Introduction
The fruticulose thallus type with a minutely shrubby habit is a very rare growth form in the family Teloschistaceae. A comprehensive survey by Poelt & Pelleter (Reference Poelt and Pelleter1984) comprised 10 such species whereas the whole family consists of c. 1000 species (Arup et al. Reference Arup, Søchting and Frödén2013). Since that study, only two more fruticulose species of Teloschistaceae have been described (Poelt & Kalb Reference Poelt and Kalb1985; Arup & Mayrhofer Reference Arup and Mayrhofer2000). Species with this growth form do not belong to a monophyletic group and derive from different genera: Austroplaca, Gondwania, Pachypeltis, Pisutiella, Polycauliona and Teloschistopsis. The affinities of two species (‘Caloplaca’ fragillima Poelt and ‘C.’ mauritanica Werner) are not yet known.
In 2019 and 2020, two authors of the present paper (DH and IS) collected lichens on the Commander Islands, east of the Kamchatka Peninsula in the Russian Far East. During the entire history of investigation of this remote archipelago lichenologists have visited it only twice (Almquist Reference Almquist and Nordenskiöld1887; Trass Reference Trass1963) and the diversity of lichens there is poorly known. Himelbrant and Stepanchikova carried out the first special lichenological studies in the region and, among other lichens, found a peculiar fruticulose species of Teloschistaceae with prominent features not corresponding to any other known fruticulose members of the family. Here we describe it as a new species.
Materials and Methods
Geographical context and sampling
The Commander Islands are the westernmost part of the Aleutian Arc and are located between the Aleutian Islands (USA) and the Kamchatka Peninsula (Russia) on the south-western margin of the Bering Sea (Fig. 1). The archipelago consists of Bering Island (1667 km2), Medny Island (186 km2), two small islets Toporkov (0.25 km2) and Ary Kamen’ (0.08 km2), and several small inshore cliffs. The islands have an ancient volcanic origin and thereby a mountainous landscape with maximal elevations of 755 m a.s.l. (Bering Island) and 647 m a.s.l. (Medny Island). The climate of the Commander Islands is oceanic, temperate and very wet. Average annual temperatures are +2.1 °C on Bering Island and +2.8 °C on Medny Island, with +10.6 °C in August and −3.7 °C in February (Mochalova & Yakubov Reference Mochalova and Yakubov2004). The combination of relief and climatic conditions favour the development of different types of tundra, valley meadows, and rocky and seashore habitats including the specific seabird colonies.

Fig. 1. Beringia and the Commander Islands shown with the known localities (black circles) of the newly described lichen Polycauliona comandorica.
Specimens were collected on four islands (Ary Kamen’, Bering, Medny and Toporkov) with the majority of the collections deposited in LE, and also in H, PRA and the personal herbarium of Frolov.
Phenotype evaluation
Measurements of morphological characters follow Vondrák et al. (Reference Vondrák, Frolov, Arup and Khodosovtsev2013). All microscopic observations are based on hand-cut sections mounted in water, without chemical treatments. Measurements are accurate to 0.5 μm for cells and 5–10 μm for larger structures. In some cases results are given as x̄ 1–x̄ 2–x̄ 3, where x̄ 1 is the minimum, x̄ 2 is the arithmetic mean and x̄ 3 is the maximum. Total number of measurements (n) is given in brackets for some characters measured. Morphological terminology follows Vondrák et al. (Reference Vondrák, Frolov, Arup and Khodosovtsev2013) and the LIAS glossary (available at https://glossary.lias.net/wiki/).
