Introduction
Maoming Basin of Guangdong Province in southeastern China is famous for its Eocene oil shale deposits belonging to the Youganwo Formation and the numerous vertebrate fossils recovered from these shales (Chow and Liu, Reference Chow and Liu1955; Liu, Reference Liu1957; Yeh, Reference Yeh1958; Chow and Yeh, Reference Chow and Yeh1962; Li, Reference Li1975; Tong and Mo, Reference Tong and Mo2010; Claude et al., Reference Claude, Zhang, Li, Mo, Kuang and Tong2012; Danilov et al., Reference Danilov, Syromyatnikova, Skutschas, Kodrul and Jin2013; Skutschas et al., Reference Skutschas, Danilov, Kodrul and Jin2014; Averianov et al., Reference Averianov, Obraztsova, Danilov, Skutschas and Jin2016). Among the known vertebrate material, many crocodylian specimens are present, nearly all of which have been referred to a single species of the subfamily Tomistominae: Maomingosuchus petrolica Shan et al., Reference Shan, Wu, Cheng and Sato2017 (=Tomistoma petrolica Yeh, Reference Yeh1958). One skull with no mandible, however, representing a short-snouted form was considered by Skutschas et al. (Reference Skutschas, Danilov, Kodrul and Jin2014) to be an indeterminate species of the Alligatoridae (referred to hereafter as the Maoming alligator). The Maoming alligator is too fragmentary to be assigned to any known species with confidence, nor can it be determined to represent a new species. Recently, four additional short-snouted crocodylian skulls recovered from the Youganwo Formation and held in the collections of the Darwin Museum, Keelung of Taiwan, China were prepared for study. All four of the short-snouted skulls were collected from the oil shale beds that form the upper part of the Youganwo Formation from which the specimens of M. petrolica and the Maoming alligator were excavated. As indicated by certain distinct features (e.g., the presence of preorbital ridges, the pointed anterior tip of the frontal, and the inclusion of the splenial in the mandibular symphysis), the four new skulls and the Maoming alligator together represent a new species belonging to the Crocodylia. Here we describe the new crocodylian, focusing on its osteology, taxonomy, and phylogeny. Its discovery not only enriches the local crocodylian assemblage but also sheds some light on our knowledge of the early history of alligatoroid crocodylians, particularly the origin of the Orientalosuchina Massonne et al., Reference Massonne, Vasilyan, Rabi and Böhme2019 and the dispersal route that the latter alligatoroid clade used to move between continents.
Geologic setting and specimen history
The museum labels associated with the four skulls described in this paper indicate that they came from the Maoming Basin, Guangdong Province, China (Fig. 1.1), but provide no additional information concerning the exact fossil locality (more information concerning the history of the specimens is provided with the online supplementary data). The Maoming Basin is ~48 km long and 18 km wide, with a northwest-southeast orientation. The basin is characterized by non-marine sediments, ranging in age from the Late Cretaceous through the Paleogene and into the Neogene. The geology of the Maoming Basin has been well studied since the mid-1990s; Nan and Zhou (Reference Nan and Zhou1996), Ye et al. (Reference Ye, Zhong, Yao, Yang and Zhang1997), and Jin (Reference Jin2008) provide detailed stratigraphic discussions; and Herman et al. (Reference Herman, Spicer, Aleksandrova, Yang, Kodrul, Maslova, Spicer, Chen and Jin2017) contains a thorough geologic overview with pertinent references cited in the bibliography.

Figure 1. Maps and photograph depicting fossil localities of Dongnanosuchus hsui n. gen. n. sp. and other orientalosuchines. (1) Mainland China and Northeastern Vietnam with a close-up of Guangdong Province and portions of surrounding territories; (2) photograph of stratigraphic section from which the specimens of Dongnanosuchus hsui n. gen. n. sp. were collected; (3) the holotype of Dongnanosuchus hsui n. gen. n. sp. before it was fully prepared; (4) relative distances among the fossil localities of orientalosuchines (1 = Dongnanosuchus hsui n. gen. n. sp., Maoming Basin, Guangdong; 2 = Eoalligator chunyii, Nanxiong Basin, Guangdong; 3 = Jiangxisuchus nankangensis, Nankang Basin, Jiangxi; 4 = Protoalligator huiningensis, Huaining, Anhui; 5 = Orientalosuchus naduongensis, Vietnam; 6 = Krabisuchus siamogallicus, Thailand). N = north; Pro = Province.
Based on field work conducted in 2009 by several of the authors at those quarries of the Maoming oil shale in the Yangmeiyong-Datouling and Shantian-Pingtiancun areas, and in particular on information obtained from one working quarry near Zhengtangcun, north of Maoming City, the probable stratigraphic position of the four skulls could be determined. We correlated the matrix associated with these specimens, a coffee-colored oil shale, to the upper part of the Youganwo Formation as exposed in the Maoming Basin (Fig. 1.2, 1.3). Interviews conducted with workers of the Sinopec Maoming Petrochemical Company (Maoming Division) as well as local residents suggest that all vertebrate fossils in the region originate high in the section in the oil shale (see online supplementary data for details). This is also supported by all previous publications of Maoming vertebrate fossils, as cited in the Introduction; notably, the incomplete alligatoroid skull reported on by Skutschas et al. (Reference Skutschas, Danilov, Kodrul and Jin2014)—which is determined here to belong to the same species as the four new skulls—was recovered from the upper section of the Youganwo Formation in association with the oil shale.
The Youganwo Formation generally has been considered to be of middle Eocene–early Oligocene age based on palynological data (Yu and Wu, Reference Yu and Wu1983; Li et al., Reference Li, Zhu, Yan, Guo and Zheng2006), or more narrowly late Eocene in age based on the presence of mammal remains, such as Lunania cf. L. youngi Chow, Reference Chow1962, and pollen (Wang et al., Reference Wang, Zhang and Jin2007; Jin, Reference Jin2008). Following a detailed study of the stratigraphy and associated flora, Herman et al. (Reference Herman, Spicer, Aleksandrova, Yang, Kodrul, Maslova, Spicer, Chen and Jin2017) considered the formation to date to the middle Eocene. The unpublished pollen data collected in our fieldwork (Wei-Ming Wang, personal communication, 2016) suggests that the oil shale of the upper part of the formation is apparently late Eocene in age (based on 11 samples) and that the lower part of the formation is possibly middle Eocene (seven samples).
Material and methods
We prepared the crocodylian specimens using mechanical tools (pneumatic chisels) and photographed them from various perspectives with a Nikon D610 digital camera. The figures were prepared using Adobe Photoshop CS6 and Illustrator CS6. We made line drawings based on the reference photographs and checked them against the original specimens. Measurements of selected skull regions were taken directly from the original specimens. The phylogenetic analyses were performed using TNT v.1.5 (Goloboff and Catalano, Reference Goloboff and Catalano2016).
Repositories and institutional abbreviations
DM = Darwin Museum, Keelung City, Taiwan, China; MMC = collection of crocodylians from the Maoming Basin in the School of Life Sciences, Sun Yat-sen University, Guangzhou, China.
Systematic paleontology
Eusuchia Huxley, Reference Huxley1875
Crocodylia Gmelin, Reference Gmelin1789 (sensu Clark in Benton and Clark, Reference Benton, Clark and Benton1988)
Alligatoroidea Gray, Reference Gray1844, (sensu Brochu, Reference Brochu2003)
Orientalosuchina Massonne et al., Reference Massonne, Vasilyan, Rabi and Böhme2019
Dongnanosuchus new genus
Type species
Dongnanosuchus hsui n. gen. n. sp., by monotype.
Diagnosis
As for type species.
Etymology
Generic name from dongnan (latinization of the Chinese for the fossil quarry located in southeastern China) + suchus (New Latin from the Ancient Greek Σοῦχος [Soûkhos], name of the Egyptian crocodile-headed god Sobek).
Remarks
Numerous fossils of crocodylians with a short snout have been recovered from the Upper Cretaceous to lower Paleogene in China. Most of these fossils are poorly preserved, however, and do not lend themselves to a robust taxonomic assessment. A notable exception is Jiangxisuchus nankangensis Li, Wu, and Rufolo, Reference Li, Wu and Rufolo2019, which is based on a nearly complete cranium associated with an incomplete mandible. Dongnanosuchus hsui n. gen. n. sp. represents another such exception and is established here based on four well-preserved crania, one of which is fossilized along with a nearly complete mandible. These specimens not only confirm the presence of the Alligatoroidea in the Maoming Basin in the late Eocene, but also represent the most complete fossil material currently known from China for a member of the Alligatoroidea.
