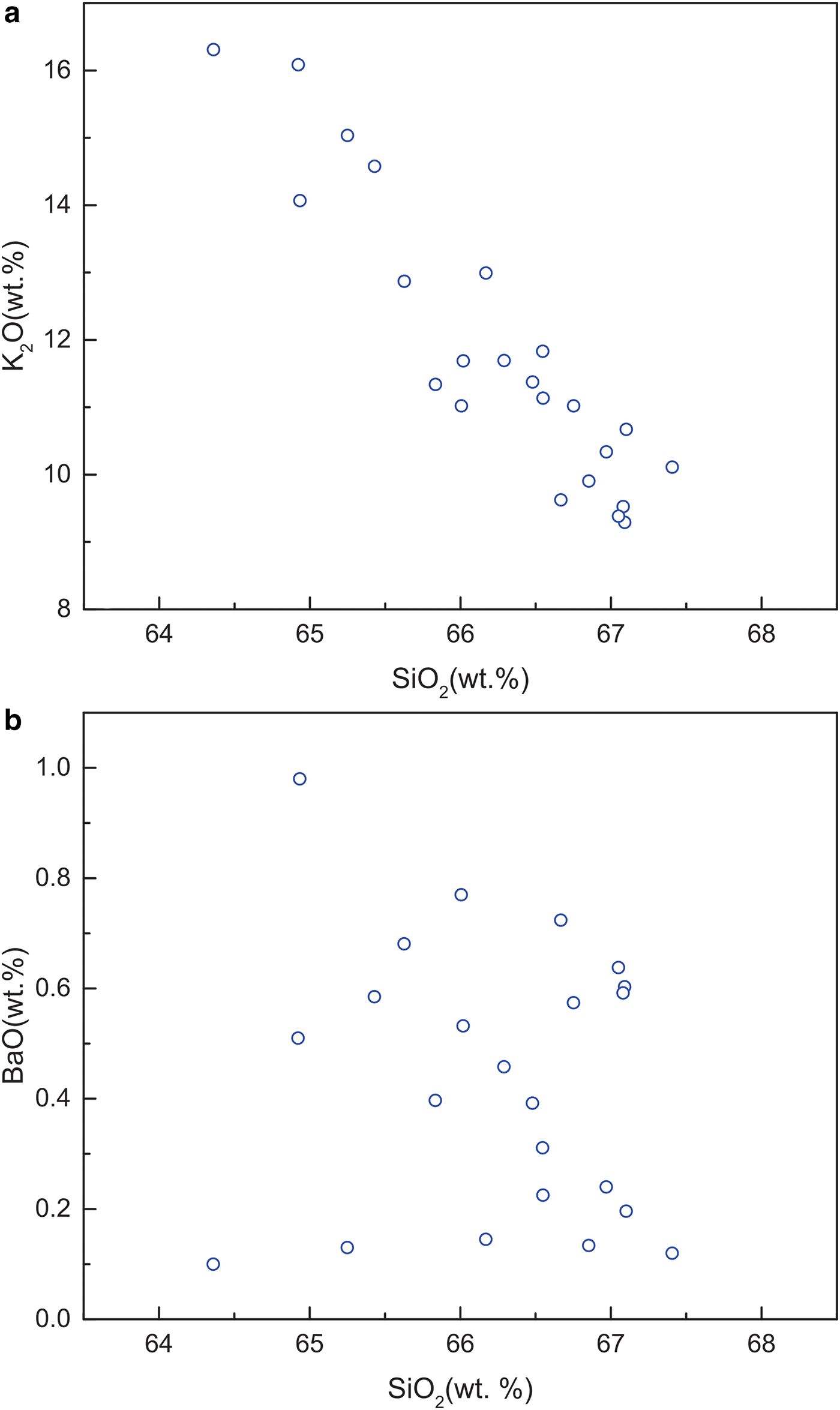
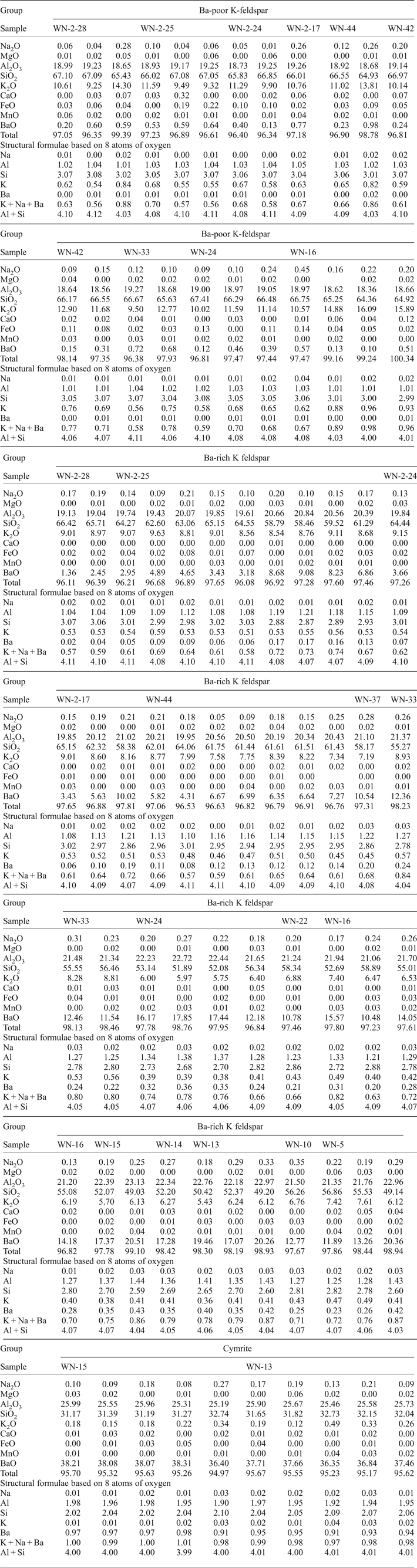
Electron microprobe analyses of most of the Ba-poor K-feldspars provide compositions that total <100 wt.% (total range, 96.34–100.34 wt.%; Table 1). K2O, SiO2 and Al2O3 are the main components, with contents ranging from 9.25–16.09 wt.%, 64.36–67.41 wt.% and 18.36–19.27 wt.%, respectively. The low BaO contents of the Ba-poor K-feldspars are limited to a range of 0.10–1.00 wt.%. The Na2O contents of the Ba-poor K-feldspars range from 0.04 to 0.45 wt.%. As shown in Fig. 4a, there is a strong negative linear correlation between K2O and SiO2 (Fig. 4a). In contrast, only a weak negative correlation can be recognized between BaO and SiO2 (Fig. 4b).
