Transcatheter closure offers a less invasive treatment option for ostium secundum atrial septal defects with comparable outcomes to surgical closure.Reference Therrien 1 Large defects often are challenging for catheter closure as they are devoid of adequate margins.Reference Warnes, Williams and Bashore 2 , Reference Santoro, Bigazzi and Lacono 3 Advancements in imaging, procedural techniques, availability of self-centring devices, and the increasing operator experience have improved the outcomes in transcatheter closure of atrial septal defects.Reference Dalvi, Pinto and Gupta 4 , Reference Amin 5 Different modified techniques of deployment are being described to improve the device alignment, thereby increasing the chances of successful occlusion of the defect.Reference Amin 5 , Reference Spies, Boosfeld and Schrader 6 Safety and efficacy of these techniques were known previously.Reference Wahab, Bairam, Cao and Hijazi 7 – Reference Pillai, Satheesh, Pakkirisamy, Selvaraj and Jayaraman 9 However, proper case selection in terms of meticulous pre-procedure imaging continues to be a prerequisite as the spectrum of anatomic variation is quite high. We planned a prospective study looking at the outcome of transcatheter closure in large atrial septal defects (defined as⩾30 mm) with anatomical complexities identified in transoesophageal echocardiography and their association with outcome of transcatheter closure of atrial septal defects.
Methods
This is a prospective observational study involving patients with large secundum atrial septal defects (size⩾30 mm) who had various morphological complexities by echocardiogram and who underwent transcatheter closure with conventional and modified techniques. It was conducted at a tertiary-level cardiac centre in south India. The study was conducted during the period from January, 2013 to December, 2016 after obtaining approval from the scientific and ethics committee of the institute.
The intervention was defined as successful when the atrial septal defect was closed with a device without major complication such as embolisation. It was declared failed when the operator fails to place a device owing to technical or anatomic reasons even after performing one or more modified techniques. The technique of device closure – conventional versus modified techniques – was at the operator’s discretion. The conventional technique involved device deployment from the left atrial chamber and opening up the left atrial disc before pulling the assembly to the septum and releasing the right atrial disc. As the learning curve with modified techniques improved during the study period, some operators directly adopted modified techniques without trying the conventional method. In some cases, a modified technique was directly used. We included patients in whom a conventional technique failed, or cases in whom the operator directly chose a modified technique. The possible associations with anatomic features of complexity were looked into. Morphological parameters of complexity included “very large” atrial septal defects – long axis in any view – measuring⩾40 mm, complete absence of retro-aortic margin, multiple defects, the presence of septal aneurysm, septal malalignment, and deficiency of inferior vena caval and posterior margins. Patients with inoperable haemodynamics owing to pulmonary vascular disease were excluded from the study. The anatomic parameters were used in a forward conditional binary logistic regression analysis to create a prediction model for outcome failure.
All adults and children underwent a pre-procedure transthoracic, as well as transoesophageal echocardiogram. General anaesthesia was used for all children who required transoesophageal echocardiogram. Right ventricular systolic pressure was estimated and documented in patients with tricuspid regurgitation. Transoesophageal imaging was performed in the standard imaging angles from 0 to 120°. The mitral and retro-aortic valves were also profiled in the 120° sweep. The parameters assessed included size of the defect, shape of the defect (elliptical or round), adequacy of margins, the number of defects, and location with regard to the retro-aortic and mitral valve. The presence of septal malalignment and septal aneurysm was also documented. The total septal length was measured in the 0° four-chamber view and 90° bi-caval view.
The various margins were described as follows:Reference Akagi 10
∙ The retro-aortic margin=measured at a 45° short axis.
∙ The mitral valve margin=measured at 0° sagittal four-chamber view.
∙ The posterior margin=measured at 120–130° adjacent to the right upper pulmonary vein.
∙ The inferior vena caval margin=measured at a 90° bi-caval view.
∙ The superior vena caval margin=measured at a 90° bi-caval view.
∙ Coronary sinus/right atrioventricular valve or inferior margin=measured at 100–120°.
