Introduction
Wild radish is the most problematic and economically damaging dicotyledonous weed of Australian cropping systems (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clark2016), threatening grain production across large areas of the wheatbelt. Due to aggressive competition, wild radish infestations are responsible for substantial grain production losses (Blackshaw et al. Reference Blackshaw, Lemerle and Young2002; Cousens et al. Reference Cousens, Warringa, Cameron and Hoy2001; Eslami et al. Reference Eslami, Gill, Bellotti and McDonald2006; Walsh and Minkey Reference Walsh and Minkey2006), estimated to be in excess of 200 million kg annually (Llewellyn et al. Reference Llewellyn, Ronning, Ouzman, Walker, Mayfield and Clark2016). The genetic diversity of this species combined with obligate out-crossing and high fecundity has allowed it to establish and adapt throughout much of the Australian wheatbelt (Bett and Lydiate Reference Bett and Lydiate2003; Conner and Via Reference Conner and Via1993). More significantly, these same attributes have contributed to the resistance-prone nature of this species such that multiple herbicide–resistant wild radish populations are now present in high frequencies across Australian cropping regions (Owen et al. Reference Owen, Martinez and Powles2015; Walsh et al. Reference Walsh, Powles, Beard, Parkin and Porter2004). Although herbicides remain the preferred and most effective method for wild radish control, the loss of herbicide options due to the evolution of herbicide-resistant weeds is a significant threat to crop production.
Mesotrione (2-[4-methyl-2-nitrobenzoyl]cyclohexane-1,3-dione) belongs to the triketone chemistry of herbicides that were developed from compounds originally isolated from the bottlebrush plant (Callistemon citrinus Stapf.), a member of the Australian myrtaceae plant family (Hellyer Reference Hellyer1968). The initial discovery in 1977 of the potential herbicidal properties of this chemical family by Zeneca herbicide company scientists followed the observation that “few weeds grew beneath bottle brush plants” (Mitchell et al. Reference Mitchell, Bartlett, Fraser, Hawkes, Holt, Townson and Wichert2001). Subsequent analyses identified a compound, leptospermone, that caused bleaching of new plant growth due to the inhibition of carotenoid production and the inhibition of photosynthetic activity (Hess Reference Hess2000). This discovery led to the development of herbicides that were found to inhibit p-hydroxyphenylpyruvate dioxygenase (HPPD), an enzyme that is integral to the formation of carotenoids, which are essential for photosynthesis.
The action of HPPD inhibitors such as mesotrione is to produce a cascade of responses that overlap with the response caused by herbicides that inhibit phytoene desaturase (PDS) and photosystem II (PS II), and that also interfere with photosynthesis. The inhibition of the HPPD enzyme, which catalyzes the conversion of hydroxyphenylpyruvate to homogentisate (Abendroth et al. Reference Abendroth, Martin and Roeth2006), prevents the synthesis of plastoquinone (PQ) and α-tocopherols (Hess Reference Hess2000; Lee et al. Reference Lee, Prisbylla, Cromartie, Dagarin, Howard, Provan, Ellis, Fraser and Mutter1997). PQ is an essential cofactor for PDS, which is integral in carotenoid biosynthesis and is the site of action (SOA) of PDS-inhibiting herbicides. Carotenoids, as reviewed by Cunningham and Gantt (Reference Cunningham and Gantt1998), play a critical role in protecting the PS II reaction center by quenching the thylakoid membrane damaging excited singlet oxygen and triplet chlorophyll. The loss of carotenoids is exacerbated by the simultaneous loss of α-tocopherols, which act similarly to carotenoids in quenching singlet oxygen to prevent peroxidation of membrane lipids (Trebst et al. Reference Trebst, Depka and Holländer-Czytko2002). Similarly, the action of PS II-inhibiting herbicides, which block electron transfer to the PS II reaction center, increases the accumulation of membrane-damaging molecules (Hess Reference Hess2000), compounding the effects of carotenoid loss. The intersecting effects of HPPD-, PS II-, and PDS-inhibiting herbicides on the PS II system has been demonstrated to produce complementary and even synergistic responses when combinations of these herbicides are used to control weeds (Abendroth et al. Reference Abendroth, Martin and Roeth2006; Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Walsh et al. Reference Walsh, Stratford, Stone and Powles2012; Woodyard et al. Reference Woodyard, Hugie and Riechers2009).