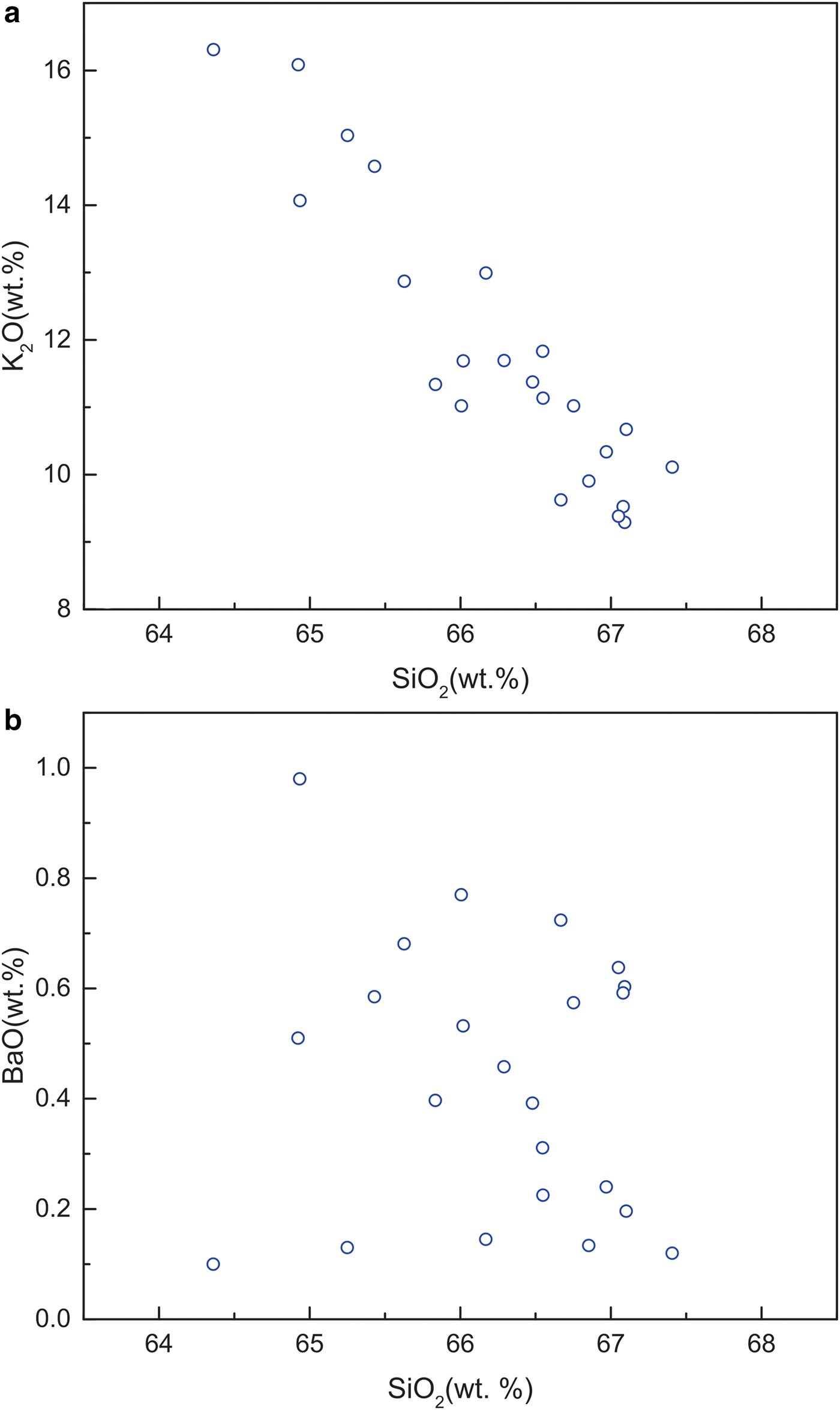
Fig. 4. Plots of the contents of main components in Ba-poor K-feldspar: (a) K2O and SiO2, a strong negative linear correlation between K2O and SiO2 is observed; (b) BaO and SiO2, only a very weak negative correlation between BaO and SiO2 can be recognized.
Table 1. Representative compositions of Ba-poor K-feldspar, Ba-rich K-feldspar and cymrite.

Formulae calculations based on eight oxygen atoms indicate that most of the Ba-poor K-feldspars are non-stoichiometric. The values of (Na + K + Ba)apfu for the Ba-poor K-feldspars range from 0.56–0.98, while those of (Al + Si)apfu range from 4.00–4.11 (Table 1). Stoichiometric Ba-rich K-feldspars were only detected in sample WN-16, and these were accompanied by non-stoichiometric Ba-rich K-feldspars (Table 1).
Ba-rich K-feldspar
Ba-rich K-feldspars are observed in most of the samples, and their modes of occurrence vary among different rock types (Fig. 2). In the mudstones, Ba-rich K-feldspars occur mainly as rims that partly or completely surround Ba-poor K-feldspar grains (Fig. 3). The rims are thicker in sample WN-16 and are discontinuous where the Ba-poor K-feldspar grains are in contact with quartz or other minerals (Fig. 3b–c). In the dolostones, Ba-rich K-feldspars commonly occur together with quartz as void fillings (Fig. 5a). In the cherts, the Ba-rich K-feldspars are dispersed mainly in the matrix, and are several to ~30 µm in size (Fig. 5b). In addition, rectangular and rhombic Ba-rich K-feldspar aggregates of ~100–500 µm in size were observed in sample WN-24 (Fig. 5c), and quartz occur in the centres of some of these aggregates (Fig. 5c). Calcite grains are observed around all the aggregates (Fig. 5c).

Fig. 5. BSE images showing the occurrences of Ba-rich K-feldspars (BRKF): (a) Ba-rich K-feldspars occur as fillings of voids in dolostone, accompanied by quartz, Sample WN-8; (b) Ba-rich K-feldspars and cymrite crystals occur as dispersive grains in chert, Sample WN-13; (c) Ba-rich K-feldspars occur as rectangular and rhombic aggregates, accompanied by sporadic calcite and quartz occurs in some of the aggregates, Sample WN-24; (d) Ba-rich K-feldspar-bearing veins in mudstone, the veins are mainly composed of Ba-rich K-feldspar and quartz, the Ba-rich K-feldspars occur as amorphous aggregates consisting of anhedral to subhedral small Ba-rich K-feldspar grains, and the Ba-rich K-feldspars in veins are associated with those (rims) in the matrix, Sample WN-42; (e) Ba-rich K-feldspar-bearing veins in dolostone, the veins are mainly composed of Ba-rich K-feldspar and quartz, some Ba-rich K-feldspars occur as euhedral rhombic grains, and the Ba-rich K-feldspars in veins are associated with those Ba-rich K-feldspar fillings in the matrix, WN-5; (f) euhedral Ba-rich K-feldspar grains in veins observed in mudstone, the euhedral Ba-rich K-feldspar grains are surrounded by or connected with Ba-rich K-feldspars with lower BaO contents, Sample WN-2-25. Mineral abbreviations: Qz = quartz, Cal = calcite, Dol = dolomite (Whitney and Evans, Reference Whitney and Evans2010).
Ba-rich K-feldspars have also been observed in cross-cutting veins where they occur together with quartz, and which are common in the mudstones and dolostones (Fig. 5d–f). The abundance of Ba-rich K-feldspar in different veins varies significantly. In most veins, the Ba-rich K-feldspars are anhedral or subhedral (Fig. 5d), but can be euhedral in other veins (Fig. 5e–f). The euhedral Ba-rich K-feldspar grains are weakly zoned in composition and are occasionally surrounded by (or connected with) Ba-rich K-feldspars with lower Ba contents (Fig. 5e–f). Notably, the Ba-rich K-feldspars in veins are commonly associated with Ba-rich K-feldspars in the matrix, indicating a genetic relationship between the two (Fig. 5d–e).
Electron microprobe analyses of most of the Ba-rich K-feldspars provide compositions that total <100 wt.% (total range, 96.08–99.10 wt.%; Table 1). BaO, K2O, SiO2 and Al2O3 are the main components, with contents ranging from 1.36–20.51 wt.%, 5.43–9.63 wt.%, 49.03–66.42 wt.% and 19.04–23.13 wt.%, respectively (Table 1). Different samples from the drilled profile show various ranges of BaO contents in the Ba-rich K-feldspars (Table 1; Fig. 2). Na2O contents of the Ba-rich K-feldspars are <0.40 wt.% (Table 1).
