Longitudinal studies of the etiology of externalizing psychopathology suggest that children with early-starting conduct problems are much more likely than their peers to engage in antisocial behavior and alcohol and substance abuse as adults (Moffitt, Reference Moffitt1993; Patterson, Reid, & Dishion, Reference Patterson, Reid and Dishion1991). Randomized prevention trials have produced compelling evidence that early intervention can interrupt this developmental progression of externalizing behavior and shift children onto more adaptive trajectories (Conduct Problems Prevention Research Group [CPPRG], 1999, 2002, 2004, 2007, 2009, 2010, 2011, 2014). A critical next step for externalizing prevention research is to identify sources of heterogeneity in intervention response, including but not limited to genetic moderators (van IJzendoorn et al., Reference van IJzendoorn, Bakermans-Kranenburg, Belsky, Beach, Brody and Dodge2011). One impetus for investigating genetic moderation of intervention effects is that identified Gene × Intervention (G × I) interactions can be translated to target “precision” interventions, for example, genetic testing to determine Warfarin treatment (Epstein et al., Reference Epstein, Moyer, Aubert, O'Kane, Xia and Verbrugge2010). However, it remains unclear whether such precision is possible in the case of complex, long-running behavioral interventions. Even if precision targeting is possible, feasibility and ethical challenges remain unresolved.
We propose an alternative reason to examine G × I interactions is that they can elucidate mechanisms through which interventions operate. Identified G × I interactions can be used to examine how risk/susceptibility within a biological substrate manifests over developmental time. Following this logic, we envision a critical role for “developmental backtracking” studies that explicate the meaning of discovered G × I. This approach builds on prior developmental analyses of genetic main effects (Belsky et al., Reference Belsky, Moffitt, Houts, Bennett, Biddle and Blumenthal2012, Reference Belsky, Moffitt, Baker, Biddle, Evans and Harrington2013; Belsky, Moffitt, & Caspi, Reference Belsky, Moffitt and Caspi2013). The broad approach we envision involves three steps that follow the initial identification of a G × I effect: (a) test genetic main effects on pretreatment manifestations of risk for the intervention target; (b) test G × I effects on proximal developmental phenotypes measured between the initiation of treatment and the time of final outcome assessment; and (c) test the hypothesis that G × I effects on proximal developmental phenotypes mediate the G × I effect on the long-term outcome. Here, we apply this developmental backtracking approach to study genetic heterogeneity in the effects of the Fast Track prevention trial, a 10-year intervention that aimed to prevent kindergarteners with early-starting conduct problems from developing persistent externalizing psychopathology. The Fast Track intervention design was based on evidence that children with early-starting conduct problems are at increased risk for long-term externalizing psychopathology due to a dynamic cascade of proximal adjustment problems in childhood and adolescence (CPPRG, 1992; Dodge, Greenberg, Malone, & CPPRG, Reference Dodge, Greenberg and Malone2008). Our aim is to elucidate the proximal processes by which genotype and the Fast Track intervention interact to produce long-term outcomes.
Background: Differential Susceptibility to Intervention
There is emerging evidence that the same children who are most vulnerable to adverse developmental outcomes are also the most likely to benefit from improvements in the quality of their environment (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Ellis, Boyce, Belsky, Bakermans-Kranenburg and van IJzendoorn2011). These children demonstrate elevated responsiveness to their social environments. In high-risk environments, these children fare poorly. However, when environmental conditions are good, they flourish. This “for better and for worse” phenomenon has been termed biological sensitivity to context (Boyce et al., Reference Boyce, Chesney, Alkon, Tschann, Adams and Chesterman1995) or differential susceptibility (Belsky, Reference Belsky1997). The sensitive/susceptible child is characterized by difficult temperament and heightened negative emotionality (Belsky, Bakermans-Kranenburg, & van IJzendoorn, Reference Belsky, Bakermans-Kranenburg and van IJzendoorn2007; Belsky & Pluess, Reference Belsky and Pluess2009) and by heightened physiological responses to social stressors (Boyce & Ellis, Reference Boyce and Ellis2005). There is also evidence that sensitivity/susceptibility may be influenced by genetic factors. Polymorphisms in genes related to neurotransmitter function have received substantial attention in this research (Bakermans-Kranenburg & van IJzendoorn, Reference Bakermans-Kranenburg and van IJzendoorn2006, Reference Bakermans-Kranenburg and van IJzendoorn2011; Belsky & Pluess, Reference Belsky and Pluess2013; Kochanska, Philibert, & Barry, Reference Kochanska, Philibert and Barry2009; Mitchell et al., Reference Mitchell, Notterman, Brooks-Gunn, Hobcraft, Garfinkel and Jaeger2011, Reference Mitchell, Hobcraft, McLanahan, Siegel, Berg and Brooks-Gunn2014; Sheese, Voelker, Rothbart, & Posner, Reference Sheese, Voelker, Rothbart and Posner2007).
A new frontier in genetically informed differential susceptibility research is the use of randomized trials (van IJzendoorn et al., Reference van IJzendoorn, Bakermans-Kranenburg, Belsky, Beach, Brody and Dodge2011). Experimental randomization of exposure (i.e., the intervention) overcomes several of the limitations of observational Gene × Environment research, including potential confounds arising from gene–environment correlation (e.g., genetically influenced selection or evocation of environments) and omitted variable bias. Initial support for the utility of the G × I design comes from studies demonstrating genetic moderation of response to single-domain, time-limited interventions focused on preschool literacy skills (Kegel, Bus, & van IJzendoorn, Reference Kegel, Bus and van IJzendoorn2011), positive parenting (Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman, & Juffer, Reference Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman and Juffer2008), and prevention of alcohol abuse among adolescents (Brody, Chen, & Beach, Reference Brody, Chen and Beach2013). Here, we apply the G × I design to the Fast Track prevention trial, a longer-running, multicomponent intervention to prevent the development of externalizing psychopathology in high-risk children in kindergarten.
The Glucocorticoid Receptor Gene as a Candidate Moderator of Intervention Response
We focused our investigation on the gene encoding glucocorticoid receptor (NR3C1; to which the hormone cortisol binds) because physiological reactivity to stress has been identified as a hallmark of differential susceptibility. The glucocorticoid receptor plays a critical role in the human stress response; cortisol binding to glucocorticoid receptors in the hippocampus, amygdala, and other limbic structures provides negative feedback to the hypothalamic–pituitary–adrenal (HPA) axis response (DeRijk, van Leeuwen, Klok, & Zitman, Reference DeRijk, van Leeuwen, Klok and Zitman2008). Glucocorticoid receptor function influences short- and long-term adaptations of the HPA axis to environmental challenge and stress (McEwen, Reference McEwen2012; Meaney, Reference Meaney2001; Sapolsky, Romero, & Munck, Reference Sapolsky, Romero and Munck2000). Dysregulated glucocorticoid signaling has been implicated in child and adult manifestations of externalizing psychopathology (Fardet, Peteren, & Nazareth, Reference Fardet, Petersen and Nazareth2012; Hawes, Brennan, & Dadds, Reference Hawes, Brennan and Dadds2009; Lopez-Duran, Olson, Hajal, Felt, & Vazquez, Reference Lopez-Duran, Olson, Hajal, Felt and Vazquez2009; McBurnett et al., Reference McBurnett, Lahey, Frick, Risch, Loeber and Hart1991; Savitz, Lucki, & Drevets, Reference Savitz, Lucki and Drevets2009; Stadler, Poustka, & Sterzer, Reference Stadler, Poustka and Sterzer2010; van Zuiden et al., Reference van Zuiden, Geuze, Willemen, Vermetten, Maas and Heijnen2011). Particularly relevant to the current study is evidence that children exhibiting low cortisol reactivity to experimental challenge respond less favorably than high cortisol-reactive children to an intervention designed to reduce disruptive behavior (van de Wiel, van Goozen, Matthys, Snoek, & van Engeland, Reference van de Wiel, van Goozen, Matthys, Snoek and van Engeland2004).