The margins were measured and documented in terms of length and strength. Any margin that was⩾4 mm and had thick echo reflection was considered adequate. The absent and floppy margins were clubbed together as “deficient” for analysis. Floppy rim was defined as any rim<3 mm, which is not thick in transoesophageal echocardiography and was considered as not strong enough to hold the device as per the existing hospital practice. Septal malalignment was defined to exist when the retro-aortic margin tends to deviate away from the plane of attachment of the non-coronary cusp. It was identified in the short-axis 45° transoesophageal echocardiogram when the atrial septal margins of the defect tend to fall apart from a straight line. Inferior vena caval rim was defined as deficient when the bi-caval 90° view fails to show a thick atrial septal rim adjacent to the inferior vena caval joining the right atrium.
An atrial septal aneurysm was described as a redundancy or saccular deformity of the atrial septum with increased mobility of the atrial septal tissue. Septal aneurysm was defined as excursion of the septal tissue, typically the fossa ovalis, of greater than 10 mm from the plane of the atrial septum into the right or left atrium or a combined total excursion towards right and left of 15 mm. We included multiple defects if the largest defect was⩾30 mm.
Local anaesthesia with sedation and transoesophageal echo guidance were used for all adult patients. General anaesthesia was used for all children, and transoesophageal echo was performed in selective children according to the discretion of the physician. Femoral venous access was used for transcatheter closure in all cases. Anti-coagulation was achieved using 100 U/kg unfractionated heparin, and activated clotting time was maintained between 250 and 300 seconds. Right heart study and haemodynamics were documented for all the patients. Crossing the atrial septal defects was performed using 5F/6F Cournand or multipurpose catheter with 0.035" hydrophilic glide wire (Terumo Inc., Tokyo, Japan). Balloon sizing with stop-flow technique was used if there was a septal aneurysm and or if there were any sizing discrepancies with transoesophageal echo finding. After crossing the defect, the catheter was parked in the left or right upper pulmonary vein. This catheter was exchanged for 0.038" super stiff wire for supporting the long device delivery sheath (Mullin’s sheath). Our choice of devices was random as per shelf availability of the particular size and did not follow any particular order. The devices used in this study include the double umbrella type discs (Amplatzer Septal Occluder (St. Jude, Minnesota, United States of America), Cocoon Septal Occluder (Vascular Innovations, Nonthaburi, Thailand) and Heart R Septal Occluders (Lifetech Scientific Limited, Schenzen, China)). Our successful deployment of device depends upon orientation of the left atrial disc parallel to the defect. As all patients had large defects, with a majority having some morphological abnormality, we upsized by 2–6 mm. In many cases, first 2-mm upsizing was chosen and then upsized in increments of 2 up to 6 mm.
Modified techniques
Different techniques studied are described as follows.
Balloon-assisted techniqueReference Dalvi, Pinto and Gupta 4
This technique is used to prevent the prolapse of the device during pulmonary vein deployment. In this technique, loaded delivery sheath is kept across the septum in the left atrium, and simultaneously a highly compliant balloon is inflated across the defect. The balloon was positioned across the defect over a 0.038" stiff wire through the contralateral femoral venous access. The balloon used for this technique involved an ultra-thin hyper-compliant balloon – that is, the sizing balloon available in sizes of 24 and 34 mm chosen according to the size of the defect – that comes along with the septal occluder kit. The balloon is inflated across the defect, and the left atrial device is deployed. Inflated balloon prevents the prolapse of the left atrial disc into the right atrium. After left atrial disc deployment, the delivery cable and sheath is pulled simultaneously to bring the left atrial disc close to the septum, with inflated balloon still kept across the defect. The right atrial disc is then deployed and the position of both the discs is confirmed by transoesophageal echocardiogram. The balloon is then deflated slowly and completely and then pulled out gently. After complete deflation of the balloon, both the discs fan out and stable position of the device across the septum is maintained.