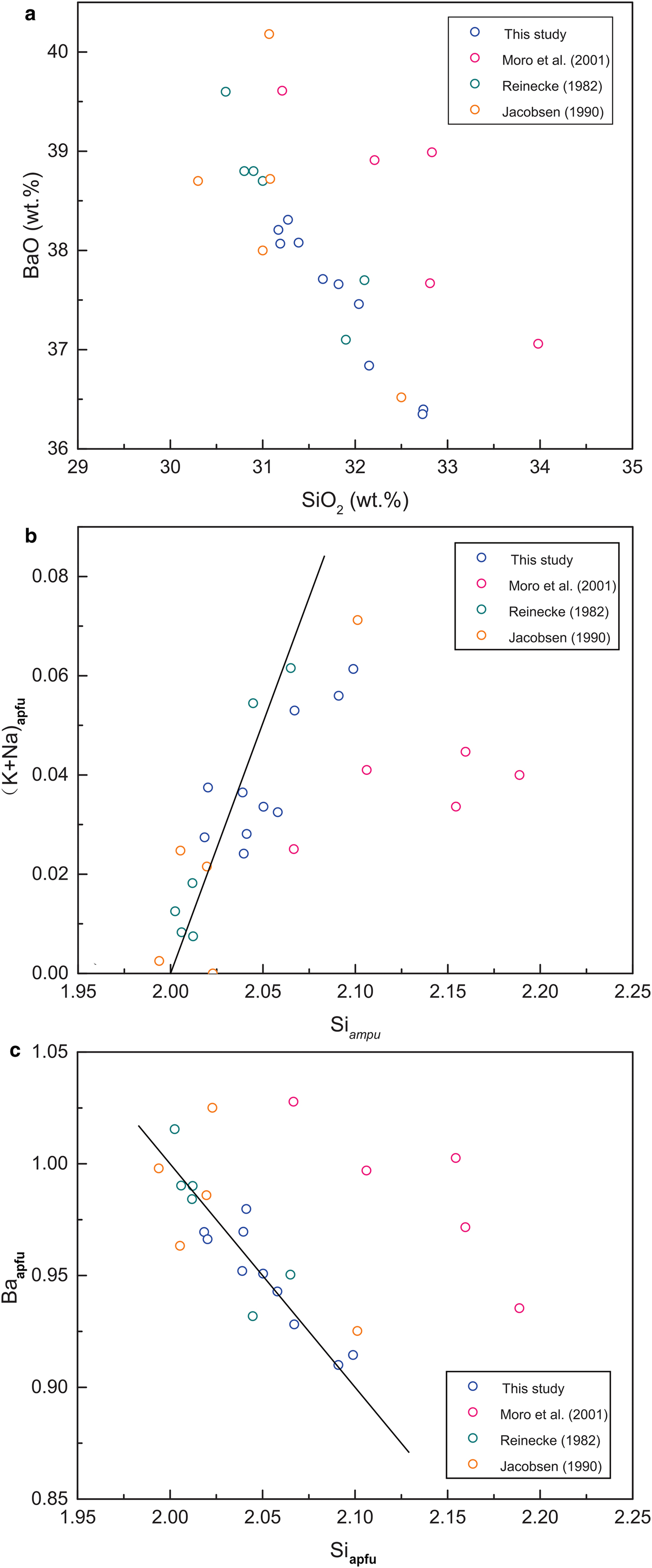
Our analytical results for K2O and SiO2 in the Ba-rich K-feldspars, and the correlation between them (Fig. 6a), differ from those reported in most previous studies (Jakobsen, Reference Jakobsen1990; Chabu and Boulègue, Reference Chabu and Boulegue1992; McSwiggen et al., Reference McSwiggen, Morey and Cleland1994; Moro et al., Reference Moro, Cembranos and Fernandez2001; Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013; Raith et al., Reference Raith, Devaraju and Spiering2014; Henry et al., Reference Henry, Will and Mueller2015). As shown in Fig. 6a, our analyses give lower K2O and higher SiO2 contents, and the correlation between them is much weaker than reported previously. Interestingly, the compositions of the Ba-rich K-feldspars reported by both Long et al. (Reference Long, Long, Zhong, Zhuang and Liu1994) and Orberger et al. (Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005) are similar to our results. The BaO and SiO2 contents of the Ba-rich K-feldspars in our samples show a strong linear correlation which resembles that reported in previous studies, regardless of the higher SiO2 contents in the present study (Fig. 6b).

Fig. 6. Plots of the contents of main components in Ba-rich K-feldspars in this and previous studies: (a) K2O and SiO2, the Ba-rich K-feldspars in most of the previous studies a strong negative linear correlation between K2O and SiO2 (Jakobsen, Reference Jakobsen1990; Chabu and Boulègue, Reference Chabu and Boulegue1992; McSwiggen et al., Reference McSwiggen, Morey and Cleland1994; Moro et al., Reference Moro, Cembranos and Fernandez2001; Xia et al., Reference Xia, Ma, Pan, Chen, Cao, Sun, Liu and Guo2005; Han et al., Reference Han, Hu and Cao2013; Raith et al., Reference Raith, Devaraju and Spiering2014; Henry et al., Reference Henry, Will and Mueller2015), whereas the data points from Long et al. (Reference Long, Long, Zhong, Zhuang and Liu1994), Orberger et al. (Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005), and this study are distributed sparsely in a single area away from the line; (b) BaO and SiO2, the Ba-rich K-feldspars of this study show a similar negative linear correlation between BaO and SiO2, the Ba-rich K-feldspars of Long et al. (Reference Long, Long, Zhong, Zhuang and Liu1994), Orberger et al. (Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005) and this study show higher SiO2 contents; (c) (K + Na)apfu and Siapfu, similar correlation characteristics to (a) are observed, the black solid line represents the theoretical compositional assemblage of hyalophane.
The formulae calculated in this study indicate that the Ba-rich K-feldspars have non-stoichiometric compositions: the value of (Na + K + Ba)apfu varies from 0.57 to 0.87, while that of (Al + Si)apfu ranges from 4.03 to 4.11. The correlation between the (Na + K)apfu and Siapfu values of the Ba-rich K-feldspars in our samples differs from that reported in most previous studies. As shown in Fig. 6c, the data points from most previous studies are closely distributed along the theoretical line of hyalophane, while those from Long et al. (Reference Long, Long, Zhong, Zhuang and Liu1994), Orberger et al. (Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005) and the present study are distributed sparsely in a single area away from the line.
Cymrite
Cymrite is observed only in two chert samples (WN-13 and 15) from the base of the Hetang Formation (Fig. 2). It is associated with Ba-rich K-feldspar and occurs mainly in the form of ~10 µm tabular crystals that are dispersed throughout the matrix (Fig. 5a and Fig. 7a). Furthermore, micro-cracks in the samples are filled with cymrite and Ba-rich K-feldspars, and the cymrite crystals locally overgrow the Ba-rich K-feldspar grains (Fig. 7a–b).

Fig. 7. BSE and SE images showing the occurrences of cymrites in chert: (a) cymrites occur as euhedral tabular crystals in voids in the matrix, Sample WN-13; (b) cymrite crystals and Ba-rich K-feldspars (BRKF) fill the micro-cracks, and occur as dispersed grains in the matrix, Sample WN-15; (c) cymrite crystals overgrowth on Ba-rich K-feldspar grains, Sample WN-15.
Electron microprobe analyses of the cymrite provide totals of <100 wt.% (total range, 94.94–95.70 wt.%; Table 1). BaO, SiO2 and Al2O3 are the main components, with contents ranging from 36.35–38.31 wt.%, 31.17–32.74 wt.% and 25.19–25.99 wt.%, respectively. The K2O and Na2O contents of the cymrite fall within the limited ranges of 0.12–0.49 wt.% and 0.09–0.27 wt.%, respectively. The BaO and SiO2 contents of the cymrites, and their correlations, are similar to those reported in previous studies (Fig. 8a) (Reinecke, Reference Reinecke1982; Jakobsen, Reference Jakobsen1990; Moro et al., Reference Moro, Cembranos and Fernandez2001).