Liquid chromatography-mass spectrometry (LC-MS) metabolite analysis
For the LC-MS analysis, 1–2 mg of air-dried lichens was ground up. The secondary substances from a sample were extracted with 0.1 ml of acetone. Extraction was carried out with constant stirring for 24 h at 20–25 °C. The high-performance liquid chromatography (HPLC) analysis was carried out using an Agilent 1290 instrument (Agilent Technologies, CA, USA). The molecular mass of ions was recorded on an Agilent 6538 UHD quadrupole-time-of-flight (qTOF) mass spectrometer with electrospray ionization (ESI). Elution was carried out in the isocratic mode. A mixture of acetonitrile and 0.1% formic acid aqueous solution in a ratio of 80:20 was used as the mobile phase. The analysis was carried out for 30 min at a flow rate of 100 μl min−1 and a column temperature of 25 °C. For separation, the ZORBAX SB-C18 column was used (150 × 0.5 mm ID, 5 μm, 80 Å). The injection volume was 1 μl and the UV detection wavelength was 270 nm. The voltage on the capillary at the ESI was 2.5 kV; capillary temperature 350 °С; nebulizer gas pressure 45 psi; sheath gas temperature (nitrogen) 225 °C with the flow rate 5 l min−1. Only negatively charged ions were registered, in the mass range of 100–1000 m/z. The resulting chromatograms were processed using the software MassHunter WorkStation v. B.04.00 (Agilent Technologies, CA, USA). To identify lichen substances, we compared the obtained molecular masses and retention times with the lichen substances standards from the Komarov Botanical Institute collection.
DNA extraction, amplification and sequencing
DNA was extracted with a CTAB-based protocol (Aras & Cansaran Reference Aras and Cansaran2006). Amplifications were made of the internal transcribed spacer regions (nrITS) and the large subunit (nrLSU) of the nuclear ribosomal RNA genes, and the small subunit of the mitochondrial ribosomal RNA gene (mrSSU). Primers for PCR amplification were ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) and ITS4 (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) for ITS, AL1R (Döring et al. Reference Döring, Clerc, Grube and Wedin2000) and LR5 (Vilgalys & Hester Reference Vilgalys and Hester1990) for nrLSU, and mrSSU1 (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999) and mrSSU7 (Zhou & Stanosz Reference Zhou and Stanosz2001) for mrSSU. The PCR settings followed Ekman (Reference Ekman2001). Sequences obtained were uploaded onto the NCBI database (GenBank); Accession numbers are provided in Table 1.
Table 1. GenBank Accession numbers and voucher information of the new Polycauliona sequences obtained in this study.

Alignments and phylogenetic analyses
Newly obtained sequences were edited in FinchTV 1.4.0 (Geospiza Inc., Seattle, WA, USA; http://www.geospiza.com) and BioEdit 7.2.5 (Hall Reference Hall1999). We compiled two alignments. The first dataset is the concatenation of the nrITS, nrLSU and mrSSU loci including the holotype of the new species along with the main genera of Teloschistaceae. The phylogeny was rooted with taxa outside the family following Arup et al. (Reference Arup, Søchting and Frödén2013). This analysis was performed only to find the taxonomic position of the new species within the family and is presented in the Supplementary Material (Fig. S1, available online). The second dataset is the nrITS alignment of the genus Polycauliona, rooted following the results of the combined analysis. Both datasets were aligned online by MAFFT v.7 (Katoh & Standley Reference Katoh and Standley2013; available at http://mafft.cbrc.jp/alignment/server/) with the L-INS-i and FFT-NS-I methods (Katoh et al. Reference Katoh, Kuma, Toh and Miyata2005) selected automatically by the program for each dataset. To exclude ambiguously aligned positions, alignments were subsequently analyzed by the automated1 algorithm as implemented in the TrimAl software package (Capella-Gutierrez et al. Reference Capella-Gutierrez, Silla-Martinez and Gabaldon2009). Phylogenetic reconstructions were carried out using Bayesian inference (BI) in MrBayes 3.2.6 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) and analyses were run on the CIPRES Web Portal (http://www.phylo.org/portal2/). Optimum partitioning of the datasets and the optimum substitution models per partition were calculated in PartitionFinder2 using the greedy algorithm and corrected Akaike Information Criterion (Lanfear et al. Reference Lanfear, Frandsen, Wright, Senfeld and Calcott2016). For the ITS alignment input a priori partitions were ITS1, ITS2 and 5.8S, with a single output subset suggested by PartitionFinder along with the GTR + I + G model. MrBayes analyses were performed using two independent runs with four MCMC chains (three cold and one heated) in each run. Trees were sampled every 500th generation. The prior settings for the ITS dataset were: rates of reversible rate matrix = Dirichlet (1.00,1.00,1.00,1.00,1.00,1.00), stationary state frequencies = Dirichlet, shape of scaled gamma distribution of site rates = Exponential (1.00), proportion of invariable sites = Uniform (0.00,1.00), partition-specific rate multiplier was not used, topology = All topologies equally probable a priori, branch lengths = Unconstrained:GammaDir (1.0,0.1000,1.0,1.0). Rate heterogeneity across partitions was allowed (ratepr = variable). The analyses were stopped when the average standard deviation of split frequencies between the simultaneous runs dropped below 0.01 (175 000 generations in the ITS analysis). In the ITS analysis PSRF of the model parameter values ranged from 0.998 to 1.005. The first 25% of trees was discarded as burn-in, and the remaining trees (528 trees in the ITS analysis) were used for construction of a 50% majority-rule consensus tree. Accession numbers of the sequences downloaded from GenBank and used in the analyses are provided in Supplementary Material Table S1 (available online).