The efficacy of HPPD-inhibiting herbicides on brassica weeds including wild radish supports the development of this herbicide for use in Australian cropping systems. Mesotrione was first introduced in the United States and Europe in 1999 as the herbicide Callisto® (Syngenta, Macquarie Park, NSW, Australia), a name derived from its plant origins (Mitchell et al. Reference Mitchell, Bartlett, Fraser, Hawkes, Holt, Townson and Wichert2001). Because grass species are more readily able to metabolize mesotrione via P450 activity, this herbicide has been successfully used for the selective control of broadleaf weeds, including Brassica spp. in maize (Zea mays L.; Mitchell et al. Reference Mitchell, Bartlett, Fraser, Hawkes, Holt, Townson and Wichert2001; Wichert et al. Reference Wichert, Foxon, Townson and Bratlett1999). There is also evidence that uptake of mesotrione is slower in grasses, which are also thought to have a less sensitive form of the HPPD enzyme (Abit and Al-Khatib Reference Abit and Al-Khatib2009; Hawkes et al. Reference Hawkes, Holt, Andrews, Thomas, Langford, Hollingworth and Mitchell2001; Mitchell et al. Reference Mitchell, Bartlett, Fraser, Hawkes, Holt, Townson and Wichert2001). Mesotrione has been found to have excellent wheat crop safety at application rates that will likely allow broadleaf weed control (Soltani et al. Reference Soltani, Shropshire and Sikkema2011). The aims of these studies were to 1) establish the efficacy of mesotrione for selective control of wild radish in wheat and 2) determine whether wild radish control was improved when mesotrione applied preemergence (PRE) was followed by bromoxynil applied postemergence (POST).
Materials and Methods
The potential for mesotrione to selectively control wild radish in wheat was investigated in a series of dose-response pot and field studies conducted over the 2012 to 2014 winter-spring growing seasons (May to August) in Western Australia.
Wild Radish Populations Used in Pot Studies
The herbicide susceptible (WARR7) population was collected in 1999 from a reserve at Yuna, Western Australia (WA; 28.33°S, 115.01°E), where there had been no known herbicide applications (Walsh et al. Reference Walsh, Powles, Beard, Parkin and Porter2004). The acetolactate synthase (ALS)-inhibiting and phenoxy herbicide-resistant (R; WARR20) population was collected from a cropping field in the Wongan Hills region, WA (30.88°S, 116.51°E; Walsh et al. Reference Walsh, Maguire and Powles2009). Subsequent generations of seed from these populations have been routinely produced on plants grown in isolation in pollen-proof enclosures at the University of Western Australia (UWA).
Dose Response Pot Trials
Mesotrione POST dose response trials (Table 1) were commenced by planting wheat or wild radish seed in equally spaced holes (10 mm deep by 6 mm wide), created with a 20-pin template, in 170-mm-diameter posts containing potting mix (50% pine bark, 25% sand, and 25% peat moss). Once seeds were placed in the planting holes they were manually smoothed over and the pots were moved to the outside growth area at the UWA in Nedlands, Australia, where they were immediately watered.
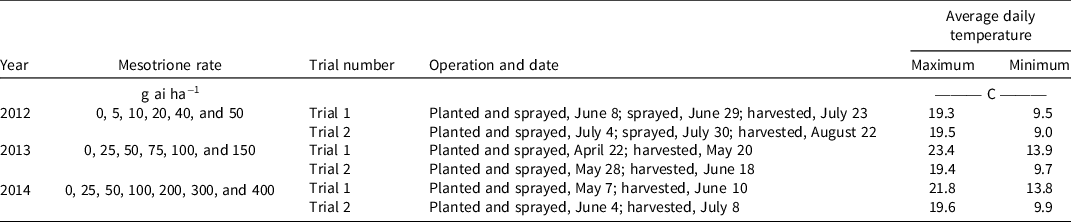
Table 1. Year, mesotrione rates, trial number, operation dates, and average daily temperatures during the evaluation of mesotrione control of wild radish in wheat in dose-response pot trials conducted in the outdoor growth facility at the University of Western Australia.
Mesotrione was applied POST (Table 1) when wild radish plants were at the two- to three- true-leaf growth stage using a cabinet sprayer fitted with dual 110° 01 flat-fan nozzles (Teejet®, Newton, Vic, Australia) with a water delivery rate of 110 L ha−1 (200 kPa, 4 km h−1).
Mesotrione PRE dose response trials (Table 1) were established by placing wheat or wild radish seeds in shallow (<5 mm) indentations created with the same 20-pin planting template. Immediately after seed placement, mesotrione PRE was applied directly to seed using the cabinet sprayer as described above. Immediately after application a 1-cm covering of potting mix was added to each of the pots, which were then moved to the outside growth area.
Pot trials were located in an outside area on the Nedlands campus of the UWA where they were exposed to winter growing season conditions that are similar to those of the Western Australian wheatbelt. Average daily maximum and minimum temperatures over the period of each trial were obtained from a nearby (6 km) Bureau of Meteorology (BOM 2021) weather station (Table 2). When rainfall was insufficient, pots were hand watered to maintain soil moisture levels at or near field capacity. Pots were located on benches in a randomized complete block arrangement with four replicates of each treatment. Each trial was repeated within the same growing season (i.e., Trial 1 and Trial 2) (Table 1). No watering or rainfall was allowed on PRE or POST treated plants/pots for at least 24 h following application. Pot trials were fertilized weekly with 2 g of a complete liquid fertilizer [N 19% (NH2 15%, NH4 1.9%, NO3 2.1%), P 8%, K 16%, Mg 1.2%, S 3.8%, Fe 400 mg kg−1, Mn 200 mg kg−1, Zn 200 mg kg−1, Cu 100 mg kg−1, B 100 mg kg−1, Mo 10 mg kg−1]. Approximately 21 to 28 d after mesotrione application, surviving plants were counted and harvested before being oven dried for 2 d at 70 C, then weighed for determination of biomass production.