Polymorphisms in NR3C1 have been associated with glucocorticoid resistance and reduced negative feedback of the HPA axis (DeRijk et al., Reference DeRijk, van Leeuwen, Klok and Zitman2008, Manenschijn, van den Akker, Lamberts, & van Rossum, Reference Manenschijn, van den Akker, Lamberts and van Rossum2009), as well as high cortisol-reactivity to stress (Kumsta et al., Reference Kumsta, Entringer, Koper, van Rossum, Hellhammer and Wust2007, Reference Kumsta, Moser, Streit, Koper, Meyer and Wust2009; van West et al., Reference van West, Del-Favero, Deboutte, Van Broeckhoven and Claes2010). At the level of psychopathology, NR3C1 variants are associated with child-onset mood disorder (Mill et al., Reference Mill, Wigg, Burcescu, Vetro, Kiss and Kapornai2009), adolescent alcohol abuse (Desrivieres et al., Reference Desrivieres, Lourdusamy, Muller, Ducci, Wong and Kaakinen2011), and adult major depression (van Rossum et al., Reference van Rossum, Binder, Majer, Koper, Ising and Modell2006; van West et al., Reference van West, Van Den Eede, Del-Favero, Souery, Norrback and Van Duijn2006; Zobel et al., Reference Zobel, Jessen, von Widdern, Schuhmacher, Hofels and Metten2008). NR3C1 variants have also been associated with differential response to environmental exposure, including greater incidence of depression among individuals exposed to adversity (Bet et al., Reference Bet, Penninx, Bochdanovits, Uitterlinden, Beekman and van Schoor2009) and irregular cortisol reactivity and behavior problems among the offspring of mothers with prenatal psychological symptoms (Velders et al., Reference Velders, Dieleman, Cents, Bakermans-Kranenburg, Jaddoe and Hofman2012).
Based on this evidence, we hypothesized that NR3C1 genotypes would differentiate individuals with a for better and for worse sensitivity to Fast Track intervention. Specifically, we hypothesized that NR3C1 genotypes would identify children with the lowest rates of externalizing psychopathology in the intervention condition and with the highest rates of externalizing psychopathology in the control condition. We found support for this hypothesis in our previous report, which showed that adult outcomes of the Fast Track intervention varied based on participants' NR3C1 genotype (Albert et al., in press). We briefly review this discovery analysis below.
G × I Discovery Analysis
Our discovery analysis tested whether the Fast Track intervention was more efficacious for children who carried specific NR3C1 variants. The outcome was externalizing psychopathology at age 25. We defined any externalizing psychopathology based on diagnostic assessment of antisocial personality disorder, attention-deficit/hyperactivity disorder, alcohol abuse disorder, marijuana abuse, and serious drug use. Analyses were conducted separately in European American and African American children in the Fast Track randomized control trial (RCT) to account for allele frequency differences between the two populations.
We selected NR3C1 test variants based on a haplotype tagging analysis, a hypothesis-free approach that surveys common variation throughout the gene (Dick, Reference Dick2011; Dick, Latendresse, & Riley, Reference Dick, Latendresse and Riley2011). Haplotype tagging identified 10 single nucleotide polymorphisms (SNPs) in NR3C1, which were genotyped in the Fast Track sample (online-only supplemental Figure S.1). We used linear probability models to test the intervention-moderating effect of each of these 10 SNPs. An adjusted Bonferroni correction was used to account for multiple testing (Nyholt, Reference Nyholt2004).
Across all genotypes, children randomly assigned to the Fast Track intervention were less likely to manifest any externalizing psychopathology at age 25 years than were children randomized to the control condition (for European American children, 46% of the treated group as compared to 61% of the control group manifested externalizing psychopathology, p = .02; for African American children, 35% in the intervention group as compared to 58% in the control group manifested externalizing psychopathology, p < .001). Among European American children, the effect of intervention was moderated by variation in NR3C1; intervention was more efficacious in preventing externalizing psychopathology for carriers of the rs10482672 A allele. Among carriers of the A allele, 18% of treated children as compared to 75% of control children manifested any externalizing psychopathology at age 25 follow-up. In contrast, for noncarriers of the A allele, 56% of treated children and 57% of control children manifested externalizing psychopathology at follow-up. Among African American children, there was no evidence that NR3C1 SNPs moderated Fast Track intervention effects.
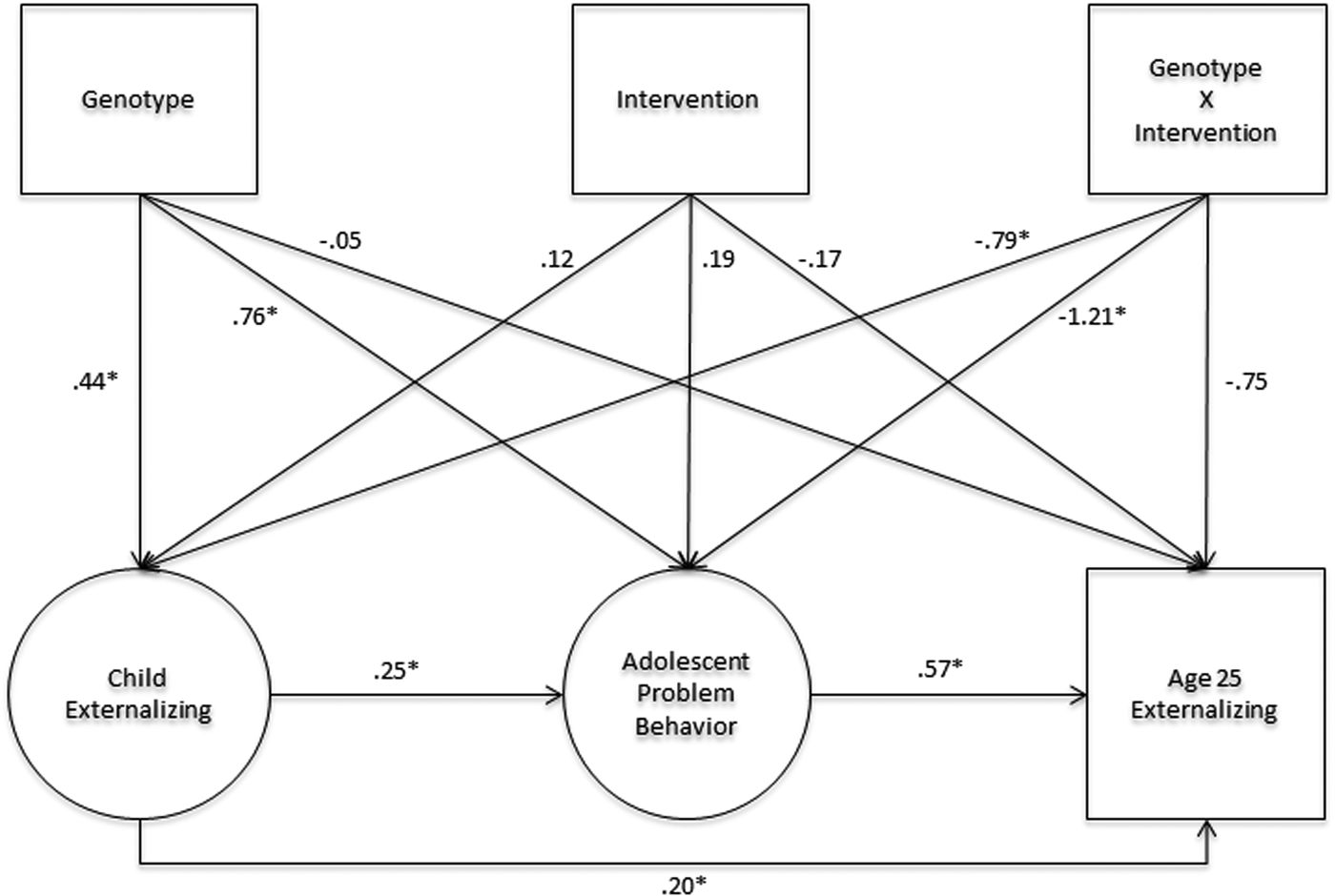
In the analyses reported in this article, we test the hypothesis that the G × I between NR3C1 SNP rs10482672 and Fast Track treatment operates via changes to children's behavior in childhood and adolescence using the three-step developmental backtracking approach outlined above. In Step 1, we test genetic main effects on the social adjustment of Fast Track participants in kindergarten, prior to their enrollment in the intervention trial. In Step 2, we test G × I effects on proximal developmental phenotypes of externalizing psychopathology during primary school and during middle and high school. In Step 3, we test our mediation hypothesis: that G × I effects on externalizing phenotypes in primary, middle, and high school mediate G × I effects on externalizing psychopathology at age 25 years. Figure 1 illustrates the conceptual framework. We interpret findings in light of developmental theories of the etiology of externalizing psychopathology and the role of the stress response system in vulnerability and in susceptibility to positive developmental influences.