Results
According to the combined analysis of nrITS, nrLSU and mrSSU (see Supplementary Material Fig. S1, available online), the new species belongs to the subfamily Xanthorioideae and is nested within the genus Polycauliona. Performing an online NCBI BLAST search with the sequences of the new species demonstrated the same result.
We have inferred a phylogeny of the genus Polycauliona based on Bayesian analysis of the nrITS alignment, including most of the sequences of the genus available in GenBank and four sequences of the new species (Fig. 2). The alignment consists of 52 sequences belonging to 27 species of the ingroup and three species of the outgroup, with a total of 525 positions after trimming. All sequences of the new species form a monophyletic, highly supported clade sister to P. verruculifera (Vain.) Arup et al. The close relationship of these two species is highly supported and is confirmed by the combined analysis (Supplementary Material Fig. S1).

Fig. 2. Phylogeny of the genus Polycauliona based on the Bayesian analysis of nrITS with sequences of the new species included (in bold, with collecting sites and GenBank Accession numbers). Some species with more than one sequence are collapsed into single terminals (numbers in parentheses correspond to the number of samples of a species in a given terminal). Two previously known fruticulose species of the genus are indicated with arrows. Numbers at branches represent posterior probability values ≥ 0.95.
Taxonomy
Polycauliona comandorica Himelbrant, Stepanchikova & I. V. Frolov sp. nov.
MycoBank No.: MB 838399
Fruticulose lichen similar to Polycauliona thamnodes but differing in having a lighter yellow to grey thallus, longer and thicker branches with a rough surface, soredia and blastidia, and in lacking apothecia.
Type: Russia, Kamchatka Territory, Aleutsky District, the Commander Islands, Medny Island, S part of Korabel'naya Bay, 54°40′55.6″N, 167°47′22.4″E, alt. 1 m, supralittoral rocks under a colony of horned puffins, 8 August 2019, D. Himelbrant & I. Stepanchikova Com-Medny-20-2019 (LE L-15420—holotype; H, PRA, hb. Frolov—isotypes). GenBank Accession numbers of the sequences of the isotype from hb. Frolov: MW432184 (nrITS), MW432187 (nrLSU), MW432186 (mrSSU).

Fig. 3. A–E, Polycauliona comandorica. A, mat of thalli with erect branches (field photograph, locus classicus). B, isotype (hb. Frolov). C, detail of branches with soralia (isotype, hb. Frolov). D, thallus with arched outwards and almost prostrate branches (field photograph, Bering Island, S part of Peregrebnaya Bay). E, more or less placodioid thallus with extremely short lobes in centre (LE L-15414, duplicate in hb. Frolov). Scales: A, D & E = 0.5 сm; B = 1 mm; С = 0.5 mm. In colour online.