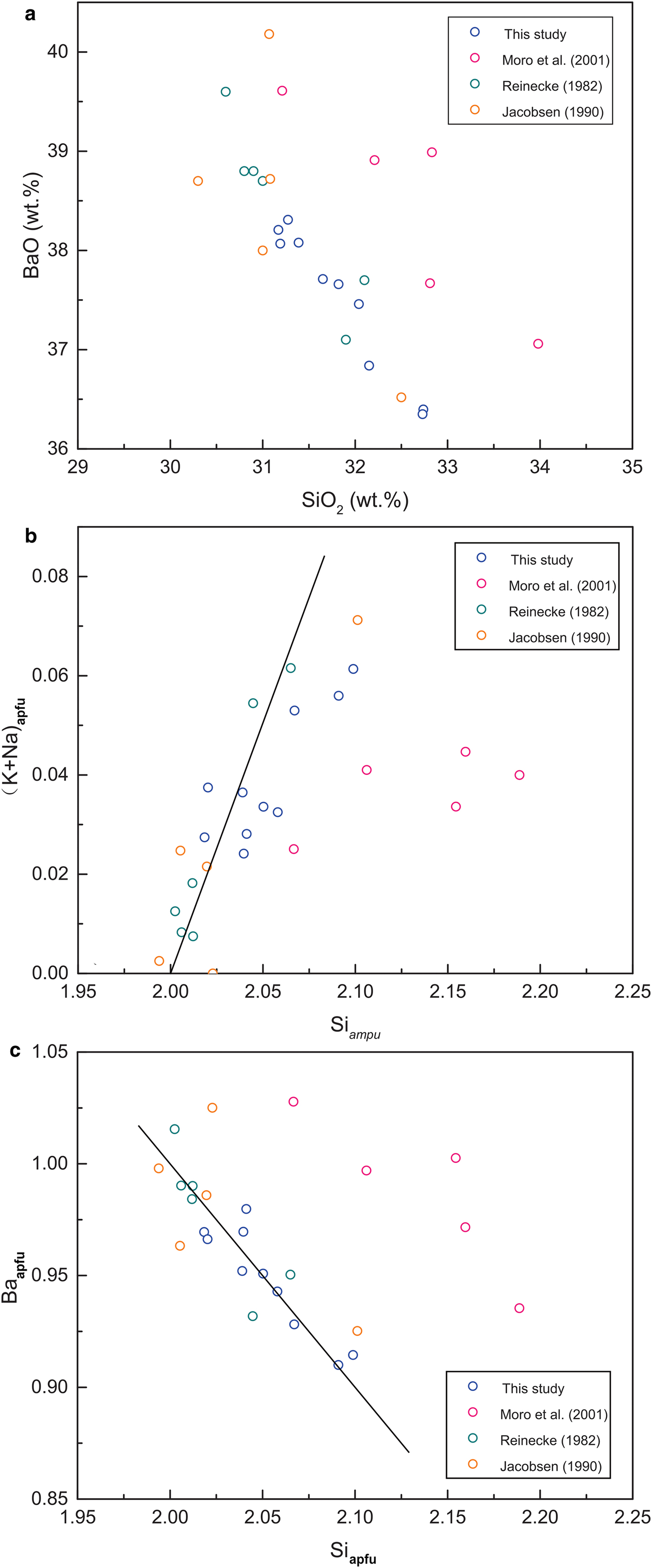
Fig. 8. Plots of the contents of main components in cymrites in this and previous studies: (a) BaO and SiO2, a strong negative linear correlation similar to previous studies is observed; (b) (K + Na)apfu and Siapfu; and (c) Baapfu and Siapfu, the data points are distributed closely along the theoretical line (black solid line) of cymrite, and strong linear correlations similar to previous studies are observed.
Formulae calculations indicate that the cymrites are stoichiometric in composition (Table 1). As shown in Fig. 8b–c, the correlations of (K + Na)apfu–Siapfu and Baapfu–Siapfu are close to the theoretical ones, and comparable to those reported previously (Reinecke, Reference Reinecke1982; Jakobsen, Reference Jakobsen1990; Moro et al., Reference Moro, Cembranos and Fernandez2001).
Causes of the non-stoichiometry
As discussed above, the Ba-poor K-feldspars and Ba-rich K-feldspars in our samples are non-stoichiometric. Normally, non-stoichiometry can be caused by analytical errors or the presence of monovalent or bivalent cations not included in the EMP analyses (Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). The strong linear correlations of K2O–SiO2 in the Ba-poor K-feldspars (Fig. 4a) and BaO–SiO2 in the Ba-rich K-feldspars (Fig. 6b) argue against the possibility of significant analytical errors in the microprobe analyses of K, Ba, Al and Si. We suggest, therefore, that some cations in the Ba-poor K-feldspars and Ba-rich K-feldspars might not have been included in the microprobe analyses, thus accounting for the non-stoichiometry.
In addition to Na+, K+, Ca2+ and Ba2+, other monovalent or bivalent cations such as Li+, NH4+, Rb+ and Sr2+ can occupy the M site in feldspars (Smith and Brown, Reference Smith and Brown1988). Ba-rich K-feldspars with high contents of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ (corresponding to as much as 56 mol.% buddingtonite) have been reported from mineralized Devonian black shales in the Selwyn Basin, Northwest Territories, Canada, for which it was suggested the
${\rm NH}_{\rm 4}^{\rm +} $ (corresponding to as much as 56 mol.% buddingtonite) have been reported from mineralized Devonian black shales in the Selwyn Basin, Northwest Territories, Canada, for which it was suggested the ![]() ${\rm NH}_{\rm 4}^{\rm +} $ originated from the decomposition of intraformational organic matter (Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). As shown in Fig. 6, our samples of Ba-rich K-feldspar and those studied by Orberger et al. (Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005) have similar compositional characteristics, including elemental correlations and non-stoichiometric features. Furthermore, the mudstones of the Hetang Formation are also rich in organic matter, with TOC contents of 2.10 to 27.13 wt.% (Chang et al., Reference Chang, Hu, Fu, Cao, Wang and Yao2016). These strong similarities indicate that the main cause of non-stoichiometry in our samples might be
${\rm NH}_{\rm 4}^{\rm +} $ originated from the decomposition of intraformational organic matter (Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). As shown in Fig. 6, our samples of Ba-rich K-feldspar and those studied by Orberger et al. (Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005) have similar compositional characteristics, including elemental correlations and non-stoichiometric features. Furthermore, the mudstones of the Hetang Formation are also rich in organic matter, with TOC contents of 2.10 to 27.13 wt.% (Chang et al., Reference Chang, Hu, Fu, Cao, Wang and Yao2016). These strong similarities indicate that the main cause of non-stoichiometry in our samples might be ![]() ${\rm NH}_{\rm 4}^{\rm +} $, and to pursue this further, sample WN-24 was analysed by Raman and IR spectroscopy. It contains large aggregates of Ba-rich K-feldspar favourable for spectroscopic analysis (Fig. 5c).
${\rm NH}_{\rm 4}^{\rm +} $, and to pursue this further, sample WN-24 was analysed by Raman and IR spectroscopy. It contains large aggregates of Ba-rich K-feldspar favourable for spectroscopic analysis (Fig. 5c).