Figure 1. (Color online) Conceptual framework for tests of direct and indirect prevention effects on adult externalizing.
Methods
Setting: The Fast Track prevention trial
The Fast Track prevention trial was implemented in the early 1990s to test whether the developmental outcomes of young children at high risk for long-term antisocial behavior could be improved through random assignment to a sustained, multicomponent behavioral intervention (CPPRG, 1999). Intervention design was based on two critical insights derived from longitudinal research on the etiology of persistent externalizing behavior (CPPRG, 1992). First, children at risk for antisocial behavior as adults are identifiable at school entry by their conduct problems in home and school settings; although not all conduct-disordered children will become antisocial adults, almost all antisocial adults have a history of childhood conduct problems (Robins, Reference Robins1966; CPPRG, 1999). Second, the pathway from early risk to later disorder is comprehensible as a dynamic cascade of adjustment problems, as failure at one developmental stage begets failure in the next, and so on, leading to increasing isolation from positive aspects of family, school, and peers (Dodge et al., Reference Dodge, Greenberg and Malone2008). High-risk children typically enter school with a risk burden that crosses multiple domains. Socioeconomic disadvantage and dysfunctional parenting contribute to escalating conduct problems at home (Dodge & McCourt, Reference Dodge and McCourt2010). Deficits in self-control and emotion regulation undermine social adjustment and academic performance at school (Moffitt, Reference Moffitt1993). These early adjustment problems increase risk for social rejection and academic failure in elementary school, association with deviant peers, and delinquency, violence, and substance abuse in adolescence and young adulthood (Dodge et al., Reference Dodge, Greenberg and Malone2008). Based on these foundational insights, the creators of the Fast Track intervention reasoned that effective prevention should begin no later than school entry, should be sustained from childhood through early adolescence, and should target the risk factors that are most salient at each developmental period (CPPRG, 1992).
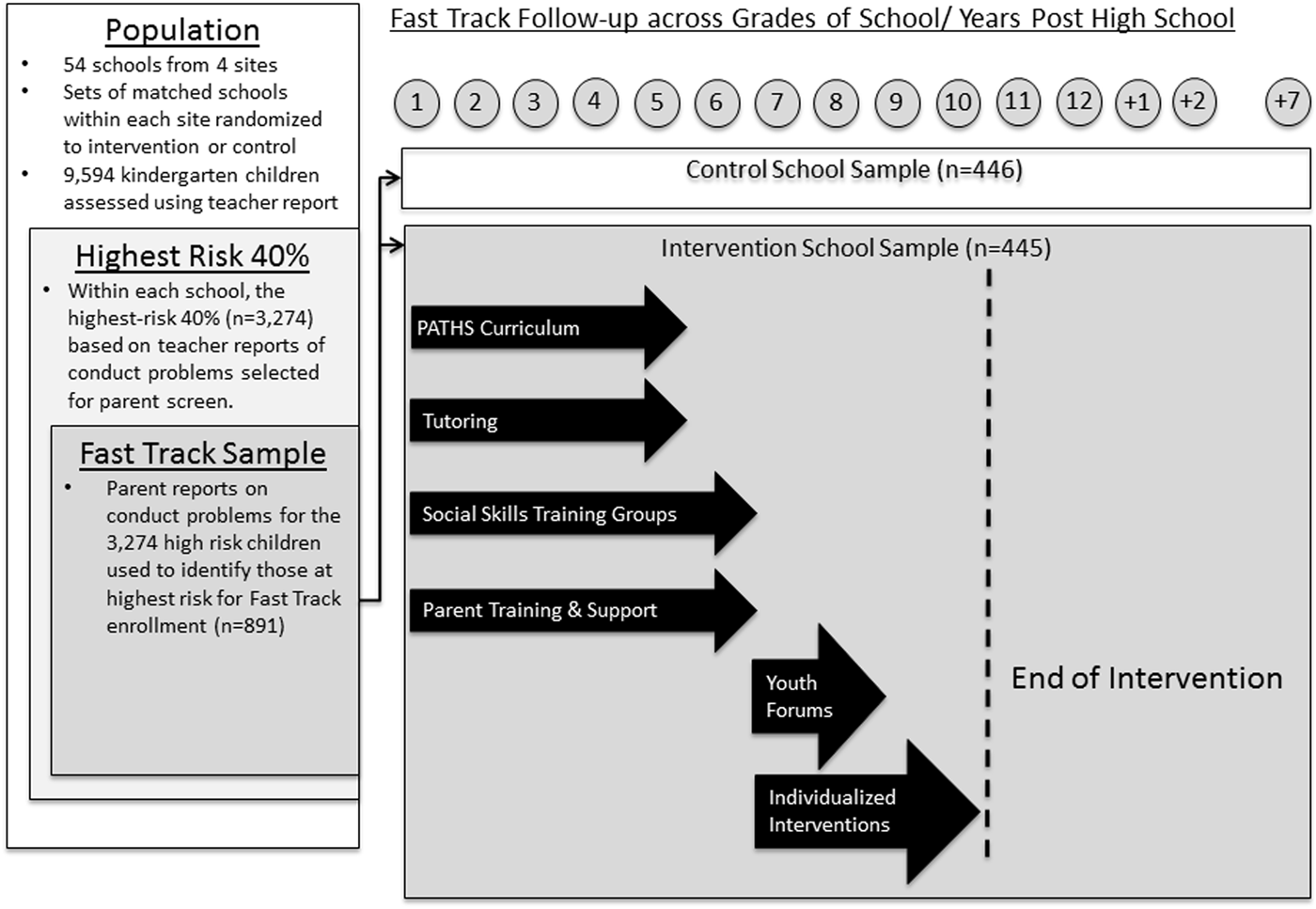
Implemented as a multisite RCT, the Fast Track trial used a multiple-gating screening procedure to select 891 children with very high levels of conduct problems at the time of school entry, and randomly assigned them to a no-treatment control condition or an intervention condition that provided them with 10 consecutive years of prevention services (Grades 1–10; see Figure 2 for further details). Programming during the elementary school years addressed the social cognitive, emotional, and self-control deficits that contribute to aggression toward peers, social rejection, academic failure, and disruptive and oppositional behavior toward authorities. Later programming targeted prevention toward salient issues at critical developmental transitions; for example, programming for the middle-school transition addressed parental supervision and adolescent decision making relevant to alcohol, tobacco, and substance use. Previously published intent to treat analyses of Fast Track demonstrated its success in reducing externalizing behavior across the elementary school, high school, and young adult years (CPPRG, 1999, 2002, 2004, 2007, 2011, 2014), with less robust effects during middle school (CPPRG, 2010). The most pronounced impacts of the Fast Track intervention have been observed in the subgroup of children who displayed the most severe conduct problems at school entry (CPPRG, 2011).