Pulmonary vein technique
In this technique,Reference Amin 5 device delivery heath is positioned at the ostium of the left/right superior pulmonary vein, and the left atrial disc is deployed. The delivery cable is kept fixed, and the sheath is retracted into the right atrium to stretch the device while keeping the left atrial disc in the pulmonary vein. When the right atrial disc comes out of the sheath and fans out in the right atrium, the left atrial disc is released by a gentle push on the delivery system. This manoeuvre keeps both the discs parallel to the septum in the respective atrium. This technique was used both for left upper and right upper pulmonary veins.
Left atrial roof deployment methodReference Amin 5
This follows the same principle as in pulmonary veins, but the left disc is opened against the left atrial roof near the origin of the pulmonary vein but not inside the pulmonary vein. This method was used when the left atrium was relatively small, preventing a successful placement of balloon alongside a large left atrial disc.
Modified/cut sheath methodReference Spies, Boosfeld and Schrader 6
Here the operator cuts the sheath tip in an oblique manner to allow for asymmetric expansion of the left atrial disc to catch the margins of the defect followed by the asymmetric deployment of the right atrial disc. This method was used in some cases of malaligned septum and cases of small left atrium.
Dilator/catheter-assisted methodReference Wahab, Bairam, Cao and Hijazi 7
With the help of a contralateral venous access, a long dilator/diagnostic catheter–wire assembly was kept across the defect to support the device delivery. This method was used in some cases of deficient posterior/retro-aortic margins with a small left atrium, where the device tends to slip back into the right atrium in the conventional approach, and the smaller left atrium is unable to accommodate the balloon with device.
In a majority of cases, conventional technique of device delivery was attempted. However, operators were allowed to use a modified technique directly as well. Balloon-assisted technique was generally used in cases of deficient posterior and or inferior vena caval rim. As it involved more consumables and dual venous access, once patients fail with conventional technique, a pulmonary vein technique was used if they had adequate inferior vena caval rim or posterior rim. If pulmonary vein technique fails, we adopted balloon assistance. In patients in whom balloon also failed, a dilator or roof method was tried if the issue was a smaller left atrium that could not accommodate the large balloon alongside an expanded left atrial disc. If no such atrial size issue existed, we declared the procedure as failed.
Post-procedure
Successful or failed intervention was documented. Morphological feature for failure if any was noted. All patients underwent transthoracic echo at 24 hours and monthly for 6 months. All patients were discharged on oral aspirin and infective endocarditis prophylaxis for 6 months. Follow-up included clinical and echocardiographic assessment until 6 months.
Statistical analysis
Data were analysed by using descriptive and inferential statistics. The distribution of the clinical characteristics, morphological profiles, comorbidity conditions, gender, social status, clinical/treatment outcome, and so on was expressed as frequency and percentages. The distribution of continuous data such as age, size of lesions, biochemical parameters, and so on was expressed as mean with standard deviation or median with range, whichever is appropriate. Fisher’s exact test was performed to find the association of morphological parameters with outcome. Independent sample t-test was used to compare the atrial septal defect size with outcome. A forward conditional (stepwise) univariate logistic regression analysis was used to identify the risk factors predicting the success and failure with modified techniques of implantation. All statistical analyses were carried out by 5% level of significance, and p value<0.05 was considered significant The analysis was performed by SPSS software version 20.0 for windows.
Results
Data on 373 patients were analysed in whom the maximum defect size measured was⩾30 mm. Mean defect size was 34.7±2.78 mm (95% confidence interval (CI) 30.67–43.1 mm) with success and 40.16±4.5 mm (95% CI 32.16–44.7) with failure (p=0.02). The mean total septal length was 38.11±0.63 (95% CI 35.21–40.56 mm) in success and 42.54±0.34 (95% CI 38.79–43.21 mm) in failure. The defect to septal ratios were 0.82 and 0.94 in success and failure groups, respectively (p=0.02). Conventional technique was initially used in 312 out of 373 cases, but proved successful only in 27 cases (8.6%). With the modified techniques, the procedure was successful in 295/346 (85.2%) patients. Baseline characteristics are given in Table 1. Complete absence of the retro-aortic margin was the most common abnormality noted in 152 (41) patients. The balloon-assisted technique was the most common technique used (Table 2). In 180 out of 205 cases (87%), the technique was successful. The pulmonary vein technique yielded success in 107/155 (69%) cases. Figure 1a to f shows echocardiographic images of a successful deployment of a large device in a patient with deficient posterior and retro-aortic margin with balloon-assisted technique. Figure 2 shows serial fluoroscopy images of transcatheter closure of a 32-mm atrial septal defect with deficient retro-aortic and floppy inferior vena caval rims. The device embolised a few minutes after deployment. It was successfully retrieved and another device deployed with balloon-assisted technique with a snare holding the device before release.