Dongnanosuchus hsui new species
Figures 2–8; Table 1

Figure 2. Photographs and line drawings depicting the Holotype (DM000001-F000001) of Dongnanosuchus hsui n. gen. n. sp. in dorsal view (1, 2) and left lateral view (3, 4). Zigzag lines indicate a broken surface. ec = ectopterygoid; en = external naris; f = frontal; fcqc = foramen for cranio-quadrate canal; if = incisive foramen; itf = infratemporal fenestra; j = jugal; l = lacrimal; m = maxilla; mt = matrix; n = nasal; ob = orbit; obr = orbital ridge; p = parietal; pf = prefrontal; pm = premaxilla; po = postorbital; pr = preorbital crest; pt = pterygoid; q = quadrate; qj = quadratojugal; so = supraoccipital; sq = squamosal; stf = supratemporal fenestra; stfo = supratemporal fossa; th = tooth.

Figure 3. Skulls of Dongnanosuchus hsui n. gen. n. sp. (1) photograph and (2) line drawing of specimen DM000001-F000002 in anterodorsal view; (3) photograph and (4) line drawing of the holotype (DM000001-F000001) in ventral view; (5) photograph and (6) line drawing of the postorbital bar of the holotype (DM000001-F000001) in dorsolateral views. Zigzag lines indicate a broken surface. bo = basioccipital; CA = Crest A; CB = Crest B; ch = internal choana; oft = occlusion fossa; pl = palatine; pm-mf = premaxillary-maxillary fossa; pm-mn = premaxillary-maxillary notch; sof = suborbital fenestra; vo = vomer; other abbreviations as in Figure 2.
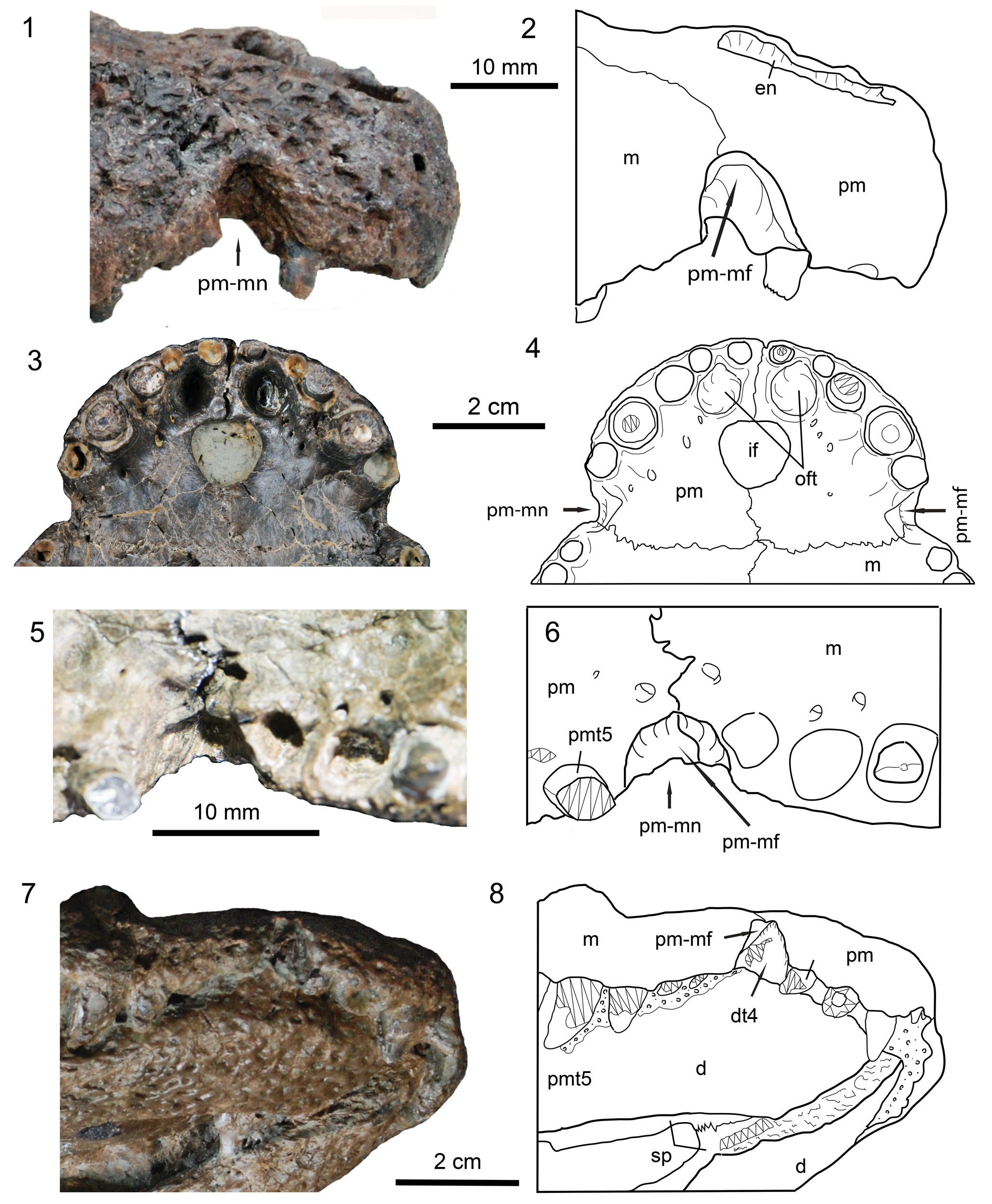
Figure 4. Detailed views of parts of the rostrum on skulls of Dongnanosuchus hsui n. gen. n. sp. (1, 3) photographs and (2, 4) corresponding line drawings of the tip of the superior rostrum of specimen DM000001-F000002 in right lateral and ventral views; (5) photograph and (6) line drawing showing the right premaxillary-maxillary fossa and notch of the holotype (DM000001-F000001) in ventral view; (7) photograph and (8) line drawing showing dentary tooth 4 sitting in the premaxillary-maxillary fossa of specimen DM000001-F000004 oriented in ventral and slightly lateral view. Zigzag lines indicate a broken surface and fields of circles/dots indicate matrix. d = dentary; dt4 = dentary tooth 4; pmt5 = premaxillary tooth 5; sp = splenial; other abbreviations as in Figures 2, 3.