Thallus usually fruticulose, not areolate in centre, yellow-orange to greenish yellow or grey with yellow spots, c. 0.5–1.5 cm diam. and up to 0.8 cm in height, appearing as rather loose cushions, rarely more or less placodioid with extremely short lobes in centre, often merging and forming large mats of several centimetres with indistinct boundaries between individuals. Branches terete, dichotomous, rarely trichotomous, erect to arched outwards, sometimes almost prostrate, nodulose, 2–8 mm long, 0.60–1.10 × 0.45–0.95 mm in sections, terminal branchlets 0.5–1.3 mm long with rounded tips; horizontal lobes often present together with vertical branches, often with uneven crenate margin, tightly attached to substratum, up to 2 mm long, c. 0.5 mm wide and 0.20–0.65 mm thick, terminal lobes 0.2–1 mm long; branches and lobes with uneven and rough or sometimes smooth surface, rarely with white pruina, often with small greenish or whitish pseudocyphellae, slightly immersed or flush with surface of branches. Cortex unevenly thickened, ‘wavy’ on centre-facing part of sections, 20–57–118 μm (n = 20) thick, prosoplectenchymatous, consisting of anticlinally arranged hyphae that sometimes form cords connecting cortex and medulla and dividing algal layer into discrete clusters; in internal part of cortex cells more elongated, 9.0–11.5–14.5 × 2.0–2.9–3.5 μm (n = 14), with walls c. 1 μm thick, in external part cells more isodiametric, 4.5–6.7–10.0 × 3.5–4.8–7.0 μm (n = 26), with walls 1.5–2 μm thick; epinecral layer sometimes present, up to 5 μm thick. Algal layer unevenly thickened, ‘wavy’ at contact with cortex, c. 15–30 μm thick, often divided into clusters, more rarely continuous, more developed in apical parts of branches and usually absent in their basal parts. Horizontal lobes usually with thinner cortex and algal layer also on lower side. Medulla of irregularly arranged hyphae, often with hollows up to 75 μm diam. Soralia common, appearing on sides of branches, never on tips, grey or yellow, contrasting or concolorous with thallus, discrete at apical part of branches, sometimes limited with indistinct rim, roundish, 0.2–0.5 mm diam., continuous at basal part of branches; soredia large, 35–62–100 μm diam. (n = 17); blastidia also common, often intermixed with soredia, roundish, 50–82–138 μm diam. (n = 23) or elongated, c. 105–125 × 60–70 μm. Prothallus rarely present, thin and whitish.
Apothecia not observed.
Pycnidia sometimes present, not abundant, seen as orange dots, immersed to somewhat raised or flush with surface of branches, up to 260 μm wide. Conidia broadly ellipsoid to bacilliform, 3.0–4.1–6.0 × 1.5–2.0–2.5 μm (n = 17).
Chemistry
Cortex, soredia and blastidia K+ purple in the orange parts of the thallus or K− in the grey parts. Thallus (specimens LE L-15414 and LE L-15419) contains parietin as a major compound, parietinic acid and emodin as additional substances, and traces of citreorosein, emodinal, emodic acid, teloschistin and fallacinal. The specimen LE L-15419 additionally contains traces of erythroglaucin and fragilin. The composition of anthraquinones corresponds to chemosyndrome A of Søchting (Reference Søchting1997).
Etymology
Named after the archipelago and the Commander Islands Nature and Biosphere Reserve to show the known geographical distribution of the new species and our gratitude to the employees of the Reserve, who have made this study possible.
Ecology and distribution
The new species grows on siliceous outcrops or rarely on peat above the outcrops, on seashores in the supralittoral zone or higher (Fig. 4A). All known localities are associated with seabird colonies (Fig. 4B) and the species seems to be ornithocoprophilous. Co-occurring lichen taxa are not diverse and include Myriolecis straminea (Ach.) Śliwa et al., Physcia caesia (Hoffm.) Fürnr., Polycauliona candelaria (L.) Frödén et al., and P. verruculifera (Vain.) Arup et al. The species is known from seven localities on four islands of the Commander Islands (Fig. 1).