Figure 1 Serial transoesophageal echocardiography images of balloon-assisted device closure of a large 38.5-mm atrial septal defect with deficient posterior margin ( a to f ). ( a ) Very large atrial septal defects measuring 38.5 mm with deficient posterior margin. ( b ) Sizing balloon occluding the defect. ( c ) Device in hour-glass shape with either discs in respective atria and waist compressed by inflated balloon. ( d ) Balloon is deflated and waist expands. ( e ) Device in good position snugly holding on after removal of the balloon. ( f ) Device after deployment.
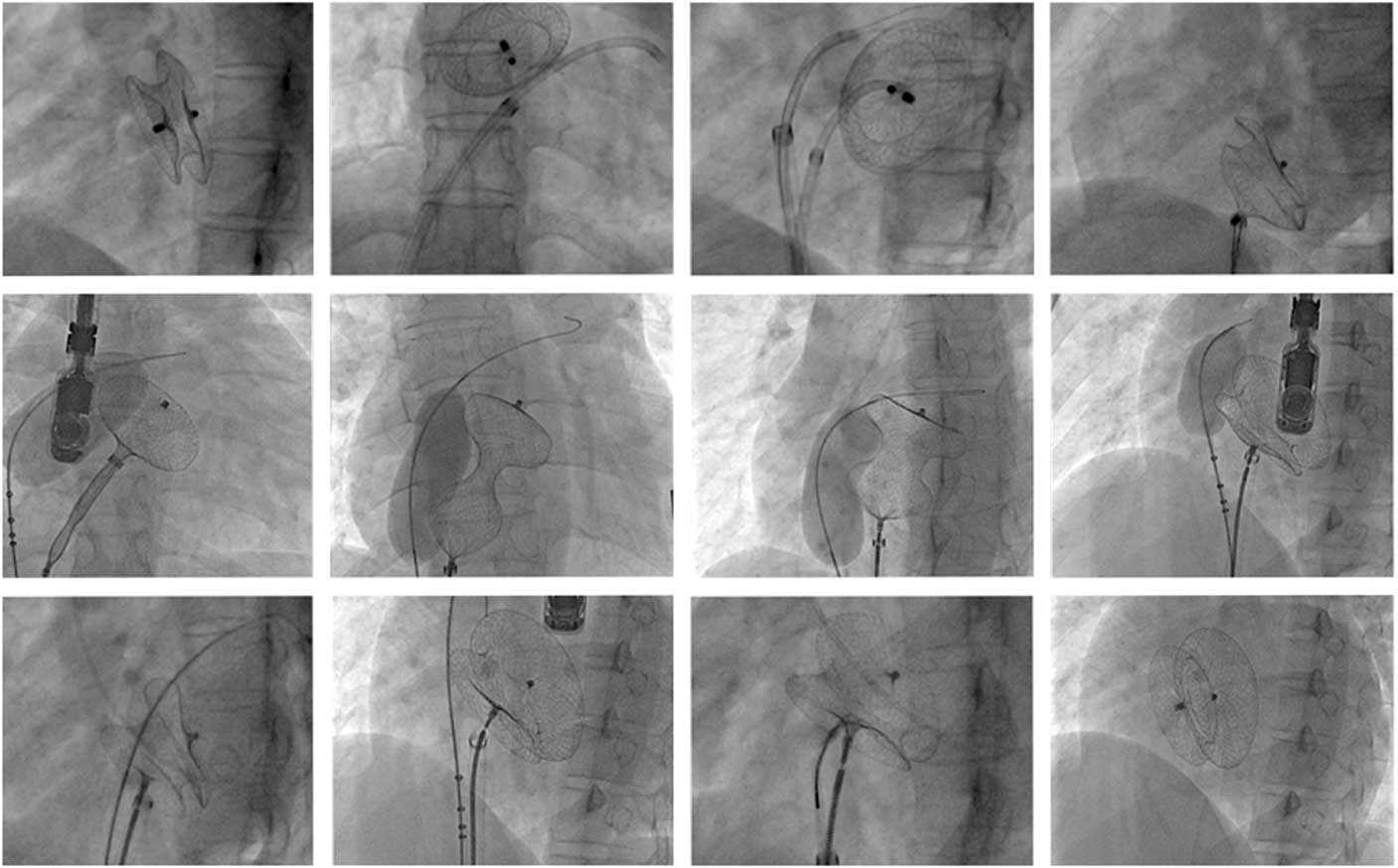
Figure 2 Serial fluoroscopy images of transcatheter closure of a 32-mm atrial septal defect with a 36-mm device. Retro-aortic and inferior vena caval rims were deficient. The device embolised a few minutes later into the pulmonary artery. It was successfully retrieved and the same device was deployed with balloon-assisted technique with a snare holding the device before release. The procedure was successful with balloon-assisted technique.
Table 1 Baseline characteristics.

CI=confidence interval; RVSP=right ventricular systolic pressure
Table 2 Modified techniques for device delivery.

PV=pulmonary vein
Figure 3a to d shows pulmonary vein deployment technique in a case of posteriorly malaligned defect (dotted arrows Fig 3a). In 45 patients, pulmonary vein technique failed and balloon technique was performed. The left atrial roof method (Fig 4) was used in four patients, and dilator support (Fig 5) was used in three patients. These methods were used after a failed balloon-assisted technique when the left atrium was small and could not accommodate a left atrial disc alongside an inflated balloon. In about 82 (23%) patients, more than one modified technique was used. In 45 patients, balloon assistance was used after failing the pulmonary vein technique. Out of the 25 patients in whom balloon assistance failed, the left atrial roof technique, dilator, or modified sheath technique was successful in 8 out of 13 cases attempted.