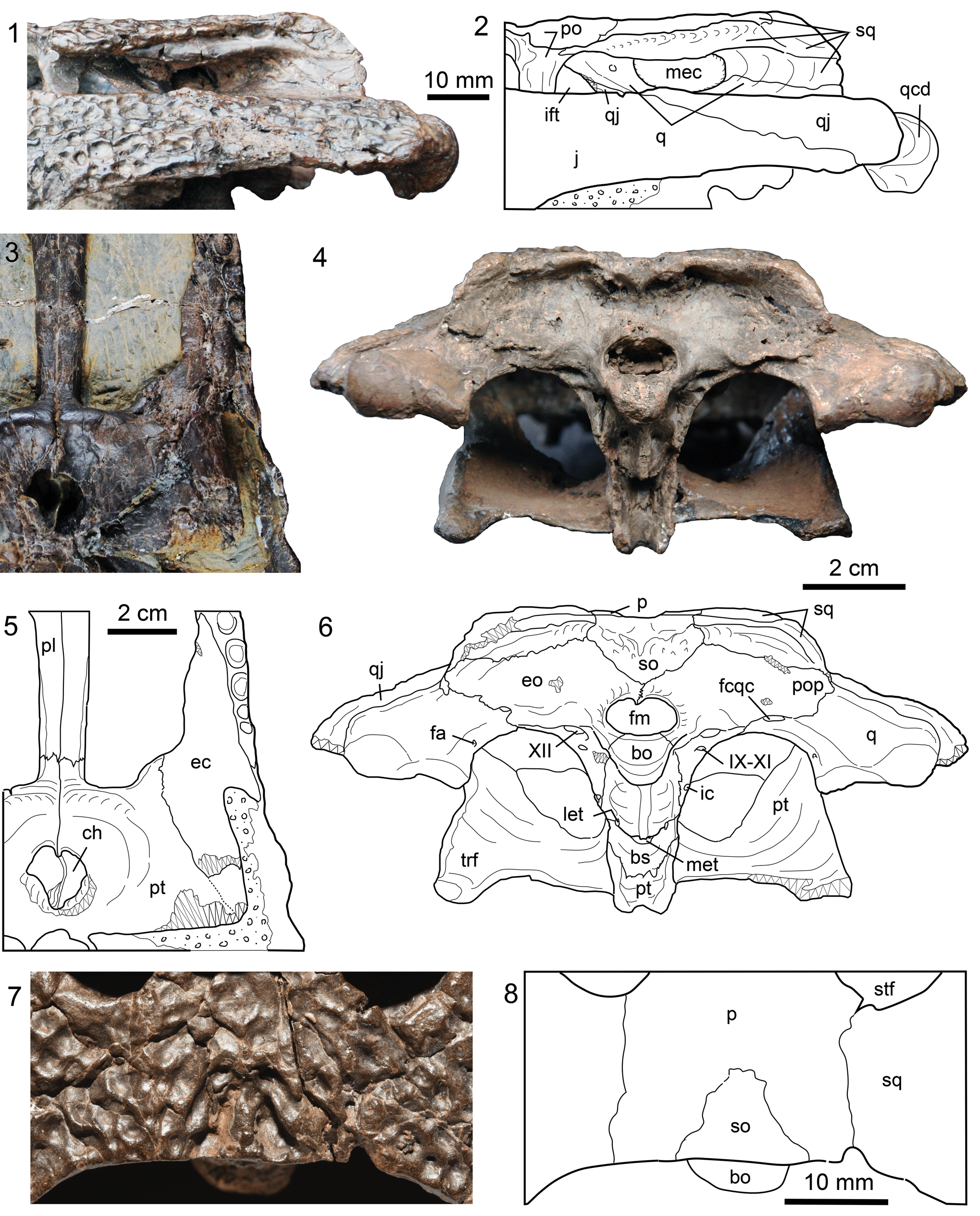
Figure 5. Skull parts of Dongnanosuchus hsui n. gen. n. sp. (1, 4) photographs and (2, 6) corresponding line drawings of the temporal region of the holotype (DM000001-F000001) in left lateral (1, 2) and occipital views (4, 6); (3) photograph and (5) corresponding line drawing of a part of the palate in ventral view (specimen DM000001-F000002); (7) a photograph and (8) corresponding line drawing of a part of the posterior skull table in dorsal view (specimen DM000001-F000003). Zigzag lines indicate a broken surface and fields of circles/dots indicate the matrix. bs = basisphenoid; eo = exoccipital; fa = foramen aëreum; fm = foramen magnum; ic = foramen for internal carotid artery; let = foramen for lateral eustachian tube; mec = middle ear chamber; met = foramen for medial eustachian tube; pop = paraoccipital process; qcd = quadrate condyle; so = supraoccipital; trf = transverse process; IX-XI = foramen for cranial nerves IX-XI; XII = foramen for cranial nerve XII; Other abbreviations as in Figures 2–4.

Figure 6. The cranium, mandible, and two teeth of Dongnanosuchus hsui n. gen. n. sp. (1) Photograph of specimen DM000001-F000004 in left lateral view with (2) the region enclosed as 1’ shown in closer view and as (3) a line drawing, highlighting the absence of the external mandibular fenestra; (4) close-up image of right maxillary tooth 4 of the holotype (DM000001-F000001) in lateral view; (5) and (6) close-up photographs of right maxillary tooth 11 of specimen DM000001-F000002 in lateral and occlusion views, respectively, showing subdued striations. Arrows direct posteriorly. Zigzag lines indicate a broken surface. afi = artificial filling; an = angular; ar = articular; bh = broken hole; blmpt = boundary limit for m. pterygoideus; fpt = facet for the insertion of M. pterygoideus; sa = surangular; sst = subdued striations; other abbreviations as in Figure 2.

Figure 7. The cranium and mandible of Dongnanosuchus hsui n. gen. n. sp. (specimen DM000001-F000004). (1) Photograph in right lateral view, with (2) a close-up image of the region bounded as 1’, and (3) its corresponding line drawing demonstrating the absence of the external mandibular fenestra. Zigzag lines indicate a broken surface and fields of circles/dots indicate the matrix. Anatomical abbreviations as in Figures 2, 4, 6.

Figure 8. The cranium and mandible of Dongnanosuchus hsui n. gen. n. sp. (specimen DM000001-F000004). (1) Photograph in ventral and slightly lateral view, with (2) a close-up view of the area bounded as 1’ and (3) its corresponding line drawing. fic = foramen intermandibularis caudalis; other abbreviations as in Figures 2–4, 6.
Table 1. Measurements of selected skull elements of Dongnanosuchus hsui n. gen. n. sp. Units = cm, † = preserved length or width, * = estimated length or width.

Holotype
DM000001-F000001, a nearly complete cranium.
Diagnosis
A small to medium-sized alligatoroid, distinguishable from others in the combination of the following characters: fourth premaxillary tooth largest; fifth maxillary tooth largest; single external naris slightly wider than long; premaxillary-maxillary notch shallow in dorsal or ventral views, but deep in lateral view; a pit/fossa present on the wall of premaxillary-maxillary notch for the fourth dentary tooth; a longitudinally oriented preorbital ridge connecting posteriorly with dorsal orbital rim and a ridge around anteroventral margin of orbit; lacrimal much longer than both frontal and prefrontal; post-supratemporal bar anteroposteriorly much longer than pre-supratemporal bar; quadrate entering the anterior exit of cranio-quadrate canal (orbito-temporal foramen) within supratemporal fossa; large suborbital fenestra nearly as long as pre-suborbital region; palatine-pterygoid suture located in front of suborbital fenestrae; ectopterygoid-maxillary suture extending forward passing through 4–5 maxillary tooth alveoli; choanae separated by a thin and inset septum; and no external mandibular fenestra.
Occurrence
Maoming Basin, Guangdong Province, southeastern China (see online supplementary data); the upper part of the Youganwo Formation, late Eocene.
Description
The holotype cranium is nearly complete, except for the anteriormost tip and the region around the choanae; its dorsal and ventral surfaces are well preserved. It has a preserved length of 20.7 cm from the anterior end of the snout to the occipital condyle. With an estimated length of ~5 mm for the missing end of the snout, the skull reached a total length (to the occipital condyle) of >20 cm in life, very similar to that of the skull of specimen DM000001-F000002 (Table 1). The maximum width of the skull achieves 12.8 cm across the posterior ends of the jugals. The snout is slightly longer than the post-snout region (measured along the dorsal midline), as in Jiangxisuchus nankangensis. The skull table is wider than long and the bar posterior to the supratemporal fossa is much broader than the bar between the orbit and the fossa (Fig. 2.1, 2.2). The occipital margin of the table does not rise up to form a ridge.
In dorsal view, the orbit is elliptic in shape and faces dorsally; it is the largest among the skull openings, more than twice as long as the supratemporal fossa. The latter is the smallest dorsal opening, even smaller than the unpaired external naris (Table 1). The naris is wider than long, with a dorsal orientation. The infratemporal fenestra is triangular in outline and taller than wide. The dorsal border of the orbit forms a pronounced ridge/rim, extending anteriorly to the preorbital ridge; the latter being very strong and extending along the dorsal midline of the lacrimal to continue anteriorly along the maxillary-nasal suture (Fig. 3.1, 3.2). It fades out gradually before reaching to the nasal-premaxillary suture. Posterolaterally, the preorbital ridge connects with an orbital ridge extending on the dorsal surfaces of the lacrimal and jugal around the anteroventral border of the orbit. The dental margin is notably concavo-convex, as in Jiangxisuchus nankangensis, with the first concavity at the premaxillary-maxillary suture and the second at the point between the seventh and eighth alveoli. In ventral view, the suborbital fenestra is roughly triangular in outline and characterized by its large size, being similar in length to the palatine or the region anterior to the fenestra (Table 1). The apple-shaped incisive foramen, which is preserved in its entirety in specimen DM000001-F000002, is medium-sized, much narrower than half the greatest width of the premaxillae (Fig. 4.3, 4.4). The internal choanae, which can be restored in specimen DM000001-F000002 (Fig. 5.3, 5.5), are divided by a thin septum that is inset a distance from the posterior edge of this internal opening. The external surface of all roof bones is well ornamented by pits and short ridges, except for the small anteromedial portion of the quadratojugal and postorbital bar. There is a pair of large, ovoid pits paralleling the midline on the posterior surface of the parietal.