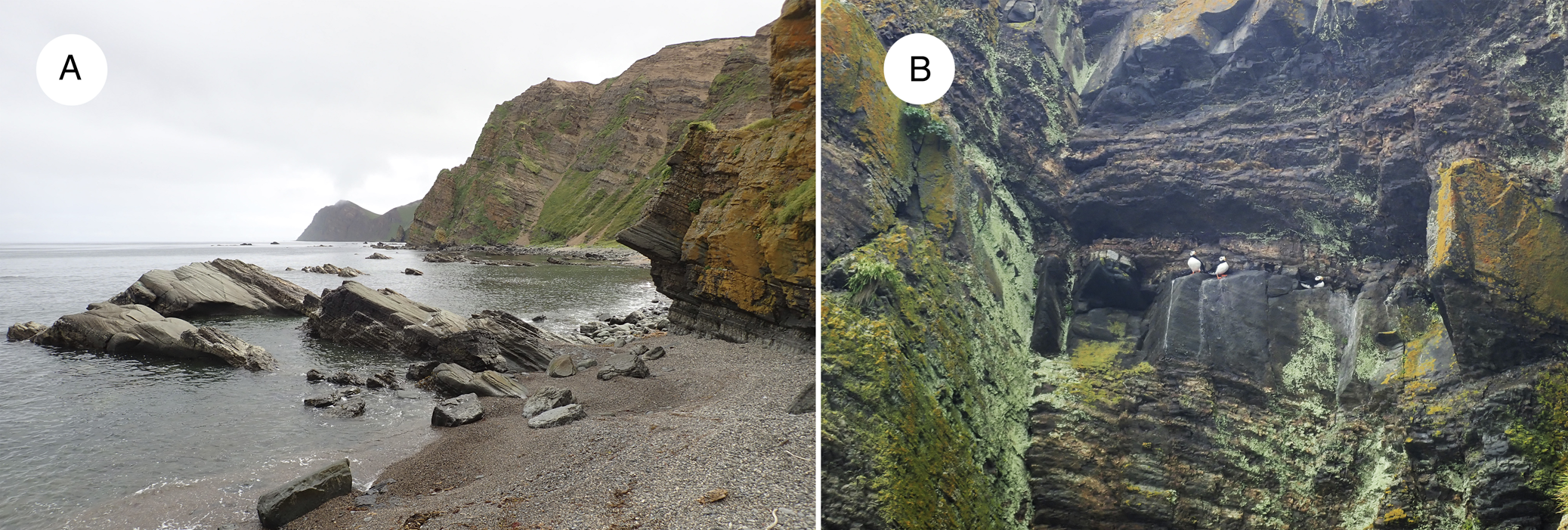
Fig. 4. A & B, type locality of the newly described Polycauliona comandorica. A, coast of the S part of Korabel'naya Bay, Medny Island. B, locus classicus with a colony of horned puffins. In colour online.
Additional material studied
Russia: Kamchatka Territory: Aleutsky District, the Commander Islands, Bering Island, S part of Peregrebnaya Bay, 54°50′09.7″N, 166°38′42.7″E, alt. 0–5 m, 2020, D. Himelbrant & I. Stepanchikova Com-Bering-20-2020 (LE L-15413); Cape Serebryannikova, 54°47′18.3″N, 166°29′12.9″E, alt. 0–4 m, 2020, D. Himelbrant & I. Stepanchikova Com-Bering-27-2020 (LE L-15414); Toporkov Island, SW coast, 55°12′16.2″N, 165°55′55.1″E, alt. 2–3 m, 2020, D. Himelbrant & I. Stepanchikova Com-Toporkov-02-2020 (LE L-15415, LE L-15419); SE coast, 55°12′11.6″N, 165°56′07.8″E, alt. 3 m, 2020, D. Himelbrant & I. Stepanchikova Com-Toporkov-03-2020 (LE L-15416); N coast, 55°12′24.1″N, 165°56′12.6″E, alt. 2 m, 2020, D. Himelbrant & I. Stepanchikova Com-Toporkov-05-2020 (LE L-15417); Ary Kamen’ Island, N summit of island, 55°12′57.7″N, 165°47′27.4″E, alt. 32 m, 2020, D. Himelbrant & I. Stepanchikova Com-Ary-69-2020 (LE L-15418).
Discussion
There are several terms used to refer to lichens that have a minutely shrubby habit. For example, Kärnefelt (Reference Kärnefelt1998), Wetmore & Kärnefelt (Reference Wetmore and Kärnefelt1998) and Arup & Mayrhofer (Reference Arup and Mayrhofer2000) use the term ‘subfruticose’. However, according to the LIAS glossary (available at https://glossary.lias.net/wiki/), the subfruticose growth form is intermediate between foliose and fruticose. In our opinion, it does not reflect the situation in Polycauliona comandorica and other minutely shrubby Teloschistaceae, where such morphotypes are mainly secondarily recruited from crustose lichens (Vondrák et al. Reference Vondrák, Frolov, Arup and Khodosovtsev2013). For the same reason, the terms ‘dwarf-fruticose’ (Ryan et al. Reference Ryan, Bungartz, Nash, Nash, Ryan, Gries and Bungartz2002) and ‘microfruticose’ are not quite correct in our case. Here we prefer to use the term ‘fruticulose’, following its definition by Vondrák et al. (Reference Vondrák, Frolov, Arup and Khodosovtsev2013). Some crustose Antarctic Teloschistaceae, for example ‘Caloplaca’ scolecomarginata Søchting & Olech, Huea coralligera (Hue) C. W. Dodge & G. E. Baker, or to a lesser extent Charcotiana antarctica Søchting et al., form thalli consisting of slim ramified and often anastomosing isidia-like branches (Ott & Sancho Reference Ott and Sancho1993; Søchting & Olech Reference Søchting and Olech2000; Søchting et al. Reference Søchting, Garrido-Benavent, Seppelt, Castello, Pérez-Ortega, de los Ríos Murillo, Frödén and Arup2014). Since in this case vertical branches are located upon the basal crustose part, we believe such thalli do not belong to the fruticulose growth form, although they seem to be an adaptation to the same environmental conditions (see below).