Figure 3 Pulmonary vein (PV) deployment technique in a patient with malaligned septum ( a to d ). ( a ) Transthoracic echo showing a posterior malalignment of the septum. The dotted lines show the malalignment. ( b ) The left atrial disc is deployed in the PV and the device is stretched across the defect. ( c ) Image showing stretched device with left atrial disc anchored to the left upper pulmonary vein. ( d ) The device in position after retraction from PV.

Figure 4 The left atrial roof method. ( a ) The left atrial disc pushed against the atrial wall and the device stretched across the defect to open out the right atrial disc as well. ( b ) The device is stretched across the atrial septal defect and the right atrial (RA) disc and pushed out. ( c ) Once RA disc is in position, a gentle pull on the cable causes the left atrial disc to fall back. ( d ) Device in position after release.

Figure 5 Dilator-assisted method in cases of small left atrium. ( a ) Dilator with wire in position in the left atrium (LA) with the LA disc opened. ( b ) Device deployed in alignment with the support of a dilator.
Table 3 shows the prevalence of different morphological features and the outcome. Retro-aortic margin was absent in 152 cases, and 122 of them had successful closure (p=0.9). The presence of septal aneurysm also did not affect the procedural success (97.5% with and 93.3% without septal aneurysm) (p=0.1), but there was a mean difference of 2.3±1.5 mm (95% CI 1.1–3.9) in measured transoesophageal echo size and balloon sizing in patients with septal aneurysm. All patients with multiple defects had a successful outcome. In total, 16 patients with multiple atrial septal defects had two devices deployed. One patient had three defects and the smallest one was left for conservative treatment. Figure 6 illustrates a case of posterior malalignment in combination with multiple septal defects successfully intervened by balloon-assisted technique.