Table 2. Herbicides, active ingredient, trade name, formulation, manufacturer, and adjuvant for pot and field studies to evaluate mesotrione for control of wild radish in wheat at University of Western Australia.
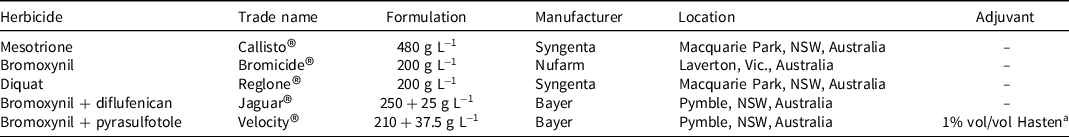
a Hasten® contains 704 g L−1 ethyl and methyl esters of vegetable oil, Victorian Chemical Company, Coolaroo, Vic., Australia.
Field Evaluation of Mesotrione
Field studies conducted in 2013 at Eradu (28.69°S 114.98°E) and 2014 at Mingenew, WA (29.19°S 115.44°E), in the northern wheatbelt region of Western Australia, evaluated the efficacy of mesotrione applied PRE to selectively control wild radish in commercial wheat fields. In collaboration with the grower owners, each of the selected sites were in fields that had wild radish infestations and were to be planted with wheat. At both locations the field-wide application of trifluralin PRE prior to wheat planting provided grass weed control in the trial sites. The experimental design in both trials was a randomized compete block with four replicates.
In 2013, mesotrione was applied PRE (0, 50, 75, 100, 150, and 200 g ai ha−1) in a spray volume of 68 L ha−1 using a vehicle-mounted boom fitted with Teejet® drift guard 002 nozzles. Plot size was 20 m × 2.25 m with each treatment replicated four times. Mesotrione treatments were incorporated by seeding (IBS) of wheat immediately after application on June 10 with a knife-point seeding system, the most commonly used tine openers in Australia (Chauhan et al. Reference Chauhan, Gill and Preston2006; D’Emden et al. Reference D’Emden, Llewellyn and Burton2008).
Wild radish emergence counts and wheat crop damage assessments were initially conducted on June 21, 11 d after planting (DAP), with final plant survival counts on August 14, 54 DAP. At each time of assessment wild radish plant densities were recorded in the entire plot area (45 m2) of each treatment. Visual ratings of wheat crop damage were based on percentage severity of herbicide bleaching and stunting effects where 0% = no damage and 100% = plant death.
In 2014, mesotrione (Table 2) was applied PRE on May 7 and was immediately incorporated by wheat planting (IBS) with a knife-point seeding system. POST herbicides were applied (Table 2) when wild radish seedlings were at the two- to four- true-leaf stage on June 5 with a vehicle-mounted boom as described above.
Wild radish plant emergence counts and wheat crop phytotoxicity assessments were performed on June 3, 4 wk after planting (WAP), just prior to the application of POST herbicides. Final wild radish survival counts and wheat crop damage ratings were conducted on June 25, 7 WAP. Ratings of wild radish biomass reductions due to residual herbicide activity were made on August 21, 14 WAP and September 23, 19 WAP.
Daily rainfall data was accessed from nearby BOM weather stations (within 10 km) to highlight rainfall amounts and occurrence throughout the growing season at each site (Figure 1; BOM 2021).
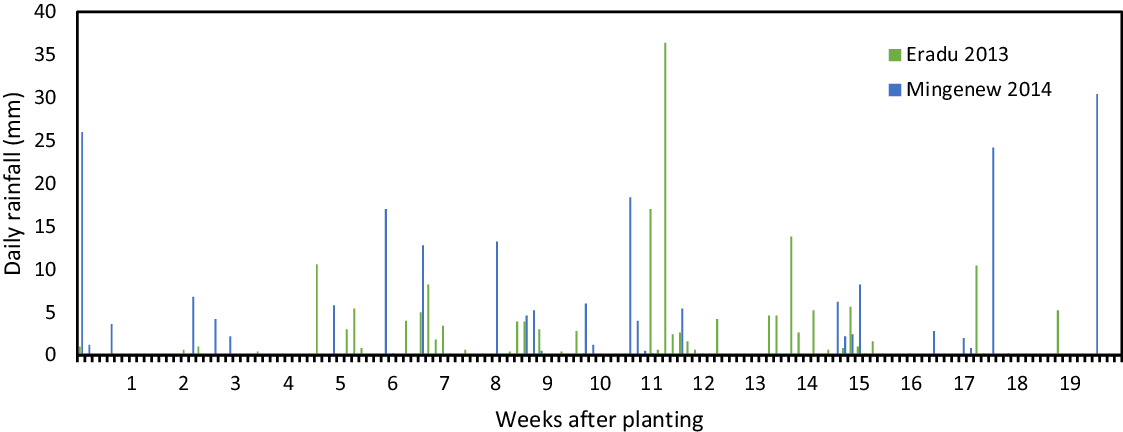
Figure 1. Daily rainfall amounts and occurrence during the 2013 and 2014 winter growing seasons at the Eradu and Mingenew field trial sites in Western Australia.