Figure 2. Fast Track randomized controlled trial design.
The Fast Track study included both longitudinal study of a community sample and an RCT of intervention with high-risk children. The intervention was a comprehensive prevention program for children at high risk for persistent antisocial behavior delivered over a 10-year period, when participating children were in the first through the tenth grades. Three successive cohorts of kindergarten children were enrolled in an RCT in 1991, 1992, and 1993 to yield a sample of 891 children (445 in the intervention group and 446 in the control group). Figure 2 illustrates the Fast Track design. Detailed description of Fast Track is available at www.fasttrackproject.org and in published evaluations (CPPRG, 1999, 2000, 2002, 2004, 2007, 2011).
Children were selected from each of four geographic sites: Durham, NC; Nashville, TN; rural PA; and Seattle, WA. Elementary schools (n = 55) in neighborhoods with very high rates of crime and economic disadvantage were divided into paired sets (one to three sets per site) matched for demographics, and one set was randomly assigned to intervention and one to control.
A multiple-gating screening procedure that combined teacher and parent ratings of aggressive–disruptive behavior was applied to all 9,594 kindergarteners in three cohorts (1991, 1992, and 1993). The first gate relied on teacher-reported classroom conduct problems, using the Teacher Observation of Child Adjustment—Revised authority acceptance score. Children scoring in the highest 40% within cohort and site were solicited for the second gate of screening: parent-rated home behavior problems, using a 22-item instrument based on the Child Behavior Checklist. Teacher and parent scores were standardized within site and summed to yield a severity of risk screen score.
Children were selected for the study based on this risk score, moving from highest down until desired sample sizes were reached within sites, cohorts, and conditions. Nine hundred seventy-nine children (10% of total) were solicited to yield a sample of 891 participating children (91% consent; intervention n = 445; control n = 446). At selection, participant mean age was 6.58 years (SD = 0.48). Ethnicity varied (51% African American, 47% European American, and 2% other ethnicity), and 69% were boys. The mean externalizing problem score for the Teacher's Report Form of the Child Behavior Checklist was 1.6 SD above the national mean. The sample was high risk in many ways: 58% had single parents, 29% of parents had not completed high school, and 35% of families were in the lowest socioeconomic class.
Written informed consent from parents and oral assent from children were obtained. Parents were paid for completing interviews, and intervention group parents were paid for group attendance. All procedures were approved by the institutional review boards of participating universities.
Elementary school phase (Grades 1–5)
During Grades 1–5, intervention families were offered group intervention during a 2-hr “enrichment program” that included children's social-skill “friendship groups,” parent-training groups, guided parent–child interaction sessions, and paraprofessional tutoring in reading. Tutors provided three additional 30-min sessions per week in reading and peer pairing to improve friendships with classmates. Teacher consultation and a Fast Track adaptation of the teacher-implemented PATHS curriculum that addresses social-cognitive skill development were implemented universally in Grade 1–5 classrooms in intervention schools (except Durham, where it was prohibited) to promote social–emotional competence. Enrichment programs were held weekly during Grade 1, biweekly during Grade 2, and monthly during Grades 3–5. In addition, home visiting helped parents generalize their skill learning and address individual needs. After Grade 1, criterion-referenced assessments adjusted the prescribed dosage to match need.
Middle and early high school phase (Grades 6–10)
During Grades 5 and 6, children received a middle school transition program and four parent–youth groups on topics of adolescent development: alcohol, tobacco, drugs, and decision making. In Grades 7 and 8, eight Youth Forums addressed vocational opportunities, life skills, and summer employment opportunities. In Grades 7–10, individualized interventions addressed parent monitoring, peer affiliation, academic achievement, and social cognition. All children received Oyserman's school to jobs, possible selves intervention aimed at examining emerging identity.
Intervention participation
Ninety-six percent of parents and 98% of children attended at least one group session during Grade 1. Of these families, 79% of parents and 90% of children attended at least 50% of prescribed group sessions. Participation decreased modestly across years, primarily due to residential moves. In Grades 7–10, intervention continued with at least 80% of all children.
High intervention fidelity was ensured by manualization, regular cross-site training, and weekly clinical supervision. Outside interventions were neither encouraged nor discouraged. The full protocol can be found at http://www.fasttrackproject.org.
Genotyping
Fast Track collected DNA from participants at the age 21 follow-up. DNA was obtained from buccal cells collected using a cytology brush. DNA extraction was performed by Penn State University. Genotyping was performed by the Virginia Institute for Psychiatric and Behavioral Genetics. Genotyping was conducted using commercially available primer and probe sequences from TaqMan Assays-on-Demand (Applied Biosystems, Foster City, CA). Duplicate genotyping produced concordance rates of 100 percent. The NR3C1 SNP rs10482672 was successfully genotyped for 94.6% of the DNA samples and was in Hardy–Weinberg equilibrium (p = 1.0).
Sample
We analyzed data from all European American Fast Track participants with available NR3C1 genotype data. (This same sample formed the basis of our earlier report, Albert et al., in press.) Of 439 European American participants enrolled in the Fast Track RCT, 62% (n = 270) provided a DNA sample that was successfully genotyped at NR3C1; 98% (N = 260) of this genetic sample provided data on Time 1 measures of psychosocial functioning, and 90% (N = 242) were interviewed at age 25 (treatment n = 114; control n = 128). Attrition analyses comparing the age 25 analytic sample of N = 242 to the complete European American Fast Track sample of N = 439 on the preintervention severity of risk score used to screen children into the Fast Track RCT found no statistically significant differences between the full Fast Track sample and the analytic sample for either treated or control children (p values = .835). Preintervention severity of risk score did not differ between control and treated children within the analytic sample (p = .237).
Measures
Interviews were conducted annually with participants and their parents and teachers during the school years of the trial, and at age 25 with participants and a peer who knew the participant well.
Preintervention measures of psychosocial functioning
Parent ratings on the Child Behavior Checklist and teacher ratings on the parallel Teacher Report Form (Achenbach, Reference Achenbach1991) were collected in the summer following kindergarten, before the start of the Fast Track RCT in first grade. We utilize T scores for the externalizing and internalizing broadband scales and the following subscales: anxious/depressed, social problems, somatic complaints, withdrawn, thought problems, delinquency attention problems, and aggression. Scores were computed as the average of parent and teacher report. We also utilize the severity of risk screen score described above.
Diagnostic assessments of child externalizing psychopathology
We used the Parent Interview version of the NIMH Diagnostic Interview Schedule for Children to assess DSM-IV disorders in the summers following children's completion of Grades 3 and 6. The Diagnostic Interview Schedule for Children is a highly structured, laptop-administered, clinical interview that is well validated for assessing disorders in children and adolescents aged 6–18 years. We used version 2.3 after Grade 3, following the published anticipated DSM-IV criteria for diagnosis, and version IV after Grade 6 (Shaffer, Fisher, Lucas, & Comer, Reference Shaffer, Fisher, Lucas and Comer2003). Condition-blind lay interviewers were trained in clinical methods and scoring accuracy by an expert clinical psychologist until demonstrating proficiency. Parent interviews were administered in the child's home with the primary parent, usually the mother.