Figure 6 ( a ) Transthoracic echo image with extreme posterior septal malalignment (“S”-shaped septum) with multiple atrial septal defects (arrows). ( b ) Anterior and posterior colour flow indicating multiple shunts. ( c ) Fluoroscopic image showing catheters crossing multiple defects under transoesophageal guidance. ( d ) Balloon-assisted delivery of the first device; the balloon is being inflated in the anterior defect to counter the malalignment and to prevent device prolapse across the posterior defect. ( e ) Subsequent successful closure of defects with two devices. The devices are oriented in different directions, indicating the extreme malalignment of the septum.
Table 3 Success rates in various techniques in different unfavourable morphologies (successful/attempted).

IVC=inferior vena caval; LA=left atrium; PV=pulmonary vein
Some patients had more than one complexity co-existing and more than one technique required for closure.
Retro-aortic rim deficiency was present in 40% cases.
Aneurysmal septum and multiple defects had high success rates of 97 and 100%, respectively.
Patients with very large defects (⩾40 mm) had high failure rates despite a modified technique. In these patients, the failure rates were 52.2%. When atrial septal defects size was 30–40 mm, the success was 95.5% (p=0.002).
Patients with malaligned septum had high failure rate of 50.5% with conventional technique. However, with balloon assistance and pulmonary vein techniques, we noted an improved success of 78%. Proportion of patients with deficient or floppy posterior margin was 164 (44%). The success rate of transcatheter closure was 68.6% in such patients. In patients with adequate posterior margin, the success rate was 95.6% (p=0.019). Inferior vena caval margin was deficient in 89 (24%) and the procedure failed in 52.6% of these patients(p<0.001). The success rate was 95.1% in patients with adequate inferior vena caval margin. The association of the presence or absence of adequate inferior vena caval margin and outcome was statistically significant with a p value <0.001 (Table 4). Deficient inferior vena caval margin, deficient posterior margin, and defect size ⩾40 mm (odds ratio of 25.3, 4.1, and 8.3, respectively) were associated with failure (Table 4).
Table 4 Prediction model for intervention failure.

CI=confidence interval; IVC=inferior vena caval; OR=odds ratio
* Univariate regression analysis
There was no procedure-related mortality. There were 15 episodes of device embolisation. All embolisations occurred in the immediate 12 hours post-implantation. Among these, 12 cases had a deficiency of posterior and inferior vena caval margins. Three cases had posterior malalignment. All successfully retrieved by endovascular approach. In a majority of patients (11/15), the device embolised to the right ventricular outflow tract and/or the pulmonary artery. In three patients, it was in the right ventricular apex and in one it was in the left atrium. In seven of these cases of embolisation, device closure was successful with a larger device. Surgical closure was performed in the eight remaining patients. There were 20 patients reported to have peri-procedural arrhythmias (30 mm; 12 had atrial fibrillation and seven had atrial tachycardia). Three patients had persistent atrial fibrillation after failed cardioversion. One patient had sustained ventricular tachycardia and had to be cardioverted.
The mean follow-up period for the study is 5.2 years, ranging from 12 months to 7.3 years). All patients had transthoracic echo follow-up monthly until 12 months after the intervention. There was no late embolisation. One patient had late erosion. She presented with aortic regurgitation, with the device intruding into the retro-aortic root 18 months after the implantation of the 36-mm Amplatzer device. She had deficient aortic rim and septal malalignment (Fig 7). The patient was asymptomatic and underwent successful open heart surgery subsequently. One patient developed atrial fibrillation, and aspirin was changed to warfarin. There were no reports of thromboembolic events. Residual shunt was present in 12 patients, which disappeared at 6 months of follow-up. None of the patients in atrial fibrillation reverted to sinus rhythm.

Figure 7 Late erosion of the device into the aorta in a patient with malaligned defect. ( a ) Four-chamber view showing the malaligned septum. ( b ) Parasternal long-axis view immediately after implantation of a 36-mm device. ( c ) Late erosion into the aorta. ( d ) Colour flow showing the eroded area with aortic regurgitation.