Data Analyses and Presentation
Data from dose-response pot studies were analyzed with the general aims of 1) determining whether PRE or POST applications of mesotrione were more effective on wild radish and 2) identifying a mesotrione application range for subsequent field trials. Therefore, one-way and two-way ANOVAs using Genstat software for Windows v. 18 (VSN International, Hemel Hempstead, UK) were used to examine wild radish and wheat survival and biomass data. Data were checked for assumptions of normality and of equal variance prior to being subjected to ANOVA. A two-way ANOVA of wild radish survival data determined that there was a significant (P < 0.05) interaction effect due to trial in the 2012 pot trials and the PRE and POST trials were subsequently analyzed separately in one-way ANOVAs. Analysis of the 2013 and 2014 wild radish survival data determined that in both years there were no interaction effects (P > 0.05), due to trial timing. The data for each year were pooled for subsequent two-way ANOVAs to determine whether there was an effect of wild radish biotype (resistant, R; and susceptible, S) on survival following mesotrione applied PRE. Wild radish and wheat population survival data and surviving wild radish and wheat plant biomass data were converted to percentage of nontreated control for presentation. One-way ANOVAs were used to examine herbicide effects on wild radish survival counts and wild radish ratings data from the 2013 and 2014 field trials. The means separation was performed using a Fisher’s protected LSD with significance set at P = 0.05.
Results and Discussion
Dose Response Pot Studies
Wild radish survival was 15%, 23%, and 33% lower, respectively, when mesotrione rates of 20, 40 and 50 g ai ha−1 were applied PRE, compared with POST applications (Figure 2A). Although an acceptable level of wild radish control (>90%) was not achieved at the rates used in this study, it was evident that as application rates increased, mesotrione PRE was increasingly more effective than POST. In contrast, when PRE and POST rates of mesotrione (e.g., 140 g ai ha−1) were compared in corn field trials, similar levels of broadleaf weed control were achieved with both application timings (Armel et al. Reference Armel, Wilson, Richardson and Hines2003a; Stephenson et al. Reference Stephenson, Bond, Walker, Bararpour and Oliver2004). It has been noted that the efficacy of mesotrione PRE in these and similar field studies is strongly influenced by the amount and timing of rainfall events (Armel et al. Reference Armel, Wilson, Richardson and Hines2003b). Soil moisture was not limiting in this pot study due to frequent watering; as such, the results presented (Figure 2A) more accurately reflect the differences in efficacy of mesotrione PRE versus POST on wild radish.

Figure 2. Effect of increasing rates of mesotrione applied preemergence (PRE) and postemergence (POST) on the survival (A) and biomass (B) of Raphanus raphanistrum and wheat in dose-response pot trials conducted during the 2012 winter growing season at the outdoor growth facility at the University of Western Australia.
Wheat survival was not reduced by mesotrione in the pot experiments regardless of application rate and timing (Figure 2A). In contrast, wheat growth was reduced by mesotrione PRE and POST, as indicated by reductions in wheat biomass (Figure 2B). Mesotrione applied POST at 5 to 40 g ai ha−1 reduced wheat growth by 15% to 20% and by 33% at 50 g ai ha−1. Wheat growth was less affected when these rates were applied PRE, resulting in biomass reductions of just 1% to 8%. Similarly, a previous field study found that mesotrione POST caused 20% wheat crop damage and 14% yield loss but there was no effect on wheat growth and yield when mesotrione was applied PRE (Soltani et al. Reference Soltani, Shropshire and Sikkema2011). The results presented here in combination with previous studies have identified that mesotrione PRE has increased efficacy on wild radish and greater wheat crop safety than POST.
High efficacy on R and S wild radish populations combined with excellent wheat crop safety were achieved with a range of mesotrione PRE rates identified in 2013 dose-response pot studies. There was no difference (P > 0.05) in survival of R and S wild radish populations at any of the mesotrione rates applied PRE (Figure 3A). As rates increased from 25 to 150 g ai ha−1 there were consistently similar reductions in survival of R and S wild radish populations. There was no effect of increasing mesotrione rates on crop safety with reductions in wheat biomass less than 10% for all rates (Figure 3B). Wild radish was not completely controlled, indicating the need for higher rates of mesotrione applied PRE than those used in 2013.