Following recommendations, at the Grade 3 assessment, criteria were solicited for the past 6 months for oppositional defiant disorder (ODD) and attention-deficit/hyperactivity disorder (ADHD), and for the past 12 months for conduct disorder (CD). Criteria were solicited for the past 12 months for all disorders at all subsequent assessments. The ADHD variable omitted DSM criteria based on age of onset and criteria in more than one setting. Criteria counts were computed for each of the three disorders (ODD, CD, and ADHD) at each assessment.
Measures of adolescent problem behavior
We assessed delinquent behaviors and alcohol and cannabis use at follow-ups from Grade 7 through 2 years post-high school. Delinquency was measured using the self-reported delinquency scale from the Denver Youth Study (Huizinga & Elliott, Reference Huizinga and Elliott1987), which measures involvement in property damage, theft, assault, and substance use. The score indexes the proportion of 25 general delinquency behaviors in which the child was involved. Alcohol use and cannabis use were measured using the Tobacco, Alcohol and Drugs (Grades 7–12) and Tobacco, Alcohol and Drugs—Revised (years 1 and 2 post-high school) assessment instruments (Bureau of Labor Statistics, 2002). For alcohol, the instrument measured the number of days consuming 5+ drinks and the number of days drunk in the past year. These two numbers were averaged to calculate days of problem drinking. For cannabis, the instrument measured days of cannabis consumption in the past month. Each score was computed as an average of the eight annual reports; participants were required to have nonmissing data for at least 50% of the assessments.
Diagnostic assessment of externalizing psychopathology at age 25 years
Externalizing psychopathology was assessed at age 25 using three standardized instruments administered to participants by condition-blind interviewers. Each participant was also invited to nominate a peer for an independent, confidential interview about the participant. Seven hundred two participants (81% of those living) and 535 peers (76% of participants, net 62% of total) provided data. Participation did not differ significantly by condition (ns = 352 control and 350 intervention). For each problem indicator defined below, we coded the problem as present (1) if either the participant or the informant interview responses met criteria, and not present (0) otherwise. The outcome of any externalizing psychopathology was defined as having any of the following externalizing mental health problems. Antisocial personality disorder and ADHD were defined by DSM-IV criterion items from the Adult Self-Report (Achenbach & Rescorla, Reference Achenbach and Rescorla2003) instrument used for participant interviews and the parallel Adult Behavior Checklist—Friend (Achenbach & Rescorla, Reference Achenbach and Rescorla2003) used for peer interviews. Alcohol abuse disorder was defined according to the Alcohol and Drug Module of the NIMH Diagnostic Interview Schedule (Robins, Helzer, Croughan, & Ratcliff, Reference Robins, Helzer, Croughan and Ratcliff1981) completed by participants and nominated peers. Marijuana abuse (defined as 27 or more days of use in the past month) and serious substance use (cocaine, crack, inhalants, heroin, LSD, PCP, ecstasy, mushrooms, speed, and other pills not prescribed by a physician in the past month) were defined from participant responses to the Tobacco, Alcohol and Drugs Version—III, a 57-item open-ended and forced-choice instrument based on measures from the National Longitudinal Study of Adolescent Health (Bureau of Labor Statistics, 2002) and from peer responses to an identical instrument adapted for this study.
Detailed documentation of all Fast Track measures is provided on the Fast Track website (http://www.fastrackproject.org/data-instruments.php).
Analyses
We conducted intent to treat analyses; that is, we treated all children randomized into the intervention condition as if they had received the full dosage of Fast Track intervention. Analyses proceeded in the three developmental backtracking steps outlined in the introduction. In Step 1, we tested genetic main effects on the social adjustment of Fast Track participants in kindergarten, prior to their enrollment in the intervention trial. In Step 2, we tested G × I effects on proximal developmental phenotypes of childhood externalizing psychopathology (Grades 3–6) and adolescent problem behavior (Grade 7 through 2 years following high school). In Step 3, we tested mediation of G × I effects on age 25 externalizing psychopathology by the developmental phenotypes analyzed in Step 2. Analyses for Step 1 were conducted using linear regression models. Analyses for Steps 2 and 3 were conducted using structural equation modeling approaches.
The structural equations used in the analysis Steps 2 and 3 modeled the childhood and adolescent outcomes as latent variables. The latent variable for childhood externalizing psychopathology was identified by six indicators corresponding to Grade 3 and Grade 6 parent-reported symptoms of CD, ODD, and ADHD. After freeing three pairs of error terms to covary (Grade 3 ADHD with Grade 6 ADHD; Grade 6 ADHD with Grade 6 ODD; and Grade 3 ADHD with Grade 6 CD), the measurement model showed adequate fit (comparative fit index = 0.99, root mean square error of approximation = 0.07). The latent variable for adolescent problem behavior was identified by indicators for alcohol use, cannabis use, and delinquency. Each indicator was calculated as the mean of self-reported behavior from annual assessments collected from Grade 7 through 2 years post high school. Because the measurement model included only three indicators, fit statistics could not be calculated. All indicators demonstrated large standardized factor loadings (>0.6). We standardized the scales of both latent variables to support interpretation of effects in terms of number of standard deviations (i.e., factor mean = 0, factor variance =1).
Step 2 structural equations modeled proximal developmental phenotypes as a function of main effects terms for genotype and intervention condition, a product term testing the G × I interaction, and a covariate for preintervention severity of risk. A simplified version of the model for a given proximal outcome is
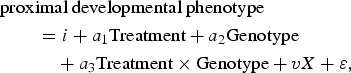 $$\eqalign{& \hbox{proximal developmental phenotype} \cr & \quad \quad =i + a_1 \hbox{Treatment} + a_2 {\rm Genotype} \cr & \quad \quad \quad + a_3 {\rm Treatment} \times {\rm Genotype} + vX + {\rm \varepsilon}\comma}$$
$$\eqalign{& \hbox{proximal developmental phenotype} \cr & \quad \quad =i + a_1 \hbox{Treatment} + a_2 {\rm Genotype} \cr & \quad \quad \quad + a_3 {\rm Treatment} \times {\rm Genotype} + vX + {\rm \varepsilon}\comma}$$where i is an intercept and X is a vector of covariates. G × I hypothesis tests were conducted with the a 3 coefficient. All analyses are reported using an additive genetic model (effects in terms of each additional susceptibility allele carried).