Skull roof.—The premaxilla is complete in specimen DM000001-F000002 and longer than wide in both dorsal and ventral views, with a constriction caused by a notch present at the lateral end of the premaxillary-maxillary suture (Figs. 2–4). The notch is shallow in both dorsal and ventral views, but very deep in lateral view. In contrast to other crocodylians, such as Leidyosuchus (Wu et al., Reference Wu, Russell and Brinkman2001), which possesses such a notch, the fourth dentary tooth fits into a pit or a fossa on the dorsal wall of the notch rather than passing through the notch to be exposed along the dorsal surface when the jaws are occluded (Fig. 4.7, 4.8). The narrow anterior processes of the nasals separate two premaxillae along the posterior margin of the naris, just as in Jiangxisuchus nankangensis. The dorsal surface of the premaxilla is slightly elevated along the lateral margin of the naris. The maxillary process of the bone is moderately long, exceeding the posterior margin of the third maxillary tooth. In ventral view, the anteroventral portion anterior to the incisive foramen is ~10 mm in length, much longer than the anterodorsal portion anterior to the external naris. The premaxillary-maxillary suture is distant from the posterior margin of the incisive foramen, >10 mm posterior to the foramen, and this suture extends laterally and slightly anteriorly from the midline, then follows a straight line to end in the premaxillary-maxillary notch. Posterior to the first and second teeth of the upper jaw, there is a deep fossa on each side lateral to the midline, which accommodates the first dentary tooth (Fig. 4.3, 4.4).
The nasal is strap-like, with a pointed anterior process and a relatively short but pointed posterior process (Fig. 2.1, 2.2). The anterior process of each nasal meets its counterpart along the midline and enters the external naris. The nasals widen slightly in the posterior direction as they approach the lacrimals; from the point of contact, they taper off to a wedge-shaped process that does not abut its counterpart directly. Rather, the posterior end of each nasal diverges posterolaterally to insert between the prefrontal and frontal, thus bounding a space that receives the tip of the anterior process of the frontal. Sutures of the nasal with the premaxilla, maxilla, lacrimal, and prefrontal form a convex line towards the lateral side.
The maxilla is the second longest bone among the cranial elements, being relatively shorter in dorsal view but longer in lateral view than the nasal (Figs. 2, 3). It narrows slightly anteriorly, but broadens across the fifth maxillary teeth and then sharply reduces in width posteriorly. The maxilla exhibits no significant elevations or depressions on the dorsal surface, save for the anterior portion of the preorbital ridge, and its maximal width is wider than that of the two nasals. The maxilla, which does not possess a narrow, posterodorsal process inserting between the nasal and lacrimal, terminates posterolaterally along a broadly inclined suture with the jugal. Sutural contacts of the maxilla with the premaxilla, nasal, lacrimal, and jugal are clear on the right side of the holotype specimen. In lateral view, the dental margin is strongly concavo-convex, forming two waves (festoons). The trough of the first wave is at the fifth maxillary alveolus and that of the second wave is at the eleventh maxillary alveolus. In palatal view, there are numerous small nutrient foramina surrounding the tooth row. There is a fossa medial to the dentition between the seventh and eighth maxillary alveoli, which serves to accommodate a dentary tooth when the jaws are closed. The maxillary-palatine suture is sharply convex toward the maxilla. The maxillary-ectopterygoid suture extends along the medial side of the posterior five of 14 maxillary alveoli. The maxilla terminates anterior to the infratemporal bar.
The lacrimal is an irregular bone in dorsal view (Fig. 2.1, 2.2). It is posteriorly broad and anteriorly narrow. It extends forward to approximately the level of the seventh maxillary alveolus. The lacrimal has a lightly convex sutural contact with the nasal and prefrontal and with the maxilla and jugal. Posteriorly, it is very concave and forms the anterior border of the orbit.
The prefrontal is much smaller than the lacrimal, about half the size of the latter (Fig. 2.1, 2.2). It is roughly rhomboid in dorsal view, with a slightly concave sutural contact with the nasal. Its anterior end reaches the middle of the lacrimal and forms the anteromedial margin of the orbit posteriorly. Sutures with the lacrimal and frontal are nearly parallel.
The unpaired frontal is longer than the nasal (Fig. 2.1, 2.2). It broadens posteriorly and narrows anteriorly, and the sharply pointed anterior process inserts into the nasals. The broadened posterior portion does not enter the supratemporal fossa. The dorsal surface of the frontal is nearly flat. The frontal-parietal suture runs nearly straight in a transverse orientation, exhibiting many fine interdigitations. The frontal forms the rimmed medial margin of the orbit. The interorbital portion is moderately broad, ~16 mm in width. Sutures with the postorbitals are slightly oblique inwards and finely interdigitated.
The postorbital comprises a dorsal body that is roughly rectangular and a slender descending process forming the dorsal half of the postorbital bar (Figs. 2, 5.1, 5.2). The dorsal surface of the body is flat and forms the rounded anterolateral corner of the skull table. It borders the posterodorsal margin of the orbit anteriorly and the anterolateral margin of the supratemporal fossa posteriorly. A tongue-like process from the anteromedial margin of the body enters the supratemporal fossa and meets the parietal within the fossa along the supratemporal fenestra. The descending process is columnar dorsally and inset medially from the skull table. It flattens ventrally and passes lateral to the ascending process of the jugal (Fig. 3.5, 3.6). The postorbital contacts the quadrate on the ventral surface of the skull table.
The single parietal lies between the supratemporal fossae. It is laterally concave in the skull roof and forms the medial floor of the fossae (Fig. 2). It rises up to form an elevated rim around the medial margin of the fossae. The interfenestral bar is broad (~15.5 mm wide), just slightly narrower than the interorbital bar. The nearly straight suture with the frontal does not enter the supratemporal fossae. The parietal contacts the quadrate broadly within the supratemporal fossa, but is excluded by the quadrate from the squamosal in the fossa (Fig. S4 in the online supplementary data). The parietal contacts the squamosal posterolaterally and the supraoccipital posteriorly behind the fossa. Dorsal ornamentation of the parietal consists of pits and ridges, and a peculiarly large and triangular pit/depression is present along the midline on both sides of the posterodorsal roof.
The anterior ramus of the jugal is much broader than, but nearly as long as, the posterior ramus. It narrows and inserts between the maxilla and lacrimal anteriorly (Fig. 2). The jugal widens posteriorly, reaching its maximum dorsoventral width at the lateral orbital margin, at which point the anterior ramus becomes narrower until it reaches the end. The jugal extends to the quadratojugal at the posteroventral corner of the infratemporal fenestra dorsolaterally, while it reaches the quadratojugal anterior to the mandibular condyle ventrolaterally. The jugal forms the entirety of the ventral margin of the infratemporal fenestra in dorsal view. The infratemporal bar is broad and lateromedially thin. The cylindrical ascending process of the jugal is inset from the surface of the bone and extends dorsally to form the lower portion of the postorbital bar (Fig. 3.5, 3.6).
Both quadratojugals are nearly complete, with no anterior process to join the formation of the infratemporal bar in dorsal view (Figs. 2, 3). In lateral view, the anteroventral portion is ornamented by pits, forming the posteroventral border of the infratemporal fenestra and extends posteriorly nearly to the lateral condyle of the quadrate, while the posterodorsal portion is unornamented and narrows dorsally, forming the greater part of the posterior margin of this fenestra where no spine is present. The posterodorsal extremity is missing in all four specimens examined for this study, which leads to an uncertainty if the quadratojugal meets the postorbital or not along the dorsal border of the infratemporal fenestra in life. In ventral view, the quadratojugal is concave. It has an anterior process that sharply tapers off anteriorly and inserts into the jugal along the posteromedial surface of the infratemporal bar. The quadratojugal-quadrate suture is nearly straight and extends posterolaterally.