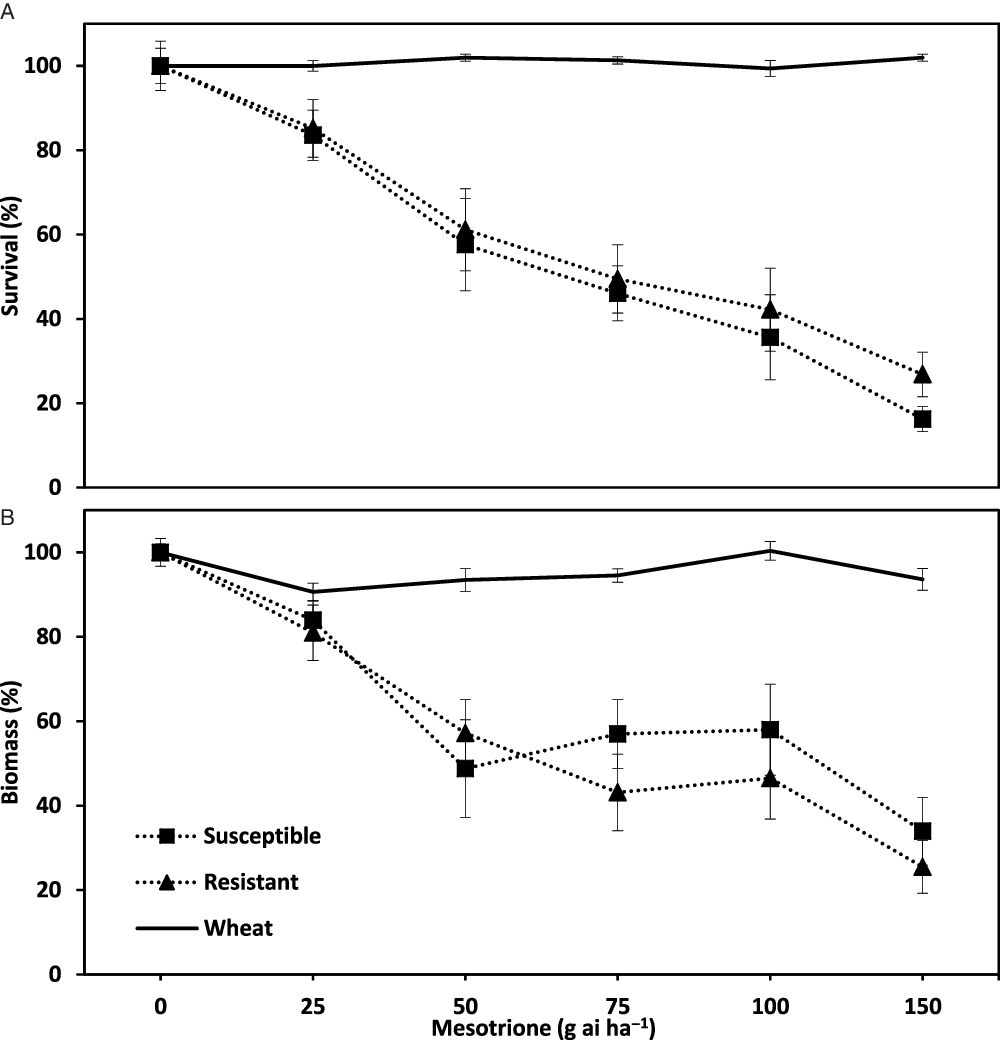
Figure 3. Effect of mesotrione applied preemergence (PRE) on the survival (A) and biomass (B) of herbicide-resistant and -susceptible populations of wild radish and wheat in dose-response pot trials conducted during the 2013 winter growing season at the outdoor growth facility at the University of Western Australia.
The extended range of rates used in the 2014 dose-response studies identified mesotrione rates that provided complete control of R and S wild radish populations with little or no effect on wheat growth. In these studies, mesotrione applied PRE at 200, 300, and 400 g ai ha−1 completely controlled R and S wild radish populations (Figure 4A). At these rates, there were only minor reductions (<5%) in wheat survival, whereas biomass levels were reduced by 5%, 17%, and 22% at the 200, 300, and 400 g ai ha−1 rates, respectively (Figure 4B). These studies identified a mesotrione rate range of >100 and <300 g ai ha−1 for use in field evaluations.