We probed significant G × I interactions to determine whether treatment effects were significantly different from zero for each of the three rs10482672 genotypes (0/1/2 A alleles). We estimated conditional treatment effects following the simple slopes approach described by Aiken and West (Reference Aiken and West1991):
Step 3 structural equations modeled the extent to which G × I effects on proximal developmental phenotypes mediated the G × I effect on age 25 externalizing psychopathology. Because this mediation analysis focused on an interaction effect, the model can formally be described as testing mediated moderation (Preacher, Rucker, & Hayes, Reference Preacher, Rucker and Hayes2007). To test mediated moderation, we fitted structural equation models that simultaneous analyzed two equations. The first equation models the mediator. In our case, this equation is identical to Equation 1, which estimates the G × I effect on a proximal developmental phenotype as a 3. The second equation estimates the effect of the G × I interaction and the proximal developmental phenotype variable on age 25 psychopathology as the following:
 $$\eqalign{& \hbox{age 25 psychopathology} \cr & = i + \acute{c}_1{\rm Treatment} + \acute{c}_2 {\rm Genotype} \cr & \quad + \acute{c}_3 {\rm Treatment} \times {\rm Genotype} \cr & \quad + b_1 \hbox{Developmental Phenotype} + vX + {\rm \varepsilon}\comma \; }$$
$$\eqalign{& \hbox{age 25 psychopathology} \cr & = i + \acute{c}_1{\rm Treatment} + \acute{c}_2 {\rm Genotype} \cr & \quad + \acute{c}_3 {\rm Treatment} \times {\rm Genotype} \cr & \quad + b_1 \hbox{Developmental Phenotype} + vX + {\rm \varepsilon}\comma \; }$$where b 1 is the effect of the proximal developmental phenotype, the second path in the indirect effect; and the ć coefficients are the “unmediated” direct effects of treatment, genotype, and Treatment × Genotype. We computed point estimates of indirect effects based on coefficient estimates derived from these equations, using the product of coefficients method (MacKinnon, Reference MacKinnon2008). The point estimate for mediated moderation is a 3 × b 1, which is the product of the Treatment × Genotype effect on the mediator (a 3) and the mediator effect on age 25 psychopathology (b 1; Preacher et al., Reference Preacher, Rucker and Hayes2007). We evaluated the statistical significance of indirect effect estimates using bias-corrected bootstrap confidence intervals (95%) based on 5,000 draws with replacement (Preacher & Hayes, Reference Preacher and Hayes2008). We estimated the magnitude of each significant indirect effect as a mediation ratio (indirect/total effects; Ditlevsen, Christensen, Lynch, Damsgaard, & Keiding, Reference Ditlevsen, Christensen, Lynch, Damsgaard and Keiding2005).
Structural equation models were estimated in Mplus version 7.1 (Muthén & Muthén, Reference Muthén and Muthén2010). Models testing effects on the binary any externalizing psychopathology outcome used the weighted least squares estimator with a probit link function. All other models used the maximum likelihood estimator. All structural equation models evaluated below showed excellent fit to the data (χ2p > .3, comparative fit index > 0.99, Tucker–Lewis index > 0.98, root mean square error of approximation < 0.02). Complete goodness of fit statistics are available in the online-only supplemental Table S.1.
Step 1. Test genetic main effects on pretreatment manifestations of risk for the intervention target
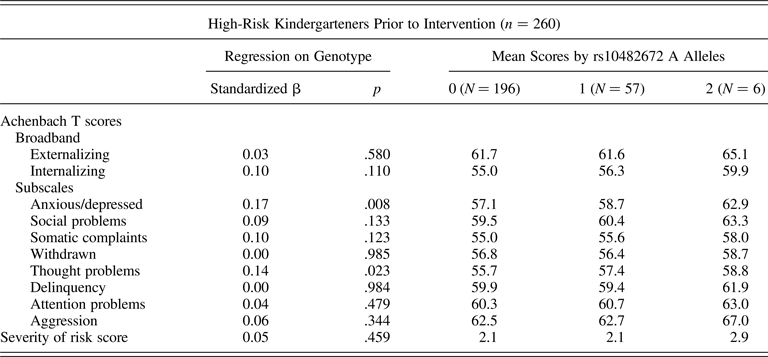
To begin our developmental analysis, we looked 20 years back in time to the initial Fast Track assessments. We asked whether children's rs10482672 genotype predicted differences in their psychosocial function at kindergarten entry, before the Fast Track intervention began. We tested whether children who carried more copies of the rs10482672 A allele exhibited worse psychosocial function as measured by the severity of risk score used to select children into the Fast Track trial and 10 target subscales of the Achenbach family of instruments. Because measurements were taken prior to randomization, analyses included the full Fast Track RCT sample (N = 260). Before the intervention began, children who carried more copies of the rs10482672 A allele were similar to their peers who carried fewer copies on the severity of risk score used to screen children into the Fast Track trial and on 8 of the 10 Achenbach scales, although in all cases, the children who carried two copies of the A allele had the highest scores. Children who carried more copies of the A allele did differ from peers who carried fewer copies on two Achenbach scales. As rated by their parents and their teachers, children who carried more A alleles exhibited more anxious/depressed behavior (β = 0.17, p = .008) and more thought problems (β = 0.14, p = .023) as compared to peers who carried fewer copies. Full regression results are included in Table 1.
Table 1. rs10482672 associations with psychosocial functioning at kindergarten entry

Note: Standardized beta coefficients can be interpreted as Pearson's r. Mean scores for Achenbach instruments are reported as T scores (population mean = 50, SD = 10). Because the Fast Track sample was selected from a high-risk segment of the population, the means of Achenbach externalizing scales for the Fast Track sample were above the general population mean of 50. The Severity of risk score is mean centered according to the scale mean observed in a normative sample drawn from the same schools as the Fast Track participants. In the normative sample, the centered scale has mean = 0 and SD = 1.5.
Because this main effect of genotype on children's anxious/depressed behavior and thought problems was not anticipated and because we conducted a relatively large number of tests, we sought to replicate the result in an independent sample, the Child Development Project (CDP; N = 363, 50% male; Dodge, Bates, & Pettit, Reference Dodge, Bates and Pettit1990). We analyzed rs10482672 genotype associations with psychosocial function in kindergarten, also measured via the Achenbach scales. In replication of what we observed in the Fast Track sample, CDP children who carried more copies of the rs10482672 A allele exhibited more anxious/depressed behavior as compared to peers who carried fewer copies (β = 0.15, p = .005). CDP children who carried more copies of the rs10482672 A allele also exhibited more withdrawn behavior (β = 0.11, p = .033) and were rated higher on the broadband internalizing scale (β = 0.13, p = .011) as compared to peers who carried fewer copies. Full details of this replication analysis are included in online-only supplemental Table S.2).