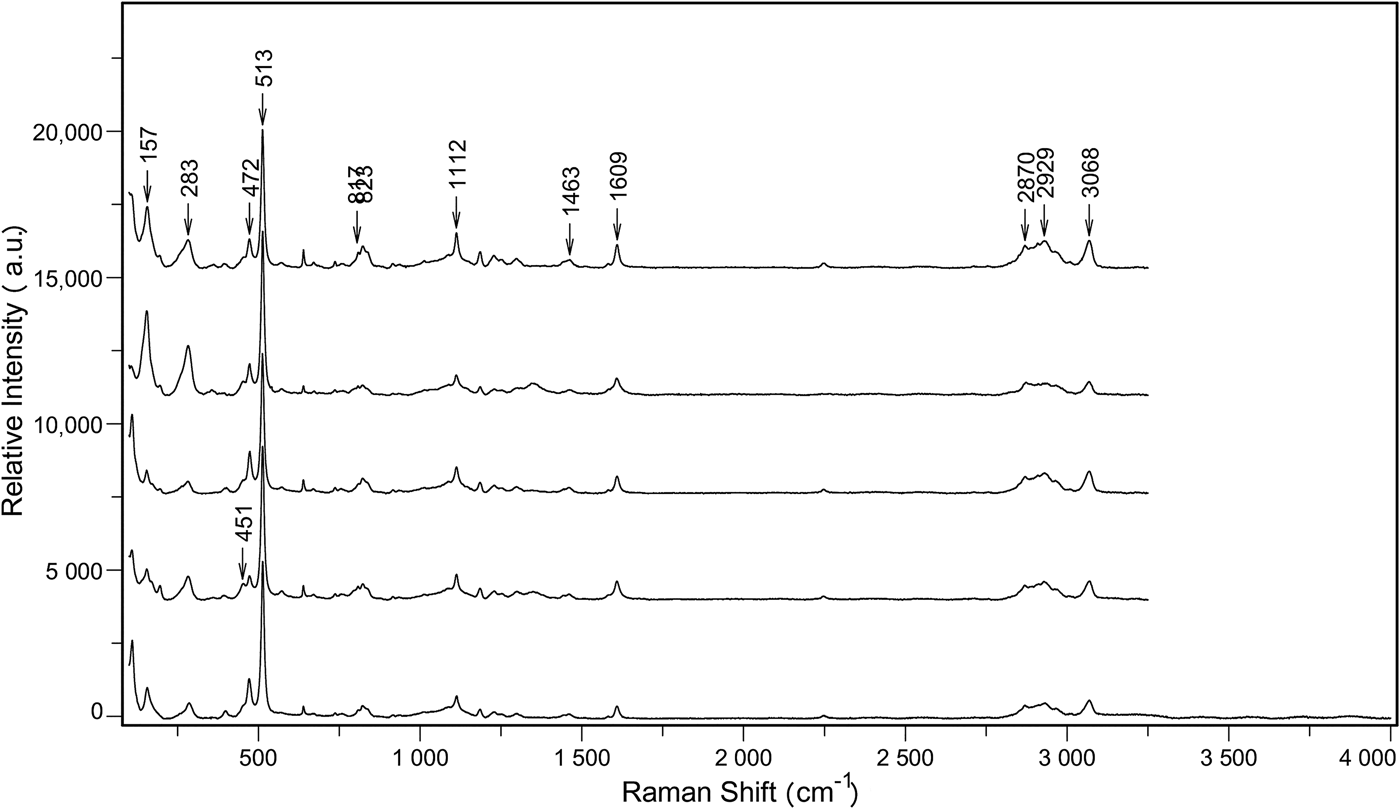
The Raman spectra for the Ba-rich K-feldspar aggregates in sample WN-24 are presented in Fig. 9. The 100–1200 cm–1 region of the spectra is characterized by peaks typical of K-feldspar (Freeman et al., Reference Freeman, Wang, Kuebler, Jolliff and Haskin2008). The strongest peak around 513 cm–1 and other peaks in the 450–520 cm–1 region (e.g. the peak around 472 cm–1) belong to the ring-breathing modes of the four-membered rings of the tetrahedra. The peaks around 157 and 283 cm–1 correspond to the rotation–transaction mode of four-membered rings and the cage-shear mode, respectively. In addition, the weak peaks around 807 and 1112 cm–1 can be assigned to the deformation and vibrational stretching modes of the tetrahedra, respectively.

Fig. 9. Raman spectra of Ba-rich K-feldspars in Sample WN-24, typical peaks associated with K-feldspar, ![]() ${\rm NH}_{\rm 4}^{\rm +} $, and H2O are observed in all the spectra and marked out in the figure.
${\rm NH}_{\rm 4}^{\rm +} $, and H2O are observed in all the spectra and marked out in the figure.
The 1400–4000 cm–1 region on the spectra includes a broad peak between 2800 and 3000 cm–1, and three other peaks around 1463, 1609 and 3068 cm–1. The broad peak between 2800 and 3000 cm–1 was also observed on the Raman spectra of tetra-n-butyl ammonium fluoride semi-clathrate hydrate, and it was attributed to the O–H vibration of the hydration water (Sakamoto et al., Reference Sakamoto, Hashimoto, Tsuda, Sugahara, Inoue and Ohgaki2008). In comparison, both the broad peak and the peak around 3068 cm–1 were observed in the Raman spectra of MgNH4PO4·6H2O (Stefov et al., Reference Stefov, Soptrajanov, Kuzmanovski, Lutz and Engelen2005). The differences in the Raman spectra of the two minerals reveal the contribution of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ to the 3068 cm–1 peak. Furthermore, the peaks around 1463 and 1609 cm–1 can be assigned, respectively to the v 4 mode of ammonium ions and δ(HOH) vibrations (Stefov et al., Reference Stefov, Soptrajanov, Kuzmanovski, Lutz and Engelen2005).
${\rm NH}_{\rm 4}^{\rm +} $ to the 3068 cm–1 peak. Furthermore, the peaks around 1463 and 1609 cm–1 can be assigned, respectively to the v 4 mode of ammonium ions and δ(HOH) vibrations (Stefov et al., Reference Stefov, Soptrajanov, Kuzmanovski, Lutz and Engelen2005).
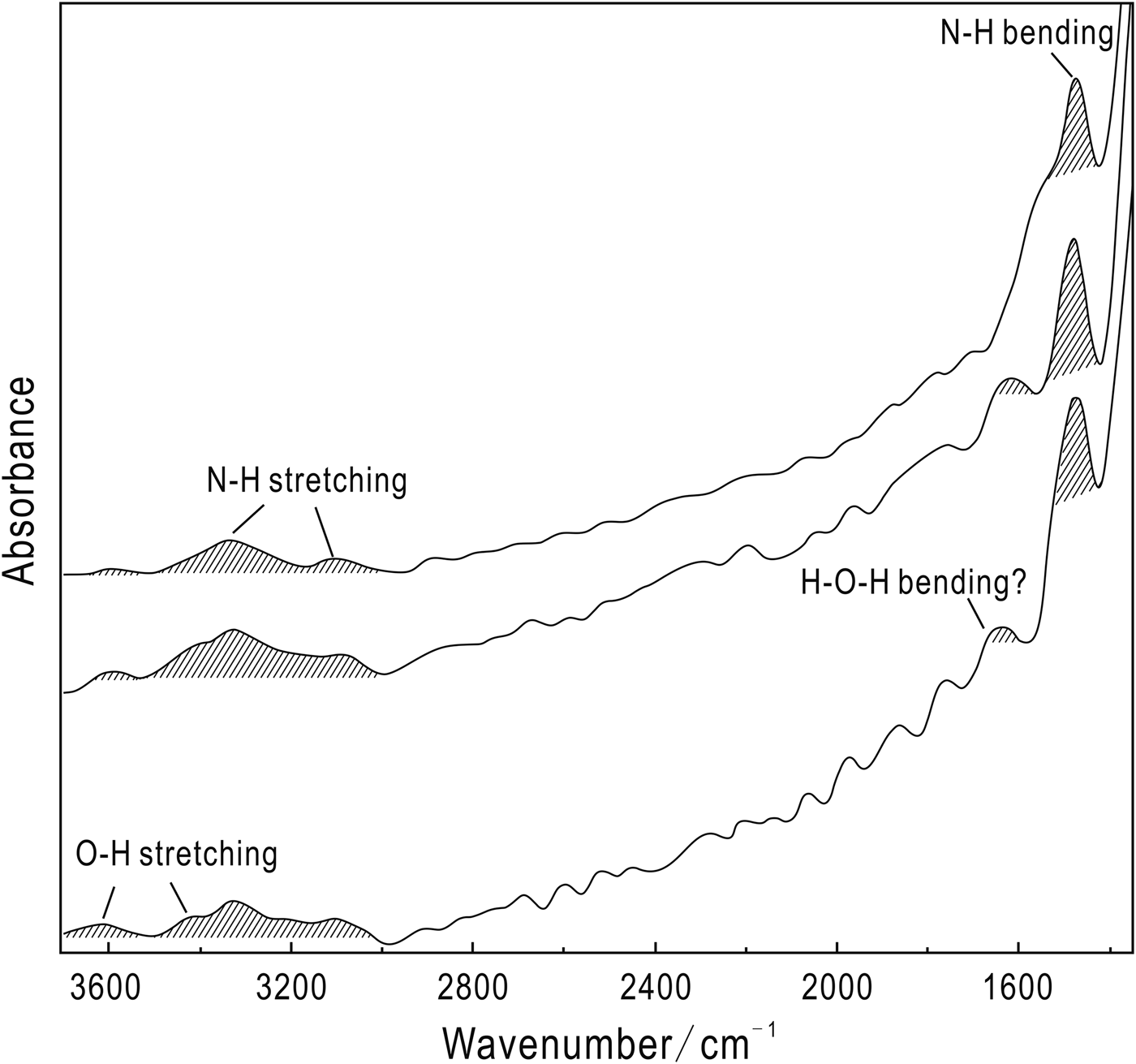
Additional evidence for the presence of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ is provided by the IR spectra of the Ba-rich K-feldspar aggregates. As shown in Fig. 10, the strong peak near 1400 cm–1 and two other peaks around 3100 and 3300 cm–1 correspond to the N–H bending and N–H stretching vibrations of
${\rm NH}_{\rm 4}^{\rm +} $ is provided by the IR spectra of the Ba-rich K-feldspar aggregates. As shown in Fig. 10, the strong peak near 1400 cm–1 and two other peaks around 3100 and 3300 cm–1 correspond to the N–H bending and N–H stretching vibrations of ![]() ${\rm NH}_{\rm 4}^{\rm +} $, respectively (Nyquist and Kagel, Reference Nyquist and Kagel1971; Boyd and Philippot, Reference Boyd and Philippot1998). A peak near 3600 cm–1 is observed in all the IR spectra, accompanied by a peak around 3400 cm–1 in some spectra (Fig. 10), and these peaks correspond to the O–H stretching vibration mode of hydration water (Nyquist and Kagel, Reference Nyquist and Kagel1971). In contrast, the peak around 1600 cm–1 in some spectra can be attributed to the H–O–H bending motion mode of hydration water (Nyquist and Kagel, Reference Nyquist and Kagel1971).