The squamosal forms the posterolateral margin of the supratemporal fossa dorsally (Figs. 2, 4). It contacts the postorbital anteriorly, passing below the latter and ending posterodorsal to the base of the postorbital bar. The squamosal is excluded by the postorbital-quadrate contact from the infratemporal fenestra (Fig. 5.1, 5.2). The squamosal extends laterally over the otic aperture to form a deep otic recess and forms the dorsal roof and posterior wall of the aperture itself. In lateral view, the squamosal presents a groove for the external ear valve musculature, which traverses the squamosal from its posterior region to the postorbital. The dorsal and ventral margins of the groove are parallel and the dorsal margin appears inflated and overhangs the ventral margin, as in some other crocodylians, such as Jiangxisuchus (Li et al., Reference Li, Wu and Rufolo2019). The squamosal contacts the parietal behind the supratemporal fossa. Its posterolateral process is well developed and its entire surface is full of pits and ridges. The process extends posterolaterally and terminates as a vertically oriented lamina-like structure against the paraoccipital process of the exoccipital posterolaterally and the dorsal surface of the quadrate body ventrally.
The quadrate, which is nearly complete on both sides (Figs. 2–5), is a complex bone, forming the anterior and ventral margins of the otic recess. As described earlier, the dorsal and posterior margins of the recess are formed by the squamosal (i.e., the quadrate-squamosal suture extends dorsally to the posterolateral corner of the recess; Figs. 2.3, 2.4, 5.1, 5.6). The dorsal surface of the quadrate body is not ornamented and the dorsal prominence, with the squamosal and paraoccipital process, encloses the cranio-quadrate foramen medially. The position of the foramen aëreum is close to the medial margin of the bone. The lateral condyle is noticeably larger than the medial condyle. In dorsal view, the anterodorsal process of the quadrate broadly enters the orbito-temporal foramen within the supratemporal fossa, which excludes the squamosal from contacting the parietal in the fossa. Ventrally, the quadrate is concave and crests A and B of Iordansky (Reference Iordansky, Gans and Parson1973) for the attachments of adductor muscles are well developed. The quadrate extends mediodorsally to meet the pterygoid and, together, underlies the basisphenoid medially.
Palate.—The paired palatines are relatively small and shorter than the long axis of the suborbital fenestra (Fig. 3.2, 3.3). The palatines comprise the medial margin of the suborbital fenestrae except for the anteromedial and posteromedial portions. The bone is strap-like, widens toward its anterior end, then narrows anteromedially. Its suture with the pterygoid is located anterior to the posteriormost level of the suborbital fenestra. The palatines are split along their suture, which exposes small portions of other bones anteriorly, which may represent the hidden vomers.
The large and broad pterygoid is divided anterior to the internal choana (Figs. 2.3, 2.4, 3.3, 3.4, 5.5, 5.6). In ventral view, its anterior portion is relatively narrower than its posterior portion. The flange is massive and extends posteriorly to form a pronounced posterolateral process projecting to a level evidently posterior to the posteromedial processes. The latter is small but well marked, overhanging the braincase in ventral view. The internal choanae are located at the posterior half of the pterygoid and divided by a thin septum that is inset from the surface on which the choanae open to join the pharyngeal cavity. The surface lateral to the choanae is depressed and no evident crest surrounds the choanae. Posterodorsally, the pterygoid underlies the basisphenoid and meets the quadrate. Anteroventrally, the divided pterygoids contribute to the medial part of the concavo-convex posterior margin of the suborbital fenestra and send a short process to join the formation of the septum between the suborbital fenestrae.
The ectopterygoid is relatively large, with its waisted portion much broader than the narrowest part of the two palatines between the suborbital fenestrae (Figs. 3.3, 3.4, 5.5). Anteromedially, the ectopterygoid forms the posterolateral margin of the suborbital fenestra where it is slightly arched dorsally. Laterally, the maxillary process abuts against the margin of the alveolar groove and extends anteriorly, passing by 4.5–5 maxillary alveoli (Figs. 3.4, 5.5). The jugal process appears much shorter than the maxillary process. Medially, the pterygoid process tapers off distally and extends posteroventrally along the lateral margin of the pterygoid flange, but it does not reach the caudal end of the flange.
Braincase.—The supraoccipital is largely exposed on the skull roof in specimen DM000001-F000003 and the exposed dorsal surface is ornamented mainly by a pair of large pits. The supraoccipital-parietal suture is roughly V-shaped (Fig. 5.7, 5.8). In the other specimens, the dorsal exposure of the supraoccipital is difficult to determine due to cracks formed in the area (Fig. 2.2; Figs. S6-S8 in the online supplementary data). However, a pair of large pits is present on the dorsal surface of the bone in the holotype and specimen DM000001-F000002 (Fig. S6 in the online supplementary data), whereases the pair of pits appears absent in specimen DM000001-F000004 (Fig. S8 in the online supplementary data). The supraoccipital appears irregularly pentagonal in occipital view (Fig. 5.4, 5.6–5.8). It bears a weak median ridge, running along the dorsal half of the bone. The dorsal third of the bone is convex and joins the formation of the thickened posterior edge of the skull roof dorsally, which overhangs the occiput. The ventral two-thirds of the bone is concave and does not enter the foramen magnum. Sutures of the bone with the parietal, squamosal, and exoccipital are clearly marked, showing no trace of the posttemporal fenestra, indicating the mature state of the skull.
The large exoccipitals form more than half the occiput and enclose the foramen magnum laterally and dorsally, separating the supraoccipital from the foramen (Fig. 5.4, 5.6). The exoccipitals are transversely divided into a large upper portion and a small lower portion. The upper portion is noticeably concave with a paraoccipital process that is stout and does not extend laterally to reach the lateralmost portion of the squamosal. The lower portion is inset from the surface of the bone; it is dorsoventrally narrow and lateromedially short. Laterally, the lower and upper portions of the exoccipital form the medial, dorsal, and ventral margins of the cranio-quadrate foramen. Medially, the lower portion dorsoventrally broadens and bears three foramina lateral to the foramen magnum. The dorsal-most foramen in occipital view is the largest and identified as the one for the exit of cranial nerve XII. The foramen slightly lateral and anterior to the former is considered to be the exit of cranial nerves IX to XI and the ventral foramen close to the margin of the bone is for the exit of the internal carotid artery (see Fig. S5 in the online supplementary data for details). Sutures with the squamosal, supraoccipital, basioccipital, and quadrate are clearly marked.
The basioccipital consists of a condyle and a plate (Figs. 3, 5). The basioccipital condyle is semicircular in posterior view, its slightly concave dorsal surface forming the floor of the braincase chamber. In ventral view, the condyle is nearly round. The basioccipital plate is nearly vertical and strongly concave, with a well-marked median ridge from the base of the plate down to the margin of the median exit of the Eustachian tube. The vertical plate slightly broadens first and then gradually narrows ventrally. It encloses the posteromedial margin of the lateral exit and the posterior margin of the median exit of the Eustachian tube.
The basisphenoid is exposed to a limited extent between the pterygoid-quadrate connection and the basioccipital in lateral view, and between the pterygoid and basioccipital in occipital view (Fig. 5.4, 5.6). It forms the anterolateral margin of the lateral exit and the anterior margin of the central exit of the Eustachian tube. The laterosphenoid and prootic are covered by matrix.
Mandible.—An additional specimen (DM000001- F000004) is the only one that has the mandible preserved (Figs. 6–8). All the bones comprising the mandible are more or less limited in terms of their exposure because the jaw is positioned tightly against the cranium. Both rami of the mandible, which possess heavily ornamented external surfaces, are damaged posteriorly and the retroarticular processes are completely absent. A broken area along the ventral side of the left ramus was filled with artificial material during preparation in order to provide greater stability to the specimen (Fig. 6). Posterolaterally, an opening observed on the left ramus is considered to be indicative of damage to the fossil in terms of uneven edges. On the right ramus, the external mandibular fenestra, which is present in most archosaurians, is absent.
The dentary is nearly complete, but its relationships with the post-dentary bones were obscured due to fusion. The symphysis is short and possibly reaches to a level posterior to the sixth dentary tooth, but the dentary portion of the symphysis ends at the level of the fourth dentary tooth (Fig. 4.8). It is broadest across the fourth dentary teeth and the symphyseal portion seems to be dorsoventrally shallow. In lateral view, the dental margin is strongly concavo-convex, as is the upper jaw.