Fruticulose Teloschistaceae occur on all continents but are found mainly in the Southern Hemisphere (Poelt & Pelleter Reference Poelt and Pelleter1984; Poelt & Kalb Reference Poelt and Kalb1985; Arup & Mayrhofer Reference Arup and Mayrhofer2000). Most of the species seem to be ornithocoprophilous, and they grow on rocks along marine coasts where much fog is induced by cold currents (Poelt & Pelleter Reference Poelt and Pelleter1984). The tendency within normally crustose lichen genera towards adopting a fruticulose or similar thallus form in often cold coastal sites manured by birds is also known in other families (Lamb Reference Lamb1968). Such separation from the rock may improve temperature conditions in the photosynthetic parts of the thallus, increase its water uptake capacity and help to avoid the deleterious effects of bird excrements and to compete for space with fast-growing ornithocoprophilous macrolichens (Jacobsen & Kappen Reference Jacobsen and Kappen1988; Ott & Sancho Reference Ott and Sancho1993; Søchting et al. Reference Søchting, Garrido-Benavent, Seppelt, Castello, Pérez-Ortega, de los Ríos Murillo, Frödén and Arup2014).
There are few inland fruticulose Teloschistaceae species. Pachypeltis cladodes (Tuck.) Søchting et al. occurs at over 2000 m elevation within the intermountain system of western North America (Wetmore & Kärnefelt Reference Wetmore and Kärnefelt1998). Pisutiella phaeothamnos (Kalb & Poelt) S. Y. Kondr. et al. is another inland species, occurring in the xerothermic environment of the Mediterranean (Poelt & Kalb Reference Poelt and Kalb1985). Austroplaca erecta (Arup & H. Mayrhofer) Søchting et al. in New Zealand also grows at 850 m above sea level; however, the area receives much fog and drizzle from the sea nearby (Arup & Mayrhofer Reference Arup and Mayrhofer2000). Furthermore, in Antarctica it is known from the seacoast around penguin colonies (Smykla et al. Reference Smykla, Krzewicka, Wilk, Emslie and Śliwa2011).
The new Polycauliona comandorica is also a coastal ornithocoprophilous lichen occurring in a region with a cool and very wet climate (see ‘Geographical context’ in Materials and Methods). It has the most northern geographical range among the coastal fruticulose Teloschistaceae (only the inland Pachypeltis cladodes reaches the same latitude).
According to our molecular data (Fig. 2; Supplementary Material Fig. S1, available online), the new species is explicitly nested within the genus Polycauliona, which does not contradict the morphological and chemical description of the genus by Arup et al. (Reference Arup, Søchting and Frödén2013). Kondratyuk et al. (Reference Kondratyuk, Kärnefelt, Thell, Elix, Kim, Jeong, Yu, Kondratiuk and Hur2014) split Polycauliona and placed P. verruculifera, the closest species to the new P. comandorica, into the monotypic genus Verrucoplaca S. Y. Kondr. et al. However, the latter genus was not accepted by Lücking et al. (Reference Lücking, Hodkinson and Leavitt2016) and was synonymized backwards under Polycauliona. Here we accept the genus Polycauliona as it was proposed by Arup et al. (Reference Arup, Søchting and Frödén2013) and consequently describe the new species in that genus.