${\rm NH}_{\rm 4}^{\rm +} $, respectively (Nyquist and Kagel, Reference Nyquist and Kagel1971; Boyd and Philippot, Reference Boyd and Philippot1998). A peak near 3600 cm–1 is observed in all the IR spectra, accompanied by a peak around 3400 cm–1 in some spectra (Fig. 10), and these peaks correspond to the O–H stretching vibration mode of hydration water (Nyquist and Kagel, Reference Nyquist and Kagel1971). In contrast, the peak around 1600 cm–1 in some spectra can be attributed to the H–O–H bending motion mode of hydration water (Nyquist and Kagel, Reference Nyquist and Kagel1971).

Fig. 10. Infrared spectra of Ba-rich K-feldspars in Sample WN-24, typical peaks corresponding to ![]() ${\rm NH}_{\rm 4}^{\rm +} $ and H2O are observed in all the spectra.
${\rm NH}_{\rm 4}^{\rm +} $ and H2O are observed in all the spectra.
In brief, the Raman and IR spectra of the Ba-rich K-feldspar aggregates show that ![]() ${\rm NH}_{\rm 4}^{\rm +} $ and hydration water are present in our samples of Ba-rich K-feldspar, and they probably indicate the presence of a buddingtonite (NH4AlSi3O8·1/2H2O) component in the Ba-rich K-feldspar (Smith and Brown, Reference Smith and Brown1988; Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). We suggest, therefore, that the occupancy of
${\rm NH}_{\rm 4}^{\rm +} $ and hydration water are present in our samples of Ba-rich K-feldspar, and they probably indicate the presence of a buddingtonite (NH4AlSi3O8·1/2H2O) component in the Ba-rich K-feldspar (Smith and Brown, Reference Smith and Brown1988; Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). We suggest, therefore, that the occupancy of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ on the M sites of the Ba-rich K-feldspars is the main cause of non-stoichiometry recorded by our EMP analyses. Moreover, although we did not carry out any direct spectroscopic measurements on the Ba-poor K-feldspars, it is reasonable to conclude that their non-stoichiometry might also be caused by the incorporation of
${\rm NH}_{\rm 4}^{\rm +} $ on the M sites of the Ba-rich K-feldspars is the main cause of non-stoichiometry recorded by our EMP analyses. Moreover, although we did not carry out any direct spectroscopic measurements on the Ba-poor K-feldspars, it is reasonable to conclude that their non-stoichiometry might also be caused by the incorporation of ![]() ${\rm NH}_{\rm 4}^{\rm +} $, given the similar non-stoichiometric characteristics of the Ba-rich K-feldspars and Ba-poor K-feldspars (depleted K and excessive Al and Si; Table 1; Fig. 4a; Fig. 6a and c), and their textural relationships (Fig. 3). The mechanisms for incorporation of
${\rm NH}_{\rm 4}^{\rm +} $, given the similar non-stoichiometric characteristics of the Ba-rich K-feldspars and Ba-poor K-feldspars (depleted K and excessive Al and Si; Table 1; Fig. 4a; Fig. 6a and c), and their textural relationships (Fig. 3). The mechanisms for incorporation of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ into the feldspars are discussed below.
${\rm NH}_{\rm 4}^{\rm +} $ into the feldspars are discussed below.
Formation mechanisms
Ba-rich K-feldspars
Two mechanisms have commonly been proposed for the formation of Ba-rich K-feldspars: (1) the replacement of authigenic baryte; and (2) as an authigenic phase crystallizing from Ba–Al–Si gels (McSwiggen et al., Reference McSwiggen, Morey and Cleland1994; Moro et al., Reference Moro, Cembranos and Fernandez2001). The replacement of authigenic baryte occurs during late diagenetic or metamorphic processes, with the baryte dissolved under a relatively reducing environment and the released barium precipitated as Ba-rich K-feldspar (Chabu and Boulègue, Reference Chabu and Boulegue1992; McSwiggen et al., Reference McSwiggen, Morey and Cleland1994). In our study we found no mineral relicts that would indicate the prior existence of authigenic baryte or textural evidence to support the replacement of baryte. We suggest, therefore, that baryte was not the precursor of the Ba-rich K-feldspar in our samples. The alternative mechanism of forming Ba-rich K-feldspars from hydrated Ba–Al–Si gels involves a late multi-stage diagenetic process that starts with hydrated Ba-silicates with higher contents of water (such as harmotome, BaAl2Si6O6·6H2O), with dehydration reactions leading to the formation of cymrite and eventually celsian (Fortey and Beddoe–Stephens, Reference Fortey and Beddoestephens1982; Jakobsen, Reference Jakobsen1990; Raith et al., Reference Raith, Devaraju and Spiering2014).
The formation of Ba–Al–Si gels is thought to be associated with hydrothermal activity (Coats et al., Reference Coats, Smith, Fortey, Gallagher, May and McCourt1980; Large, Reference Large1980; Fortey and Beddoe–Stephens, Reference Fortey and Beddoestephens1982; Russell et al., Reference Russell, Hall, Willan, Allison, Anderton and Bowes1984; Jakobsen, Reference Jakobsen1990; McSwiggen et al., Reference McSwiggen, Morey and Cleland1994; Moro et al., Reference Moro, Cembranos and Fernandez2001). Large amounts of Ba and SiO2 could be released into seawater during exhalative hydrothermal activity and then precipitated on the seafloor as a silica gel under reducing conditions (Large, Reference Large1980). Ba-rich K-feldspar-bearing cherts have been reported by Fortey and Beddoe–Stephens (Reference Fortey and Beddoestephens1982) and Jakobsen (1990), who suggested that the Ba-rich K-feldspars were of exhalative hydrothermal origin. Similarly, the Ba-rich K-feldspars in our samples of chert could have originated from Ba–Al–Si gels that formed during exhalative hydrothermal activity. The dispersed nature of the grains of Ba-rich K-feldspar (Fig. 5a) and the reducing sedimentary environment of the chert are consistent with this mechanism of formation (Chang et al., Reference Chang, Hu, Fu, Cao, Wang and Yao2016).