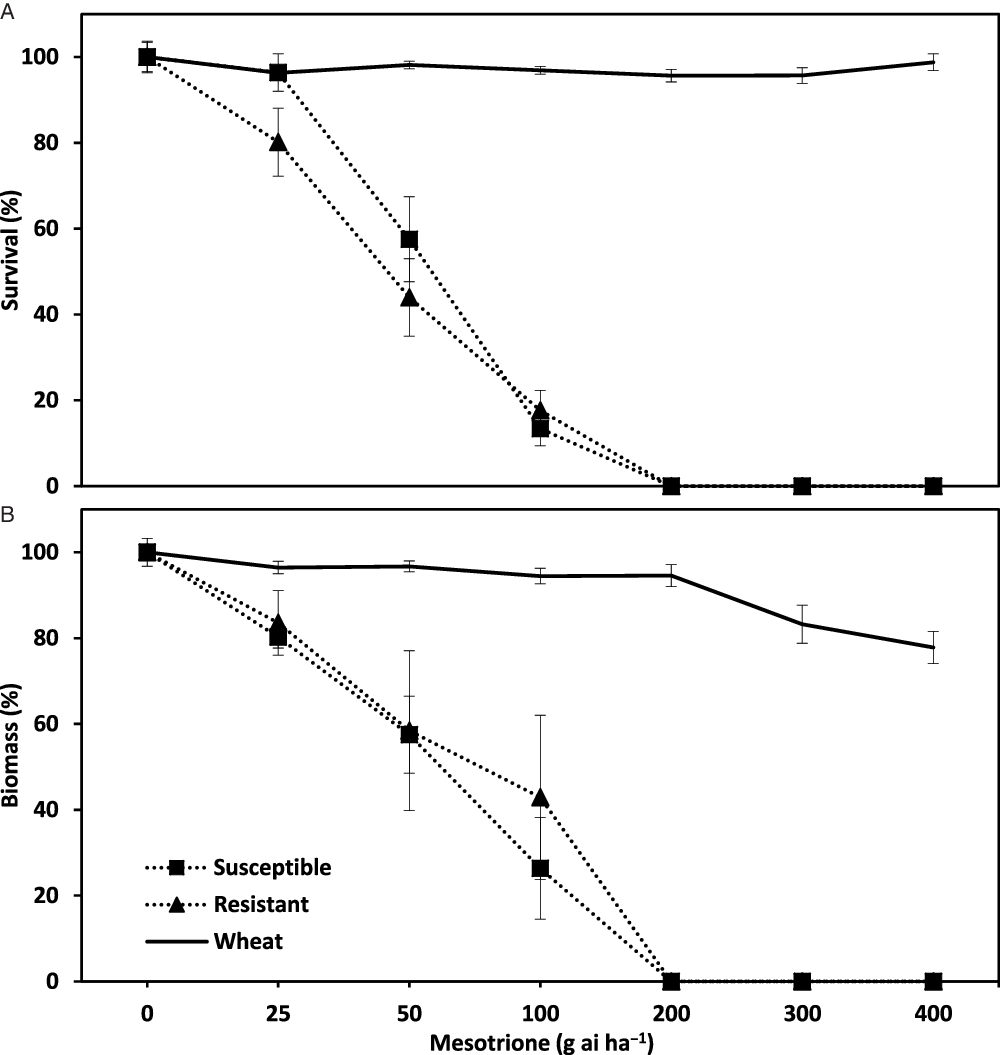
Figure 4. Effect of mesotrione preemergence (PRE) on the survival (A) and biomass (B) of herbicide-resistant and -susceptible populations of wild radish populations and wheat in dose-response pot trials, conducted during the 2014 winter growing season at the outdoor growth facility at the University of Western Australia.
The similar response of R and S wild radish populations to mesotrione PRE indicated the potential for this herbicide to control populations that have evolved resistance to a range of commonly used herbicides. In the 2013 and 2014 pot trials, there were no differences (P > 0.05) in the survival or biomass responses between R and S wild radish populations to mesotrione applied PRE (Figures 2 and 3). Similarly, mesotrione has been shown to be effective on ALS- and PS II-inhibiting herbicide resistant biotypes of a range of weed species (e.g., waterhemp and Palmer amaranth (Amaranthus spp.), common lambsquarters (Chenopodium album), annual sowthistle (Sonchus oleraceus), black nightshade (Solanum nigrum), and common cocklebur (Xanthium strumarium; Sutton et al. Reference Sutton, Richards, Buren and Glasgow2002; Woodyard et al. Reference Woodyard, Hugie and Riechers2009). Despite this weed control opportunity, mesotrione use needs to be judicious as there are instances of evolved resistance to HPPD-inhibiting herbicides in field-collected weed populations (Lu et al. Reference Lu, Yu, Han, Owen and Powles2020; Ma et al. Reference Ma, Kaundun, Tranel, Riggins, McGinness, Hager, Hawkes, McIndoe and Riechers2013; Oliveira et al. Reference Oliveira, Jhala, Gaines, Irmak, Amundsen, Scott and Knezevic2017).