In Polycauliona there are two previously known fruticulose species (indicated with arrows on Fig. 2), both occurring on the coast of western North America. Polycauliona thamnodes (Poelt) Arup et al. is the most similar to the new P. comandorica. It also forms the loose cushions with erect to arched outwards terete branches with rounded tips and algal cells arranged in clusters (Poelt & Pelleter Reference Poelt and Pelleter1984; Wetmore & Kärnefelt Reference Wetmore and Kärnefelt1998). This species differs in lacking vegetative propagules, producing apothecia, having slightly shorter (up to 5 mm vs up to 8 mm) and slightly thinner (0.4–0.7 mm vs c. 0.6–1 mm) branches, a darker orange colour, and a more southern distribution (Baja California, Mexico). Another fruticulose Polycauliona, P. coralloides (Tuck.) Hue, differs in lacking vegetative propagules, producing apothecia, having thinner branches (c. 0.2–0.4 mm vs c. 0.6–1 mm), a more southern distribution (northern Baja California, Mexico to northern Oregon, USA), and in avoiding bird-manured sites (Arup Reference Arup1995; Wetmore & Kärnefelt Reference Wetmore and Kärnefelt1998). Forms of P. comandorica lacking well-developed erect branches and resembling lobate lichens (Fig. 3E) could be confused with young thalli of P. verruculifera, having short lobes and a similar anatomical structure of the upper cortex, and an algal layer characteristic of the maritime lichens (Poelt & Romauch Reference Poelt, Romauch, Frey, Hurka and Oberwinkler1977). The latter species, however, forms isidia and never roundish soralia or blastidia; in addition, horizontal lobes of P. comandorica are still terete and usually have a cortex and algal layer also on their lower side, whereas lobes of P. verruculifera are not terete and have a cortex only on the upper side. Other fruticulose Teloschistaceae, three more species in the Northern Hemisphere (‘Caloplaca’ mauritanica, Pachypeltis cladodes and Pisutiella phaeothamnos) and seven species in the Southern Hemisphere, are normally fertile and never form vegetative propagules (Poelt & Pelleter Reference Poelt and Pelleter1984; Poelt & Kalb Reference Poelt and Kalb1985; Arup & Mayrhofer Reference Arup and Mayrhofer2000).
As follows from the phylogenetic reconstructions based on both the combined and the nrITS alignments (Fig. 2; Supplementary Material Fig. S1), fruticulose Polycauliona comandorica pairs with the lobate P. verruculifera. Both lichens grow on the Commander Islands side by side; however, the latter species is distributed much more widely, occurring on the coasts of all northern seas in the Holarctic (e.g. Arup Reference Arup1995), whereas the geographical range of P. comandorica is probably much smaller. Both species form vegetative propagules and P. verruculifera is often fertile. Remarkably, in the genus Polycauliona there is another example of a fruticulose-lobate pair of species, namely the fruticulose P. thamnodes and the lobate P. brattiae (W. A. Weber) Arup et al. (Fig. 2). Both species are fertile and do not form vegetative propagules. The geographical ranges of the species do not overlap but are adjacent to each other. The fruticulose P. thamnodes is known only from Baja California, whereas lobate P. brattiae has a wider and more northern distribution, from southern California to northern Oregon (e.g. Arup Reference Arup1995; Wetmore & Kärnefelt Reference Wetmore and Kärnefelt1998).
Acknowledgements
We would like to thank Evgeny Mamaev and the team from The Commander Islands Nature and Biosphere Reserve for their full support of the expeditions and friendly help during field studies. We also thank Alexey Shavarda who helped with the LC-MS analysis and chromatogram interpretation. IF worked within the framework of the national project of the Institute Botanic Garden (Russian Academy of Sciences, Ural Branch) and project АААА-А20-120040890002-8 of the Sakhalin Branch of Botanical Garden-Institute FEB RAS, and was also supported by the Russian Foundation for Basic Research (RFBR grant no. 19-04-00074). The studies of DH and IS were carried out within the framework of the institutional research project ‘Flora and systematics of algae, lichens, and bryophytes of Russia and phytogeographically important regions of the world’ (no. 121021600184-6) of the Komarov Botanical Institute RAS and supported by the RFBR (grant no. 18-05-60093). The study of IP was carried out within the framework of the institutional research project no. АААА-А18-118032390136-5 of the Komarov Botanical Institute RAS and no. АААА-А21-121012190035-9 of the Institute for Biological Problems of the Cryolithozone SB RAS.
Author ORCID
Ivan V. Frolov, 0000-0003-4454-3229.
Supplementary Material
To view Supplementary Material for this article, please visit https://doi.org/10.1017/S0024282921000268