Step 2. Test G × I effects on proximal developmental phenotypes measured between the initiation of treatment and the time of final outcome assessment
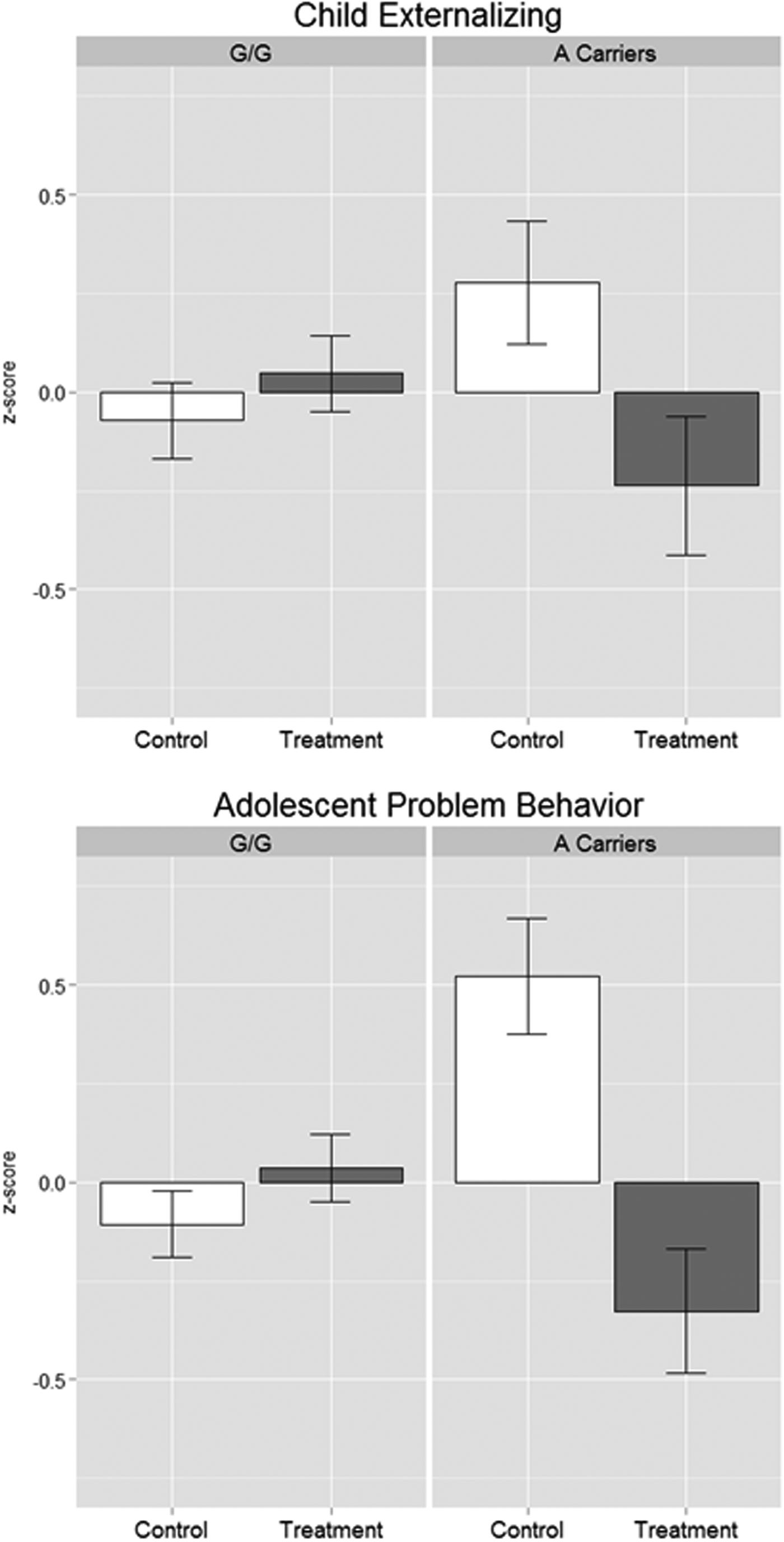
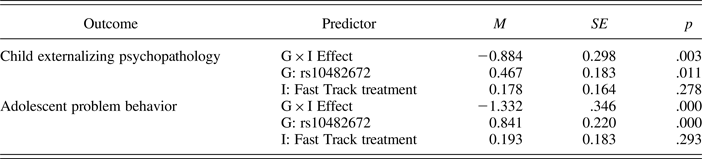
The second step in our developmental analysis examined G × I effects on proximal developmental phenotypes measured during childhood and adolescence. We began with an analysis of externalizing psychopathology in elementary school (Grades 3–6). In parallel to our analysis of age 25 psychopathology, we observed a for better and for worse G × I interaction between rs10482672 genotype and Fast Track treatment predicting children's externalizing psychopathology (Figure 3a). For each additional copy of the susceptibility allele a child carried, the Fast Track treatment decreased childhood externalizing psychopathology by 0.88 SD (p = .003). We quantified treatment effects for each genotype as described in Equation 2. There was no effect of Fast Track treatment on childhood externalizing psychopathology for children who carried no copies of the susceptibility allele (p = .278). For children who carried one susceptibility allele, the Fast Track treatment decreased childhood externalizing psychopathology by 0.71 SD (p = .007). For children who carried two susceptibility alleles, the Fast Track treatment decreased childhood externalizing psychopathology by 1.59 SD (p = .003). Full regression results are included in Table 2.

Figure 3. Gene × Intervention differences in latent factor means for child externalizing psychopathology and adolescent problem behavior. Standardized factor scores were extracted from unconditional confirmatory factor analysis models of child externalizing psychopathology and adolescent problem behavior, respectively. Factor indicators for the child externalizing factor include parent-reported symptom counts for conduct disorder, oppositional defiant disorder, and attention-deficit/hyperactivity disorder, ascertained via in-person diagnostic interviews in the summers following the child's third- and and sixth-grade years. Factor indicators for the adolescent problem behavior factor include three child-report scales, each of which aggregates reports across eight assessment years spanning the Grade 7 and 2 years post-high school. The three scales are (a) alcohol use, operationalized as number of past year binge-drinking days; (b) cannabis use, as number of past-month days of any use; and (c) self-reported delinquency (general). Measurement details are provided in the Methods Section.
Table 2. Gene × Intervention (G × I) interaction analyses of proximal developmental phenotypes

Note: The reported values are the coefficient estimates, standard errors, and p values estimated from structural equations modeling Gene × Intervention effects on child externalizing psychopathology and adolescent problem behavior. Models were adjusted for the baseline severity of risk score.
Next, we followed children in the Fast Track trial through their adolescent years. We again observed a for better and for worse G × I interaction between rs10482672 genotype and Fast Track treatment predicting adolescents' problem behavior from Grade 7 through the 2 years following the end of high school (Figure 3b). For each additional copy of the susceptibility allele a child carried, the Fast Track treatment decreased adolescent problem behavior by 1.33 SD (p < .001). There was no effect of Fast Track treatment on adolescent problem behavior for children who carried no copies of the susceptibility allele (p = .293). For children who carried one susceptibility allele, the Fast Track treatment decreased adolescent problem behavior by 1.14 SD (p < .001). For children who carried two susceptibility alleles, the Fast Track treatment decreased adolescent problem behavior by 2.47 SD (p < .001). Full regression results are included in Table 2.
Step 3. Test the hypothesis that G × I effects on proximal developmental phenotypes mediate the G × I effect on the long-term outcome
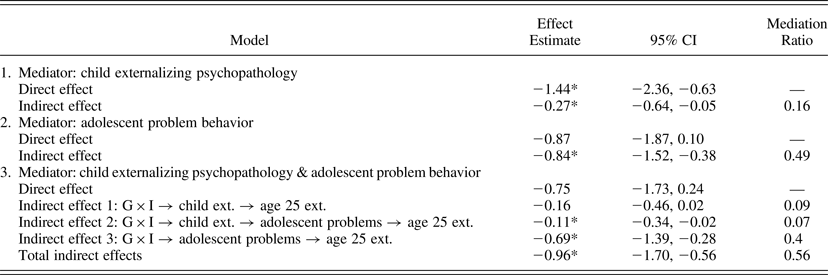
The third step in our developmental analysis tested the hypothesis that the G × I effects on proximal developmental outcomes analyzed in Steps 2 and 3 mediated the ultimate G × I effect on age 25 externalizing psychopathology. We estimated the mediated moderation equations (i.e., Equations 1 and 3) using both proximal developmental outcomes as mediators in turn. Developmental phenotypes measured in childhood and adolescence were statistically significant mediators of the G × I effect on age 25 externalizing psychopathology, accounting accounted for 16% and 49% of the total G × I effect, respectively (Table 3).
Table 3. Mediation of Gene × Intervention interaction (G × I) effects on age 25 externalizing psychopathology by G × I effects on proximal developmental phenotypes

Note: The models tested mediated moderation. Direct effects refer to the G × I effects on age 25 externalizing psychopathology that were independent of any G × I effect on the proximal developmental phenotype. Indirect effects refer to G × I effects on age 25 externalizing psychopathology that were mediated by the proximal developmental phenotypes. Models 1 and 2 tested mediated moderation for the two developmental phenotypes in turn. Model 3 tested mediated moderation when both developmental phenotypes were included simultaneously. In Model 3, indirect effect 3 can be interpreted as a portion of the G × I effect on age 25 externalizing psychopathology that is attributable to G × I effects on adolescent problem behavior only (i.e., not accounted for by G × I effects on child externalizing psychopathology).
*Effect estimates that are statistically significant.