In contrast, the cross-cutting Ba-rich K-feldspar-bearing veins in the mudstones and dolostones demonstrate that the Ba-rich hydrothermal fluids also infiltrated the strata along cracks and formed Ba-Al-Si gels (Fig. 5d–f). Furthermore, on the basis of textural relationships we suggest that the hydrothermal fluids that formed the veins penetrated through voids into the matrix, leading to the formation of Ba-rich K-feldspar rims in the mudstones and Ba-rich K-feldspar void fillings in the dolostones (Fig. 5d–e). The infiltration of the hydrothermal fluids might have occurred when the sediments were relatively unconsolidated, so that the fluids could move easily through the inter-granular spaces of the sediments and form abundant micro-scale veins (Fig. 5d–e). Compositional zoning of the Ba-rich K-feldspar grains in the veins could be a result of heterogeneous crystallizing conditions (Fig. 5e–f), while the more intense zoning of the Ba-rich K-feldspar aggregates could be attributed to Ba-depleted Ba-rich K-feldspars forming overgrowths on early-formed Ba-enriched grains of Ba-rich K-feldspar (Fig. 5f).
In addition, the peculiar occurrence of the Ba-rich K-feldspar aggregates in sample WN-24 suggests a distinctive formation process. The regular shapes of the aggregates might have been inherited from crystallized precursor minerals, and the consistent occurrence of calcite around the aggregates suggests a genetic relationship (Fig. 5c). Consequently, we suggest tentatively that the regularly shaped aggregates were originally calcite or dolomite crystals. These crystals would have been dissolved easily by acidic and Ba-rich hydrothermal fluids, and Ba–Al–Si gels would then have been precipitated in the resulting voids. A small amount of Ca-enriched fluid might have been preserved, leading to the formation of the calcite around the aggregates.
In brief, we suggest the Ba-rich K-feldspars in our samples formed from Ba-rich hydrothermal fluids in multiple stages, and that the intensity of the hydrothermal activity fluctuated during the various stages. The Ba-rich K-feldspar-bearing cherts were possibly formed when hydrothermal activity was intense, and when the hydrothermal fluids were transported to the seafloor. The sedimentary strata would have been infiltrated by the hydrothermal fluids as they ascended. At other stages, the hydrothermal activity might have been insufficiently intense enough to form bedded chert, and at times most of the hydrothermal fluids might simply have infiltrated the pile of sedimentary strata. The results of early-stage hydrothermal activity could have been overprinted by late-stage activity, which means that the Ba-rich K-feldspars in any one sample might have formed during multiple stages of hydrothermal activity.
The contrasting BaO contents of the Ba-rich K-feldspars in different samples might therefore be related to variable intensities of hydrothermal activity at different stages (Fig. 2). As shown in Fig. 2, the BaO contents of the Ba-rich K-feldspars are highest at the bottom of the Hetang Formation and decrease upwards to the calcareous mudstone layer, indicating that hydrothermal activity was intense at the beginning of the Cambrian, but gradually weakened thereafter.
During the degradation of sedimentary deposits organic matter can form, which can then be dissolved in pore fluids; moreover, hydrothermal fluids can boost the formation of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ from organic matter (Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). Therefore, large amounts of
${\rm NH}_{\rm 4}^{\rm +} $ from organic matter (Orberger et al., Reference Orberger, Gallien, Pinti, Fialin, Daudin, Grocke and Pasava2005). Therefore, large amounts of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ could have been derived from young organic-rich sedimentary strata such as the late Neoproterozoic Doushantuo Formation (Yang et al., Reference Yang, Zhu, Zhang, Zhao and Lv2015) and the Hetang Formation, and then incorporated into hydrothermal fluids. The hydrothermal fluids would then have transported the
${\rm NH}_{\rm 4}^{\rm +} $ could have been derived from young organic-rich sedimentary strata such as the late Neoproterozoic Doushantuo Formation (Yang et al., Reference Yang, Zhu, Zhang, Zhao and Lv2015) and the Hetang Formation, and then incorporated into hydrothermal fluids. The hydrothermal fluids would then have transported the ![]() ${\rm NH}_{\rm 4}^{\rm +} $ into voids in the sediments and contributed to the formation of the Ba-rich K-feldspars.
${\rm NH}_{\rm 4}^{\rm +} $ into voids in the sediments and contributed to the formation of the Ba-rich K-feldspars.
A multi-stage mechanism of formation is consistent with the tectonic setting of extension during the Ediacaran–Cambrian transition (Wang and Li, Reference Wang and Li2003). Multiple stages of hydrothermal fluids from a deep-seated heat source could have been driven upwards along faults under enhanced edge-driven convection during that period (Chen et al., Reference Chen, Wang, Qing, Yan and Li2009). Except for the aforementioned baryte and vanadium deposits, polymetallic sulfide deposits of hydrothermal origin and various occurrences of chert associated with multi-episodic hydrothermal activities have also been reported previously (Steiner et al., Reference Steiner, Wallis, Erdtmann, Zhao and Yang2001; Jiang et al., Reference Jiang, Yang, Ling, Chen, Feng, Zhao and Ni2007; Chen et al., Reference Chen, Wang, Qing, Yan and Li2009; Wang et al., Reference Wang, Chen, Wang, Yan, Zhou and Wang2012), further supporting the idea that hydrothermal activities were prevalent in South China during the Ediacaran–Cambrian transition.
Ba-poor K-feldspars
In contrast to the Ba-rich K-feldspars, most Ba-poor K-feldspars in our samples occur independently of any Ba-bearing veins in the mudstones (Fig. 3 and Fig. 5d–f), and no Ba-poor K-feldspars have been observed in association with the Ba-rich K-feldspar aggregates in sample WN-24 (Fig. 5c). These observations suggest that the Ba-poor K-feldspars might not have formed directly from the hydrothermal fluids responsible for the Ba-rich K-feldspars. Rather, the presence of stoichiometric Ba-poor K-feldspars in sample WN-16 supports the idea that the Ba-poor K-feldspars were originally stoichiometric K-feldspars within the mudstone (Table 1). These K-feldspars might then have interacted with the infiltrating Ba-rich hydrothermal fluids, leading to the formation of the Ba-poor K-feldspars. However, as only small amounts of hydrothermal fluid could have infiltrated the sediments, the interactions between the K-feldspars and the hydrothermal fluids would have been limited and incomplete. As a result, most of the Ba-poor K-feldspars still occur as normal grains rather than dissolved relicts, and the core areas of some large K-feldspar grains have retained their original compositions (WN-16; Table 1).