Field Trials
Mesotrione PRE rates identified in dose-response pot trials were similarly effective on wild radish and safe on wheat in field trials. At the 2013 field site at Eradu there was sufficient rainfall (>20 mm) over the 3 wk prior to planting to ensure adequate soil moisture for wild radish emergence and herbicide activity regardless of low rainfall in the 2 wk after planting (Figure 1; BOM 2021). With adequate soil moisture levels, there was similar mesotrione efficacy on the resident wild radish population as was observed in the pot studies. The two highest mesotrione PRE rates of 150 and 200 g ai ha−1 provided ≥90% control of a wild radish populations in the 2013 field trial (Figure 5A). At the first time of assessment, 11 DAP, the 75 and 100 g ai ha−1 rates provided ≥90% control of wild radish, but by 8 WAP the level of control had reduced to 78% and 85%, respectively. There was no change in wild radish control between the 11 DAP and 8 WAP for the 150 and 200 g ai ha−1 rates, highlighting the extended residual activity of mesotrione. Mesotrione at rates of 150 and 200 g ai ha−1 caused appreciable levels of crop injury of 24% and 33%, respectively at 11 DAP (Figure 5B). At 54 WAP, visual crop damage was negligible for all but the highest mesotrione rate.
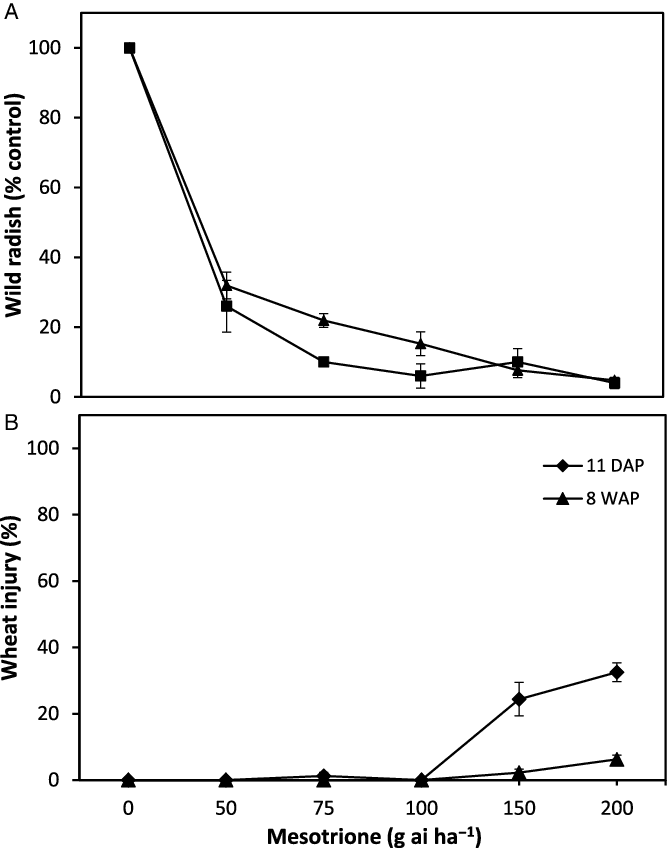
Figure 5. Effect of mesotrione preemergence (PRE) on (A) wild radish emergence and (B) visual ratings of wheat crop damage assessed at 11 d after planting (DAP) and 8 wk after planting (WAP) in a field trial, at Eradu, Western Australia, in 2013.
In the 2014 field trial, mesotrione PRE provided similar wild radish control at rates of 100 g ai ha−1 and higher as observed in the 2013 field trial. At the Mingenew site there was significant rainfall (>20 mm) just after planting to ensure wild radish germination and herbicide activity (Figure 1; BOM 2021). Wild radish emergence was reduced by 86% and 90% by mesotrione PRE rates of 100 and 125 g ai ha−1, respectively, at 11 DAP (Table 3). At 7 WAP, wild radish control was reduced by approximately 30% when mesotrione was applied PRE at the aforementioned rates. Both the 2013 and 2014 field trials highlighted the efficacy and duration of mesotrione PRE rates of 100 g ai ha−1 and higher in substantially reducing wild radish emergence. However, in both trials these rates did not completely prevent wild radish seedling establishment that occurred following rainfall events throughout the growing season (Figure 1). These results indicate the value of mesotrione PRE for the control wild radish, but also highlight the need for supporting POST herbicide applications.