The final step in our developmental analysis tested the hypothesis that a portion of the G × I effect on age 25 externalizing psychopathology was mediated by a unique G × I effect on adolescent problem behavior, net of the G × I effect on childhood externalizing psychopathology. We estimated a structural equation model that simultaneously evaluated mediation of G × I effects on age 25 externalizing psychopathology by the two proximal developmental mediators, as illustrated in Figure 4. A portion of the G × I effect on the adolescent developmental phenotype was independent of any G × I effect on the childhood developmental phenotype. In turn, this independent G × I effect on the adolescent developmental phenotype accounted for 40% of the total G × I effect on age 25 externalizing psychopathology. Point estimates and confidence intervals for direct and indirect effects are included in Table 3, Panel 3.

Figure 4. Path estimates of unique effects of genotype, intervention, and G × I effects on child, adolescent, and adult outcomes. The structural equation model showed strong fit to the data (χ2 = 64.20, df = 58, comparative fit index = 0.99, root mean square error of approximation = 0.02). All regressions covaried for the preintervention risk score used to screen children into the intervention. Latent variables for child externalizing psychopathology and adolescent problem behavior are standardized (factor mean = 0, variance = 1). Age 25 externalizing is modeled as a latent variable (mean = 0, variance = 1); scores represent the probability of positive case status on the binary any externalizing psychopathology outcome variable. *p < .05.
Discussion
We conducted a three-step developmental backtracking analysis to investigate mechanisms mediating genetic heterogeneity in the effects of the Fast Track intervention. We previously observed that carriers of the rs10482672 A allele responded to Fast Track in a for better and for worse fashion: at age 25 years, A carriers had the lowest risk of externalizing psychopathology if they were randomized to the Fast Track trial treatment arm and the highest risk of externalizing psychopathology if they were randomized to the control arm. Our developmental backtracking analyses revealed some evidence that A carriers were at increased risk at baseline, before the intervention began, although this risk manifested, not as externalizing psychopathology, but as anxious/depressed and thought problems symptoms. As we followed the children forward in time, the G × I effect emerged early on in the course of the intervention. G × I effects were detected for externalizing psychopathology measured at Grades 3–6. These effects persisted and grew larger in adolescence. During these intermediate developmental stages, A carriers in the treatment arm manifested the lowest levels of the developmental externalizing phenotypes, while A carriers in the control arm manifested the highest levels of the developmental externalizing phenotypes. In turn, G × I effects on child and adolescent developmental phenotypes mediated over half of the total G × I effect observed at the age 25 follow-up.
These findings have implications for how interventions to prevent externalizing psychopathology are theorized, designed, and evaluated, and for future research into the differential susceptibility hypothesis. The primary implication of our study for theories of early childhood intervention is that significant heterogeneity exists in how children at risk to develop externalizing psychopathology respond to a complex, long-running intervention like Fast Track, and that this heterogeneity has something to do with stress biology, specifically glucocorticoid signaling. Glucocorticoid signaling is traditionally studied in relation to internalizing psychopathology (e.g., Owens et al., Reference Owens, Herbert, Jones, Sahakian, Wilkinson and Dunn2014). Consistent with this literature, we find that in two independent samples of intervention naive kindergarteners, those who carried the NR3C1 susceptibility allele experienced elevated anxiety/depression symptoms. It is a novel contribution of our study that NR3C1 variation and, by implication, glucocorticoid signaling, may represent an important dimension in the responsiveness of childhood externalizing psychopathology to preventive intervention.
With respect to the design and evaluation of interventions, our study offers two lessons. First, effective intervention takes time. Although a portion of the G × I effect was manifest already during elementary school, the majority was not detected until later in adolescence, when the full 10 years of intervention had been delivered. One implication of this finding is that population delivery of improved early-childhood education may not fully address the needs of the children who benefited from the Fast Track intervention. Second, intervention effects may grow even beyond the completion of treatment. The total G × I effect on age 25 externalizing psychopathology exceeded the portion that was mediated by childhood and adolescent developmental phenotypes. This result is broadly consistent with other randomized trials of early childhood interventions that show effects of increasing magnitude over developmental time (even beyond the end of intervention delivery; Campbell et al., Reference Campbell, Conti, Heckman, Moon, Pinto and Pungello2014; Eckenrode et al., Reference Eckenrode, Campa, Luckey, Henderson, Cole and Kitzman2010; Heckman, Reference Heckman2006). Evaluations of the effectiveness and, in particular, the cost effectiveness of intervention with young children may not have full information until those children have grown to adulthood.
In terms of the translational significance of our finding, we wish to be clear that our data do not indicate that genetic testing can discern a child's susceptibility to interventions like Fast Track. The value of our genetic analysis is instead to point toward a dimension of children's physiology that may provide clues as to whether they are likely to benefit from intervention and why. Important next steps are to evaluate exactly how the polymorphism we studied relates to glucocorticoid signaling in children at risk to develop externalizing psychopathology and how glucocorticoid signaling phenotypes, such as cortisol response, may forecast outcomes for children receiving interventions to prevent or treat externalizing symptoms.
With respect to differential susceptibility research, our findings offer provocative supporting evidence for the hypothesis that heightened sensitivity of the stress response system is at the core of the susceptibility phenotype. NR3C1 is established as a gene encoding individual differences in the HPA-axis response to social stressors (DeRijk et al., Reference DeRijk, van Leeuwen, Klok and Zitman2008, Kumsta et al., Reference Kumsta, Entringer, Koper, van Rossum, Hellhammer and Wust2007, Reference Kumsta, Moser, Streit, Koper, Meyer and Wust2009; Manenschijn et al., Reference Manenschijn, van den Akker, Lamberts and van Rossum2009; van West et al., Reference van West, Del-Favero, Deboutte, Van Broeckhoven and Claes2010). We found evidence that a common NR3C1 variant modified Fast Track intervention response in the classic for better and for worse pattern. Future differential susceptibility research should incorporate NR3C1 genotypes alongside those of the traditional neurotransmitter genes.
We acknowledge limitations. First, our sample was small and included only European American Fast Track participants. We focused our analysis on this group because this is the group within which we detected the original G × I effect. Now that we have documented the G × I effect within the context of a randomized trial, larger scale analyses relying on observational data can be conducted to evaluate the robustness of the finding. Second, our mediation analyses focused on behavioral outcomes in development (children's externalizing symptoms and adolescents' problem behaviors), not psychological processes. Further research is needed to uncover specific effects of the G × I on socioemotional development that gave rise to these behavioral changes. Third, our study is right-censored at age 25 years. We do not know if the reduction in externalizing psychopathology at age 25 will persist. Fast Track participants are now aging out of the developmental period during which externalizing symptoms are most common in the general population. An important further test of the G × I will be whether it extends to the prevention of the most damaging and costly life course persistent cases (Moffitt, Reference Moffitt1993). Fourth, our study is not able to specify which component of the Fast Track intervention interacted with genotype to influence behavioral outcomes. G × I studies based on single-component interventions are needed to test hypotheses regarding genetic susceptibility to specific environmental exposures.
Differential susceptibility research is still in its early stages. Studies testing differential susceptibility hypotheses in the context of randomized trials serve as an acid test of the hypothesis because they enforce strict independence between individuals' susceptibility and the environment to which they are exposed. There is an interest in using differential susceptibility research to design precision interventions. At least insofar as long-running, high-cost interventions such as Fast Track are concerned, such precision targeting is, at best, ethically fraught. Much more research is needed to develop and refine the screening necessary to even consider such a project. More realistic, in our view, is the use of differential susceptibility research to inform developmental theories of how environments affect children's development. We know that children respond to their environments in different ways. As our study begins to illustrate, a powerful contribution of differential susceptibility research is to elucidate how and why these divergent responses come about.
Supplementary Materials
The supplementary materials in this article can be found online at http://journals.cambridge.org/dpp.