The right splenial is mostly exposed (Figs. 4.8, 8). Anteriorly, it joins the formation of the symphysis. In medial view, the bone is anteriorly narrow and posteriorly broad. It is difficult to determine if there are any foramina because of the many cracks running across the surface. Posteriorly, the splenial-coronoid suture is not exposed. Ventrally, the splenial encloses the anterodorsal border of the foramen intermandibularis caudalis, which is large and elliptical. Only the ventral portion of the splenial-dentary suture is visible.
The surangular is only visible in lateral view, and its sutures with neighboring bones are clear only with the angular. The surangular forms the upper half of the posterior part of the mandible. It is unclear whether the surangular extends to the end of the retroarticular process due to damage. The majority of the lateral surface is heavily ornamented except for the posteriormost portion, which serves as the attachment site for the m. pterygoideus posterior.
The angular forms the posteroventral part of the mandible. As is the case for the surangular, the greater portion of the lateral surface of the bone is ornamented except for a region at the posteriormost end that served for insertion of the m. pterygoideus posterior. Damage to the specimen prevents determining whether the angular reaches the end of the retroarticular process posteriorly. In medial view, the angular forms the posteroventral border of the foramen intermandibularis caudalis and possibly meets the coronoid posterior to the foramen.
A small portion of the left articular, forming part of the mandibular glenoid in lateral view (Fig. 6), is the only part of this bone available on the specimen for examination. The coronoid cannot be assessed due to the poor preservation of this area.
Dentition.—The dental margin of the upper jaw is concavo-convex in lateral view, corresponding to the “drapes” of the two festoons formed by the shape of the maxilla (Fig. 6.1). The dentition is not complete, but the exact tooth number of the premaxilla and maxilla can be recovered based on the preserved teeth and alveoli of the holotype and specimen DM000001-F000002. There are five premaxillary teeth, as suggested by the left premaxilla of specimen DM000001-F000002, which bears two alveoli (the second and fifth) and three incomplete teeth. As indicated by the size of the alveoli and the teeth, the fourth premaxillary tooth is the largest, followed by the third and fifth teeth successively. The first and second teeth are nearly identical in size to one another, both being about half the length of the fifth. The complete teeth (such as the third of an additional specimen, DM000001-F000004) are conical and slightly lingually curved; they bear subdued striations converging on the apex, but have no serrations along their anterior and posterior carinae.
The maxillary dentition is better preserved in the holotype, with most teeth (some complete) preserved (Fig. 3.3, 3.4). The fifth tooth is the largest, followed by the eleventh, the latter being very similar to the fourth premaxillary tooth in size. Tooth size increases towards the fifth, then decreases sharply towards the eighth, followed by a sharp increase to the eleventh, after which the general size of the teeth again decreases. As shown by actual teeth or the alveoli present on three specimens, all elements of the dentition—including the posteriormost five teeth—are separately implanted, indicating that the holotype (specimen DM000001-F000001) and specimen DM000001-F000003 represent fully grown adults (Cong et al., Reference Cong, Hou and Wu1984). The dentition of an additional specimen (DM000001-F000004) is mostly concealed due to occlusion of the jaw, but its similar size (20.1 cm long from the anterior end of the snout to the posterior border of the skull table) in comparison with specimen DM000001-F000002 (21.5 cm long) also indicates that the specimen is most probably an adult (Fig. 6.1). As indicated by the preserved teeth, the first nine maxillary teeth are similar to those of the premaxilla in morphology except for the size, with fine striations covering the crown (Fig. 6.4). Starting from the tenth tooth (on the right maxilla of specimen DM000001-F000002), the bases of the maxillary teeth become slightly constricted and the crown becomes low and globular (Fig. 6.5, 6.6). As described earlier, an occlusion pit or fossa is present just medial to the first and second premaxillary alveoli and between the seventh and eighth maxillary alveoli.
The exact number of teeth in each dentary could not be determined because much of the upper surface of the mandible is obscured in specimen DM000001-F000004. The large caniniform tooth fitting into the premaxilla-maxillary fossa on the left side is most probably the fourth dentary tooth, as suggested by the interdigitated pattern of the concavo-convex dental margin opposite to the five premaxillary teeth (Fig. 4.7, 4.8). Posterior to the fourth tooth, a set of four successive teeth is exposed, but these teeth all exhibit damaged crowns, preserving no significant features. The nearly complete fourth tooth is similar to that of the fifth maxillary tooth in morphology.
Etymology
Specific name in honor of Dr. Wei-Chieh Hsu who allowed the authors to study the specimens held by the Darwin Museum.
Materials
DM000001-F000002, a cranium with the left quadrate condyle damaged (Fig. S6 in the online supplementary data); DM000001-F000003, an incomplete cranium with the anterior tip of the snout missing (Fig. S7 in the online supplementary data); DM000001- F000004, a crushed but nearly complete skull with the mandible occluded (Fig. S8 in the online supplementary data); and MMC 001, an incomplete skull with a damaged dorsal surface (= the Maoming alligator of Skutschas et al., Reference Skutschas, Danilov, Kodrul and Jin2014).
Remarks
As suggested by the following phylogenetic analyses, Dongnanosuchus hsui n. gen. n. sp. is an orientalosuchine. Comparison is made here first with the other taxa of the Orientalosuchina and then with other crocodylians possessing a short snout from the Upper Cretaceous and the lower Paleogene of China. The orientalosuchine Eoalligator chunyii Young, Reference Young1964 (Wu et al., Reference Wu, Li and Wang2018) is from the lower Paleocene of the Nanxiong Basin of Guangdong (~500 km northeast of Maoming). Dongnanosuchus hsui n. gen. n. sp. clearly differs from Eoalligator chunyii; in the latter taxon, the premaxillary-maxillary notch is absent, and the interfenestral septum is relatively narrow. The orientalosuchine Jiangxisuchus nankangensis is from the Upper Cretaceous of the Nankang Basin of Jiangxi (~600 km northeast of Maoming). Dongnanosuchus hsui n. gen. n. sp. is also very different from Jiangxisuchus nankangensis. In Jiangxisuchus nankangensis, the preorbital ridge is absent, the prefrontal is much larger than the frontal, the frontal enters the supratemporal fossa, the palatine-pterygoid suture is parallel to the posterior margin of the suborbital fenestra, and the splenial is excluded from the symphysis. Compared with another Chinese orientalosuchine, Dongnanosuchus hsui n. gen. n. sp. cannot be referred to Protoalligator huiningensis Wang et al., Reference Wang, Sullivan and Liu2016 (= Eoalligator huiningensis Young, Reference Young and Young1982) from the Paleocene of Huaining, Anhui (~1300 km northeast of Maoming) because the latter has no preorbital ridge, no inclusion of the splenial in the symphysis, and exhibits an open external mandibular fenestra.
In geological age, Dongnanosuchus hsui n. gen. n. sp. is similar to that of Orientalosuchus naduongensis Massonne et al., Reference Massonne, Vasilyan, Rabi and Böhme2019 (the type species of the Orientalosuchina) and Krabisuchus siamogallicus Martin and Lauprasert, Reference Martin and Lauprasert2010. Orientalosuchus naduongensis was discovered from the middle to upper Eocene of the Na Duong Basin of Vietnam (~400 km west of Maoming) and Krabisuchus siamogallicus was found in the upper or uppermost Eocene of Krabi, Thailand (~1,950 km southwest of Maoming). Dongnanosuchus hsui n. gen. n. sp. is similar to Orientalosuchus naduongensis in that there is a preorbital ridge, the lacrimal extends anteriorly to a much greater degree than the prefrontal and frontal, and the palatine-pterygoid suture is well anterior in position from the posterior margin of the suborbital fenestra, but they are distinguishable from each other in many other structures. The most striking of these differences are summarized here. In Dongnanosuchus hsui n. gen. n. sp., the preorbital ridge forms a ridge system with the dorsal orbital rim and a ridge around the anteroventral orbit, the interorbital septum is slightly broader than the septum between the supratemporal fenestrae, the splenial enters into the symphysis, and the external mandibular fenestra is closed. All the aforementioned structures display an opposite condition in Orientalosuchus naduongensis. Krabisuchus siamogallicus is not complete, but it is clearly different from Dongnanosuchus hsui n. gen. n. sp. in that the orbital margins are not rimmed, the palatine-pterygoid is parallel to the posterior margin of the suborbital fenestra, and the external mandibular fenestra is not closed.