Table 3. Effect of preemergence and postemergence herbicides on wild radish densities and control in a field trial at Mingenew, Western Australia, in 2014.a,d
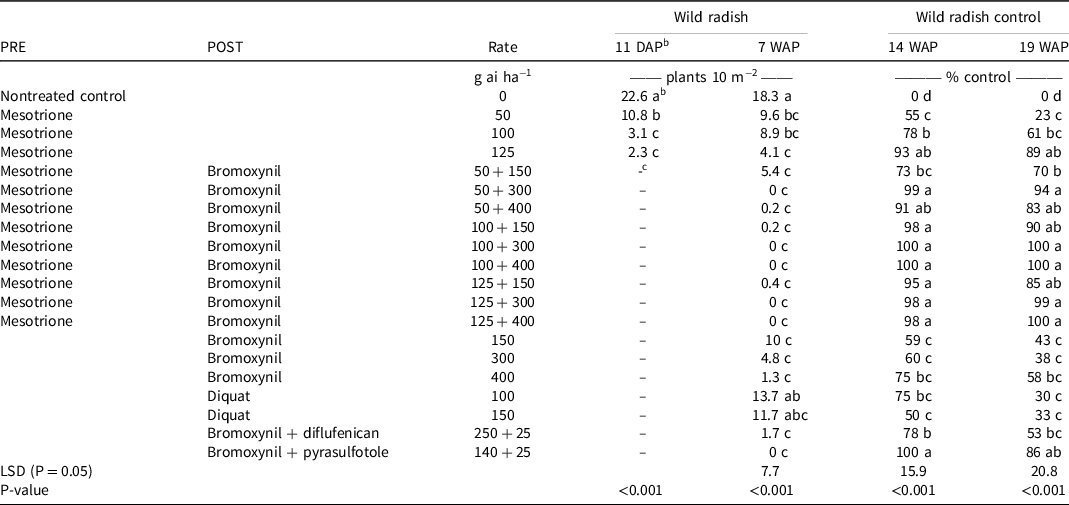
a Means followed by same letter within a column are not significantly different (P > 0.05).
b Wild radish counts conducted at 11 DAP were prior to the application of POST herbicides.
c Cells with a dash indicate where PRE herbicides were not applied.
d Abbreviations: DAP, days after planting; POST, postemergence; PRE, preemergence; WAP, weeks after planting.
Mesotrione applied PRE and bromoxynil applied POST provided season-long and selective control of wild radish in wheat. At the first time of assessment (11 DAP), mesotrione at the highest rate (125 g ai ha−1) provided good but incomplete control (90%) of wild radish (Table 3). At 7 WAP, wild radish populations were 78% lower (P < 0.05) than the nontreated control at all mesotrione PRE rates, highlighting the prolonged residual activity of this herbicide. Despite this, wild radish plant densities did increase over this period. Bromoxynil, bromoxynil plus diflufenican, or bromoxynil plus pyrasulfotole applied at 4 WAP provided excellent control (P < 0.05) of wild radish at 7 WAP. Wild radish control ratings at 14 and 19 WAP indicated that, except for bromoxynil plus pyrasulfotole, these treatments did not control wild radish plants that continued to emerge over this period. The combination of bromoxynil plus pyrasulfotole applied POST provided complete control of wild radish until the final assessment at wheat crop anthesis, 19 WAP. At this stage, mesotrione plus bromoxynil combinations were the most effective. Specifically, 100 g ai ha−1 mesotrione applied PRE plus 300 or 400 g ai ha−1 bromoxynil applied POST provided season-long control of wild radish. Additionally, the mesotrione 125 g ai ha−1 PRE plus bromoxynil 300 or 400 g ai ha−1 POST combination also provided ≥98% control of wild radish over the growing season. At 14 and 19 WAP, visual assessments of wheat determined that none of the treatments caused any crop phytotoxicity (data not presented). These results highlighted the potential for combinations of mesotrione PRE and bromoxynil POST to provide season-long control of wild radish populations in Australian wheat crops.
The combination of mesotrione PRE and bromoxynil POST will likely provide reliably high levels of wild radish control due to the potential for synergy as a consequence of their overlapping activities in disrupting PS II activity. As demonstrated in these studies, the application of mesotrione PRE can provide excellent (90%) early season control of wild radish populations at rates that had little or no impact on wheat growth. As indicated by results from the field studies, an additional POST herbicide (e.g., bromoxynil) was needed to provide season-long control of wild radish. As demonstrated here, the combined effect of an HPPD-inhibiting herbicide, mesotrione PRE, and the PS II-inhibiting herbicide, bromoxynil POST, consistently provided excellent wild radish control due to the efficacy of the individual herbicidal effects as well as possible synergistic interactions (Abendroth et al. Reference Abendroth, Martin and Roeth2006; Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Walsh et al. Reference Walsh, Stratford, Stone and Powles2012). As with all herbicides, the threat of resistance evolution in weed species is ever-present; however, herbicide combinations are a proven strategy in at least delaying this process (Beckie and Reboud Reference Beckie and Reboud2009; Busi et al. Reference Busi, Powles, Beckie and Renton2020; Lagator et al. Reference Lagator, Vogwill, Mead, Colegrave and Neve2013). The combinations of HPPD and PS II inhibitors are particularly robust due to the potential for synergistic activity with evidence that in some cases this has overcome resistance in weed populations (Hugie et al. Reference Hugie, Bollero, Tranel and Riechers2008; Walsh et al. Reference Walsh, Stratford, Stone and Powles2012; Woodyard et al. Reference Woodyard, Hugie and Riechers2009).
Acknowledgments
We thank the Grains Research and Development Corporation and Syngenta for funding this research. We also gratefully acknowledge the technical support with pot studies provided by UWA students. No conflicts of interest have been declared.