Figure 8 depicts the diagrammatic representation of various techniques used.

Figure 8 Pictorial representation of various techniques. ( a ) Serial diagrams showing the steps of balloon assistance method. ( b ) Pulmonary vein method. ( c ) Left atrial (LA) roof technique. ( d ) Dilator-assisted technique. LV=left ventricle; RA=right atrial; RV=right ventricle; TV=tricuspid valve.
Discussion
Transcatheter closure of large atrial septal defects with a maximal native diameter ⩾30 mm is challenging, and alternative special techniques for deployment of the device are usually required.Reference Akagi 10 This study involves large atrial septal defects (⩾30 mm) attempted with various modified techniques and outcomes analysed with respect to different anatomic variables. Three parameters proved statistically significant in procedural outcomes – namely, very large defect size measuring⩾40 mm, deficient posterior margin, and deficient inferior vena caval margin. We had very few cases of deficient superior caval vein and deficient coronary sinus margins (<1%).
In our experience, spanning more than three decades with transoesophageal echocardiographic imaging of atrial septal defects, most of the anatomic complexities are more common with larger defects like the ones in this study. Nearly 80% patients had either a deficient posterior rim or inferior caval rim or septal malalignment. This made the success of conventional technique very low (8%) in this series. The larger mean defect size of 35.3 mm with 22% patients having a defect size ⩾40 mm was another feature in this group that could have played a role in lowering the success of conventional technique. Absent retro-aortic margin was the most common anatomic variation reported in the literature,Reference Amin 5 , Reference Varma, Benson and Silversides 8 , Reference Akagi 10 , Reference McElhinney, Quartermain, Kenny, Alboliras and Amin 11 and we had about 42% of patients with this deficiency. This anomaly was not a factor for procedural failure in our series. The presence of septal aneurysm or multiple defects did not show an outcome difference either. However, the aneurysm was found to result in significant discrepancy in the measurement of defect size. We found a mean size difference of 2.3±1.5 mm in patients with and without septal aneurysm between transoesophageal echocardiogram and the balloon stop-flow technique.
Septal malalignment is a rarely reported entity in transcatheter closure.Reference Spies, Boosfeld and Schrader 6 We found that the lack of alignment of septum primum and secundum in the form of L or S shape interferes with proper disc alignment. For a successful closure with a double umbrella device, the expanded left atrial disc has to fall in line with the septal plane so that the right atrial disc can be released to abut the right side of the septum. The left atrial disc should hold against the defect on the left atrial side for the waist to expand across the defect and the subsequent expansion of the right disc.Reference McElhinney, Quartermain, Kenny, Alboliras and Amin 11 A balloon-assisted technique was helpful in cases with non-linear septal planes.Reference McElhinney, Quartermain, Kenny, Alboliras and Amin 11 With the modified techniques, the success rate was 78%. We feel that the combination of absent retro-aortic margin with malalignment is unfavourable as it eventually precludes any sort of left atrial disc alignment against the septum, causing it to tilt tangentially across the defect and balloon assistance helps in the type of anomaly.