In comparison with other uppermost Cretaceous–upper Eocene crocodylians of China, Dongnanosuchus hsui n. gen. n. sp. clearly differs from Maomingosuchus petrolica from the same strata of the Maoming Basin; the latter is a longirostral taxon of the Tomistominae (Shan et al., Reference Shan, Wu, Cheng and Sato2017). Asiatosuchus nanlingensis Young, Reference Young1964 (Wu et al., Reference Wu, Li and Wang2018) and Planocrania datangensis Li, Reference Li1976 were collected from the uppermost Cretaceous–lowermost Paleocene of the Nanxiong Basin. Dongnanosuchus hsui n. gen. n. sp. is neither comparable with Planocrania datangensis, which has a moderately elongated snout with ziphodont teeth (Brochu, Reference Brochu2013, figs. 10, 11), nor with Asiatosuchus nanlingensis, which possesses a fenestrated and elongated mandible (Wu et al., Reference Wu, Li and Wang2018, figs. 1A, C, 2A, B).
Asiatosuchus grangeri Mook, Reference Mook1940, from the upper Eocene of Inner Mongolia, is considered to represent a basal crocodyloid in many later studies, including the phylogenetic analyses presented here. Asiatosuchus grangeri is known only from a mandible, but it differs from Dongnanosuchus hsui n. gen. n. sp. in having a long symphysis, an open external mandibular fenestra, and a splenial that does not enter the symphysis. Zhang (Reference Zhang1981) named and described Wanosuchus atresus based on a left ramus of a mandible from the Paleocene of Anhui Province as a sebecosuchian, which has not been included in any phylogenetic study so far. This species also lacks an external mandibular fenestra, but the broadened and strongly dorsally arched posterior portion of the mandible, the long mandibular symphysis, and the exclusion of the splenial from the symphysis are different from the corresponding features of Dongnanosuchus hsui n. gen. n. sp. Li and Wang (Reference Li and Wang1987) described Alligator luicus Duméril and Bibron, Reference Duméril and Bibron1836, based on a skull and some postcranial elements from the middle Miocene, Shandong Province. The divided external nares, the presence of the external mandibular fenestra, and no preorbital ridge clearly distinguish Alligator luicus from Dongnanosuchus hsui n. gen. n. sp. Dong (Reference Dong1974) named Dzungarisuchus manacensis based on a mandibular ramus from the upper Eocene of Xinjiang and considered it as a crocodyline. The elongated symphyseal region and same-sized alveoli are not comparable to those of Dongnanosuchus hsui n. gen. n. sp.
Phylogenetic analyses
Data matrices and analysis
Since a number of different data matrices have been recently used in phylogenetic studies of crocodyloids or alligatoroids, we conducted dual comparative analyses to explore the phylogenetic relationships of Dongnanosuchus hsui n. gen. n. sp. based on two separate data matrices that have been employed in the literature for the analysis of East and Southeast Asian taxa. The first analysis was conducted using a modified form of the data matrix used by Li et al. (Reference Li, Wu and Rufolo2019). The latter was elaborated from data matrices of Brochu (Reference Brochu, Rowe, Brochu and Koshi1999, Reference Brochu2011), Martin and Lauprasert (Reference Martin and Lauprasert2010), Brochu and Storrs (Reference Brochu and Storrs2012), Skutschas et al. (Reference Skutschas, Danilov, Kodrul and Jin2014), Narváez et al. (Reference Narváez, Brochu, Escaso, Pérez-García and Ortega2016), Wang et al. (Reference Wang, Sullivan and Liu2016), and Wu et al. (Reference Wu, Li and Wang2018), which resulted in a more complete matrix that incorporates information from Asian crocodylians as well as non-crocodylian taxa that are more derived than Bernissatia fagesii Dollo, Reference Dollo1883. With the inclusion of Bernissatia fagesii and Theriosuchus pusillus Owen, Reference Owen1879, we designated Goniopholis simus Owen, Reference Owen1878 to represent the outgroup in the analysis, as was done by Narváez et al. (Reference Narváez, Brochu, Escaso, Pérez-García and Ortega2016). We added newly described alligatoroids (Orientalosuchus naduongensis, Protocaiman peligrensis Bona et al., Reference Bona, Ezcurra, Barrios and Blanco2018, Deinosuchus schwimmeri Cossette and Brochu, Reference Cossette and Brochu2020) and the newly restudied Bottosaurus harlani von Meyer, Reference von Meyer1832 (Cossette and Brochu, Reference Cossette and Brochu2018) and Deinosuchus riograndensis Colbert and Bird, Reference Colbert and Bird1954 (Cossette and Brochu, Reference Cossette and Brochu2020) into the data matrix. As argued earlier, the Maoming alligator from the Maoming Basin (Skutschas et al., Reference Skutschas, Danilov, Kodrul and Jin2014) was treated as belonging to the new species described here, Dongnanosuchus hsui n. gen. n. sp. In total, the data matrix includes 123 taxa and 191 morphological characters (see online supplementary data). In this data matrix, characters 54, 81, 137, and 156 were modified with the addition of a new state, as in Cossette and Brochu (Reference Cossette and Brochu2020, characters 47, 72, 117, 135, respectively); coding changes for these characters were made accordingly for the relevant taxa, as detailed in the online supplementary data. Coding changes involving certain characters were made for four Chinese taxa—Jiangxisuchus nankangensis, Protoalligator huiningensis, Eoalligator chunyii, and Maomingosuchus petrolica originally coded by Shan et al., Reference Shan, Wu, Cheng and Sato2017—based on further examination of the specimens in preparation for this study (see online supplementary data). We coded Dongnanosuchus hsui n. gen. n. sp. based on the four new skulls with our version in comparison to that of Skutschas et al. (Reference Skutschas, Danilov, Kodrul and Jin2014) in characters 91, 97, and 131. For character 91, we changed the code from state 1 to state 0; for character 97, from state 0 to state 1; and for character 131, from state 1 to state 0 in the matrix employed here. As for character 91, preservation makes it difficult to determine whether a premaxillary-maxillary notch, which is evident in the new skulls referred to Dongnanosuchus hsui n. gen. n. sp., is also present in the Maoming alligator. The preorbital ridges (character 97) are present in the Maoming alligator as described by Skutschas et al. (Reference Skutschas, Danilov, Kodrul and Jin2014), although it is not as strong as in the new skulls due to a dorso-ventral compression. Skutschas et al. (Reference Skutschas, Danilov, Kodrul and Jin2014, fig. 2) shows that the anterior tip of the frontal (character 131) is pointed in the Maoming alligator rather than broad and complex, which is very similar to the condition seen in the new skulls.
The second data matrix used for this study was derived from that of Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019), which itself represents a modified form based on the data matrices constructed for many previous studies such as Brochu and Storrs (Reference Brochu and Storrs2012), Jouve et al. (Reference Jouve, Bouya, Amaghzazc and Meslouh2015), Narváez et al. (Reference Narváez, Brochu, Escaso, Pérez-García and Ortega2015), Hastings et al. (Reference Hastings, Reisser and Scheyer2016), Wang et al. (Reference Wang, Sullivan and Liu2016), Cossette and Brochu (Reference Cossette and Brochu2018), and Li et al. (Reference Li, Wu and Rufolo2019). As enumerated in Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019) and employed here, characters 54, 81, 137, and 156 also were also altered to reflect the definitions presented in in Cossette and Brochu (Reference Cossette and Brochu2020, characters 47, 72, 117, 135, respectively) and coding changes for these characters were made accordingly for the relevant taxa (see online supplementary data). Compared with a number of previous studies, Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019) made coding changes to some characters for certain taxa based on published descriptions. For a small number of taxa, including the Chinese species Protoalligator huiningensis, coding changes were made based on the observation of actual specimens. Not being able to directly examine specimens of all the involved taxa, the character coding changes of Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019) were followed here except for the four aforementioned Chinese taxa (see the preceding discussion concerning the first data matrix) because those specimens were available for observation. The character coding employed here for these four Chinese taxa differs from that utilized by Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019) (see online supplementary data). There were no changes in character coding for another Chinese taxon, Asiatosuchus nanlingensis. With Dongnanosuchus hsui n. gen. n. sp. replacing the Maoming alligator and the addition of Protocaiman peligrensis and Deinosuchus schwimmeri, the second data matrix included 116 taxa and 202 characters. Of the 202 characters, characters 1–50, 52–147, and 150–191 matched those used in the first analysis in definition. Characters 51, 148, and 149 were different due to the addition of an extra state; coding of these three characters, as applied to newly added taxa that were also included in the first analysis, was thus changed accordingly (see online supplementary data). In addition, the second data matrix selected Bernissatia fagesii as representing the outgroup and did not include Goniopholis simus, Theriosuchus pusillus, and some of the non-crocodylian taxa that were concerned in the first data matrix.