During these interactions, Ba2+ and ![]() ${\rm NH}_{\rm 4}^{\rm +} $ in the hydrothermal fluids would have substituted for K+ in the K-feldspars. The ionic radius of Ba2+ is 0.144 nm, similar to that of
${\rm NH}_{\rm 4}^{\rm +} $ in the hydrothermal fluids would have substituted for K+ in the K-feldspars. The ionic radius of Ba2+ is 0.144 nm, similar to that of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ (0.143 nm) (Honma and Itihara, Reference Honma and Itihara1981). The substitution of K+ by Ba2+ occurs in the form of a coupled exchange between (K, Si) and (Ba, Al) (Gay and Roy, Reference Gay and Roy1968), which requires a high activation energy and is obviously more difficult than substituting K+ by
${\rm NH}_{\rm 4}^{\rm +} $ (0.143 nm) (Honma and Itihara, Reference Honma and Itihara1981). The substitution of K+ by Ba2+ occurs in the form of a coupled exchange between (K, Si) and (Ba, Al) (Gay and Roy, Reference Gay and Roy1968), which requires a high activation energy and is obviously more difficult than substituting K+ by ![]() ${\rm NH}_{\rm 4}^{\rm +} $ (Raith et al., Reference Raith, Devaraju and Spiering2014). Therefore, relatively large amounts of K+ might have been substituted by
${\rm NH}_{\rm 4}^{\rm +} $ (Raith et al., Reference Raith, Devaraju and Spiering2014). Therefore, relatively large amounts of K+ might have been substituted by ![]() ${\rm NH}_{\rm 4}^{\rm +} $ during the interactions, while the substitution of K+ by Ba2+ would have been more limited. As the SiO2 content of buddingtonite is higher than that of K-feldspar, the SiO2 content of Ba-poor K-feldspar will increase with the substitution of K+ by
${\rm NH}_{\rm 4}^{\rm +} $ during the interactions, while the substitution of K+ by Ba2+ would have been more limited. As the SiO2 content of buddingtonite is higher than that of K-feldspar, the SiO2 content of Ba-poor K-feldspar will increase with the substitution of K+ by ![]() ${\rm NH}_{\rm 4}^{\rm +} $, and this is consistent with the obvious negative correlation between SiO2 and K2O in the Ba-poor K-feldspars (Fig. 4a). In contrast, a decrease in SiO2 content accompanies the substitution of K+ by Ba2+, as celsian has a lower SiO2 content than K-feldspar. This process would be expected to produce a negative correlation between the SiO2 and BaO contents of the Ba-poor K-feldspars. However, as the substitution of K+ by Ba2+ is much weaker than that by
${\rm NH}_{\rm 4}^{\rm +} $, and this is consistent with the obvious negative correlation between SiO2 and K2O in the Ba-poor K-feldspars (Fig. 4a). In contrast, a decrease in SiO2 content accompanies the substitution of K+ by Ba2+, as celsian has a lower SiO2 content than K-feldspar. This process would be expected to produce a negative correlation between the SiO2 and BaO contents of the Ba-poor K-feldspars. However, as the substitution of K+ by Ba2+ is much weaker than that by ![]() ${\rm NH}_{\rm 4}^{\rm +} $, the negative correlation would be obscured by the changes in SiO2 content caused by the substitution of K+ by
${\rm NH}_{\rm 4}^{\rm +} $, the negative correlation would be obscured by the changes in SiO2 content caused by the substitution of K+ by ![]() ${\rm NH}_{\rm 4}^{\rm +} $, and this explains why only a weak negative trend can be recognized on the BaO–SiO2 plot (Fig. 4b).
${\rm NH}_{\rm 4}^{\rm +} $, and this explains why only a weak negative trend can be recognized on the BaO–SiO2 plot (Fig. 4b).
Cymrite
Cymrite, BaAl2Si2O8·nH2O, where n = 0.5–1.0 (Back et al., Reference Back, Mandarino and Fleischer2008), is a hydrated silicate mineral rarely found in nature (e.g. Hsu, Reference Hsu1994; Moro et al., Reference Moro, Cembranos and Fernandez2001). Its occurrence is usually associated with metasedimentary or hydrothermal ore deposits, as well as late-stage low-grade metamorphism (Matsubara and Kato, Reference Matsubara and Kato1991; Hsu, Reference Hsu1994; Moro et al., Reference Moro, Cembranos and Fernandez2001; Sorokhtina et al., Reference Sorokhtina, Chukanov, Voloshin, Pakhomovsky, Bogdanova and Moiseev2008). It can crystallize under hydrothermal conditions corresponding to a low-grade dynamothermal metamorphism, and develops as crystals in rock cavities (Matsubara and Kato, Reference Matsubara and Kato1991; Hsu, Reference Hsu1994; Moro et al., Reference Moro, Cembranos and Fernandez2001; Sorokhtina et al., Reference Sorokhtina, Chukanov, Voloshin, Pakhomovsky, Bogdanova and Moiseev2008).
In our samples, the cymrite occurs as tabular crystals that are dispersed in the intergranular voids or micro-cracks of chert (Fig. 5a and Fig. 7), and this occurrence suggests it formed as a result of hydrothermal activity (Sorokhtina et al., Reference Sorokhtina, Chukanov, Voloshin, Pakhomovsky, Bogdanova and Moiseev2008), consistent with the mechanism of formation proposed for the Ba-rich K-feldspars in the chert (see the section ‘Formation mechanisms’). However, thin section observations and EMP analyses have shown that metamorphic minerals such as chlorite or muscovite are absent from our samples, indicating a lack of any significant regional metamorphism (Bucher and Grapes, Reference Bucher and Grapes2011). Therefore, the presence of cymrite in our samples demonstrates that it can be stable under very low P–T (sub-metamorphic) conditions (Graham et al., Reference Graham, Tareen, Mcmillan and Lowe1992; Hsu, Reference Hsu1994). Moreover, no celsian has been detected in the cymrite-bearing cherts, possibly because the dehydration of cymrite requires higher P–T conditions.
Micro-cracks would have assisted in the transportation and accumulation of Ba-rich fluids, where abundant cymrite and Ba-rich K-feldspars were formed (Fig. 7b). BaAl2Si2O8·4H2O is believed to be the precursor mineral of cymrite (Jakobsen, 1990), and the occurrence of both Ba-rich K-feldspar and BaAl2Si2O8·4H2O in unmetamorphosed rocks of the Navarana Fjord area, North Greenland, demonstrates that the formation of Ba-rich K-feldspar can precede the formation of cymrite under very low P–T conditions. This explains the overgrowth of cymrite on the Ba-rich K-feldspar, as shown in Fig. 7c.
Conclusions
Barium-rich silicates in the black shale sequence of the Hetang Formation, Anhui Province, South China, include Ba-poor K-feldspar, Ba-rich K-feldspar and cymrite. The Ba-poor K-feldspars have BaO contents that are <1.00 wt.%, and occur as grains in mudstone. The BaO contents of the Ba-rich K-feldspars are much higher, ranging from 1.36 to 20.51 wt.%. The Ba-rich K-feldspars occur mainly as rims around Ba-poor K-feldspar grains in mudstone, as void fillings in dolostone, and as dispersed grains in chert. Veins composed of Ba-rich K-feldspar and quartz are common in the mudstone and dolostone. The Ba-poor K-feldspars and Ba-rich K-feldspars have non-stoichiometric compositions, and EMP analyses yield compositions that total <100 wt.%. We suggest this is due to the incorporation of ![]() ${\rm NH}_{\rm 4}^{\rm +} $ and H2O into the feldspars, based on the results of Raman and IR spectroscopy. The cymrite in our samples occurs as tabular crystals that are dispersed in chert, and it has compositions that are comparable to those reported in the literature (Reinecke, Reference Reinecke1982; Jakobsen, Reference Jakobsen1990; Moro et al., Reference Moro, Cembranos and Fernandez2001).
${\rm NH}_{\rm 4}^{\rm +} $ and H2O into the feldspars, based on the results of Raman and IR spectroscopy. The cymrite in our samples occurs as tabular crystals that are dispersed in chert, and it has compositions that are comparable to those reported in the literature (Reinecke, Reference Reinecke1982; Jakobsen, Reference Jakobsen1990; Moro et al., Reference Moro, Cembranos and Fernandez2001).
We suggest that the formation of the Ba-silicates in our samples was related to multiple episodes of hydrothermal activity. The Ba-poor K-feldspars probably formed through interactions between pre-existing K-feldspars and Ba-rich hydrothermal fluids. The Ba-rich K-feldspars in the mudstones and dolostones might have formed mainly as a result of the infiltration of Ba-rich hydrothermal fluids into the sediments when they were still poorly consolidated. The Ba-rich K-feldspars and the cymrite in the cherts might have formed during diagenesis from Ba–Al–Si gels of exhalative hydrothermal origin. These proposed mechanisms of formation are consistent with a tectonic setting of extension in South China during the Ediacaran–Cambrian transition (Wang and Li, Reference Wang and Li2003; Chen et al., Reference Chen, Wang, Qing, Yan and Li2009).
Acknowledgements
This study was supported financially by National Natural Science Foundation of China (Grant No. 41230312 and 41573054) and Major State Basic Research Development Program of China (973 Project, Grant No. 2012CB214803). Special thanks to Dr. Michael M. Raith and Dr. Zuzana Tasaryova for their suggestions on re-checking the compositions of the minerals and the explanations of the special compositional characteristics. Also, we want to thank Dr. Wenlan Zhang, Dr. Bin Wu and Haoran Dou for their help with EMP analyses, and Dr. Tong He for his help with IR spectra acquisition. The samples studied were provided by the East China Branch and Petroleum Bureau, SINOPEC, which is deeply appreciated.