Posterior and inferior vena caval margins are often regarded crucial for holding the device.Reference Santoro, Bigazzi and Lacono 3 , Reference Akagi 10 , Reference McElhinney, Quartermain, Kenny, Alboliras and Amin 11 Complete absence of the entire posterior and inferior vena caval margins essentially rules out a device intervention in our practice. However, if either of them is adequate, the case was attempted. The device can be successfully placed if the lower half of the semi-circular or C-shaded ridge – comprising the posterior margin above and inferior vena caval margin below – is strong. Absent or floppy posterior margin poses difficulty in device alignment.Reference Amin 5 , Reference McElhinney, Quartermain, Kenny, Alboliras and Amin 11 Combined absence of diametrically opposite anterior and posterior septal margins is a challenge to deploy the device, as most often the left disc tilts down.Reference Amin 5 We find the Balloon-assisted technique useful in this situation as it temporarily holds the left disc while the waist is deployed followed by right disc. In our experience, this works very well as the inflated balloon holds on the whole system until the whole device is delivered and device tends to be stable in such cases when the inferior half of the “C” or the inferior caval margin is good and strong to support the device. However, if this margin is not good, then the device will eventually slip out in to right atrium as soon as the balloon is deflated. In total, 80% of patients who had 40-mm device embolisation in this series had deficiency of inferior caval and or posterior margins. A Snare technique (to hold the screw of the right disc) was sometimes used to hold the device before deployment as a fall-back option.Reference Butera, Lovin, Basile and Carminati 12 Inferior vena caval margin is a crucial determinant of device stability,Reference Pillai, Rangaswami Balasubramanian, Selvaraj, Saktheeswaran, Satheesh and Jayaraman 13 and the margin close to coronary sinus is rarely deficient in published series.Reference Pillai, Rangaswami Balasubramanian, Selvaraj, Saktheeswaran, Satheesh and Jayaraman 13 , Reference Pedra, Pedra and Costa 14 In many cases, transoesophageal echo can only show the absence or presence of a margin and it cannot provide accurate data about the strength of the margin.
Modified techniques as described were used in successful delivery of the device in 82% cases. This was similar to our findings earlier in a retrospective cohort.Reference Amin 5 Deployment technique was modified upon discretion of the operator, to orient the left atrial disc parallel to the septum, with some operators preferring pulmonary vein technique over balloon-assisted technique and vice versa. We predominantly used balloon assistance and pulmonary vein technique in our patients. This was a not in a randomised manner. However, we found that many patients in the larger atrial septal defect group we studied required more than one technique to receive an overall success of 82% that we recorded. More often than not many anatomic complexities co-existed. In all, 82 patients out of 346 (23%) had multiple techniques used. In patients in whom balloon assistance failed, 50% had smaller left atrium, which precluded optimal balloon expansion and opening of the left atrial side of the device. We found that the roof method, dilator assistance, and modified sheath offered success in 8/13 of such patients. Irrespective of the type of modified techniques, an absent or deficient inferior vena caval or posterior margin and a very large sized defect predicted failure.
The balloon-assisted technique was the most successful technique in our experience (87%). In our opinion, it gives the operator a better control of device delivery in almost all morphological variations such as deficient inferior rim, deficient posterior rim, and septal malalignment. The only drawback we find is that it incurs additional vascular access and the added expenses on the balloon and accessories. In addition, it fails in cases where the left atrium is very small to accommodate the balloon, as described earlier. The left atrial roof method and dilator-assisted support methods were used only in a very few cases with relatively small left atrium after balloon technique failed. The pulmonary vein technique was useful for posterior malalignment and septal aneurysm cases. The technique had a success of 67%, but in our opinion it requires more learning curve in the release left atrial disc that is supposed to stretch across the defect into the right atrium after getting anchored to the pulmonary vein origin. In cases in which the left atrial dimensions are long, the whole right atrial disc tends to get deployed in the left atrium, causing failure of technique. Furthermore, the initiation of the left disc delivery deep inside the pulmonary vein ostium may cause failure of the disc to get released out of the ostium.
Study limitations
This is single-centre observational study, and the outcomes cannot be generalised. Only two-dimensional data were available. We did not use three-dimensional echo technology as the protocol was not ready for clinical use in our hospital when this study began. We could not analyse the crucial information as to which specific anatomic variant better fits in with a specific modified technique as there were individually small in number. Some patients had more than one complex morphological variable, which is a confounding factor.
Conclusions
The modified techniques for device deployment offer substantial chances of success in transcatheter closure of large secundum atrial septal defects (82%) with anatomic complexity. Malalignment of the septum, absent retro-aortic margin, and the presence of multiple defects or aneurysmal septum offered good success with modified techniques. Very large defect (⩾40 mm) and deficiency of posterior and inferior margins were associated with high failure rate.
Acknowledgement
None.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflicts of Interest
None.
Ethical Standards
This study was approved by our Institutional Scientific and Ethics Committee Board. Written informed consent was obtained from all patients enrolled.