The only available specimen of Asiatosuchus nanlingensis is highly fragmentary, rendering it impossible to assess character states with any confidence for most characters; the taxon was therefore excluded in both analyses conducted here, as was the case in the second analysis of Li et al. (Reference Li, Wu and Rufolo2019). In the two analyses, a New Search Technology of TNT v.1.5 standard version was preferred over the conventional Traditional Search Method in terms of the large data matrix. We set the maximum for trees to 10,000 and analyzed using 1000 random seeds of tree fusing. Multistate characters were unordered and all characters were equally weighted as in Li et al. (Reference Li, Wu and Rufolo2019) and Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019).
Results
The first phylogenetic analysis of 122 taxa yielded two most parsimonious trees (MPTs), each with a tree length of 906 steps, a consistency index (CI) of 0.288, and a retention index (RI) of 0.776. The second phylogenetic analysis of 115 taxa yielded six MPTs, each with a tree length of 967 steps, a consistency index (CI) of 0.289, and a retention index (RI) of 0.754. As shown in the strict consensus trees (Fig. 9; Figs. S9, S10 in the online supplementary data), both analyses recognized Dongnanosuchus hsui n. gen. n. sp. as an alligatoroid within the Orientalosuchina, although internal phylogenetic relationships were not comparable. Both analyses also suggested the basal position of the Orientalosuchina within the Globidonta. Due to the relatively higher support values and greater compatibility with other studies, we preferred the results obtained by the first analysis, which included more terminal taxa and found six unequivocal synapomorphies (characters 47-0, 61-1, 70-1, 104-1, 131-1, and 177-1), to support the alligatoroid status of Dongnanosuchus hsui n. gen. n. sp.. Of the six synapomorphies, the states of characters 47, 131, and 177 are reversal and the states of characters 61 and 70 could not be determined for Dongnanosuchus hsui n. gen. n. sp. due to poor preservation. The globidontian status of the Orientalosuchina was supported by three unequivocal synapomorphies (characters 39-1, 47-1, and 150-2), of which character 39 is indeterminate in Dongnanosuchus hsui n. gen. n. sp. With the inclusion of Dongnanosuchus hsui n. gen. n. sp., the monophyletic Orientalosuchina recognized by Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019) was further confirmed. This was suggested by four unequivocal synapomorphies: characters 64-1 (surangular-dentary suture intersecting external mandibular fenestra at posterodorsal corner), 70-1 (foramen aërum setting in from margin of retroarticular process), 143-1 (postorbital contacting quadratojugal, but not quadrate, medially), and 159-1 (squamosal extending ventrolaterally to lateral extent of paraoccipital process). Of the four synapomorphies, character 64 is not applicable to Dongnanosuchus hsui n. gen. n. sp. and characters 70 and 143 could not be assessed based on the specimens currently available for this newly erected taxon.
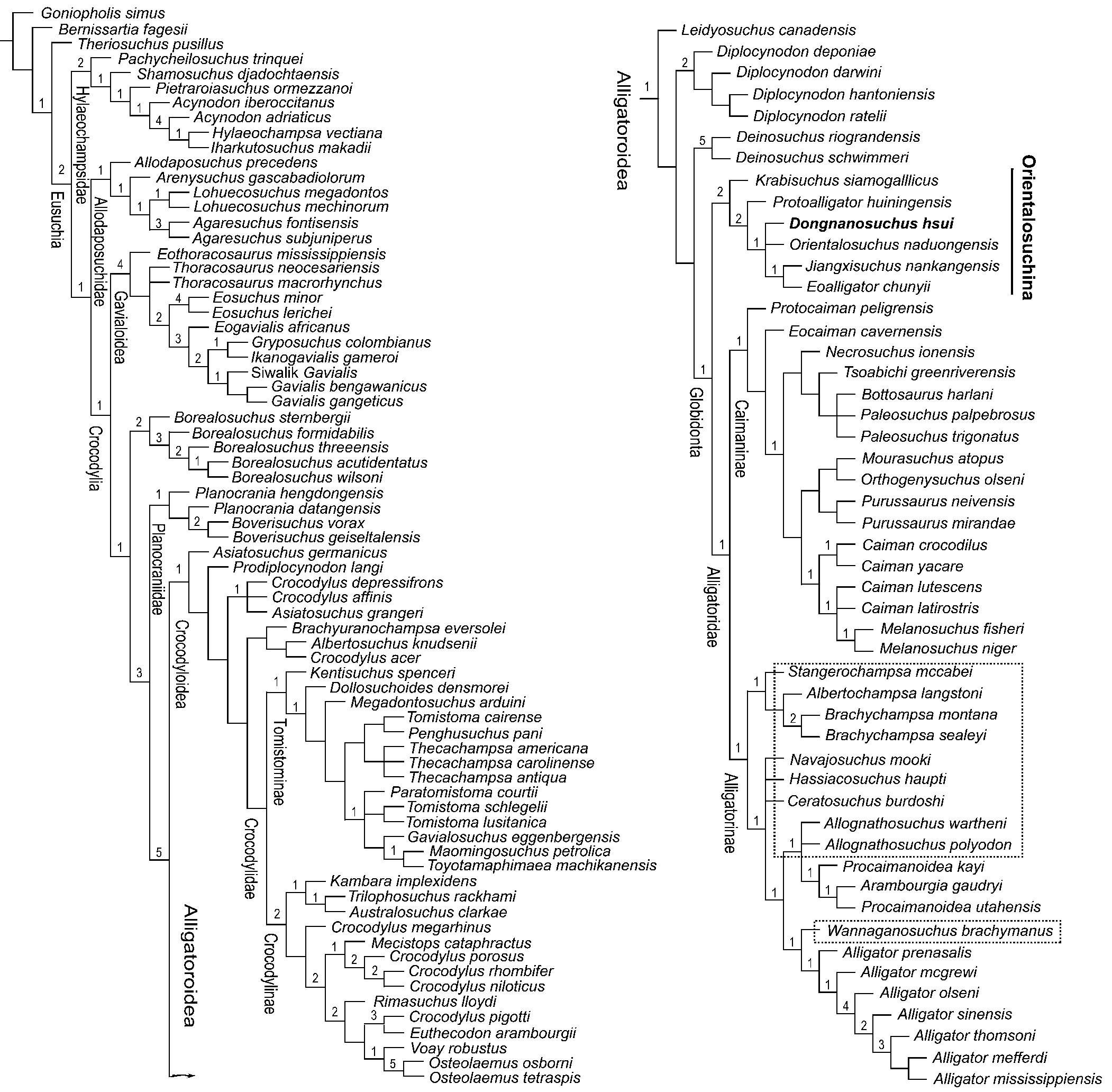
Figure 9. Strict consensus of the two MPTs obtained by the analysis of the first data matrix (123 taxa and 191 characters, with Asiatosuchus nanlingensis excluded from the analysis). Taxa enclosed by boxes are the nine North American alligatorids and one German species mentioned in the text. Bremer support values are listed at each node, showing a weak level of support for the clades of interests.
Remarks
Although both analyses grouped the Orientalosuchina in the Alligatoroidea as the basal most clade of the Globidonta, phylogenetic relationships for certain other groups or taxa within the Eusuchia differed between the two analyses. The most striking of the differences were related to the relationships of the Hylaeochampsidae, Allodaposuchidae, Gavialoidea, and ten alligatoroids that included nine from the Upper Cretaceous and lower Paleogene of North America and one from the Eocene of Germany (enclosed by squares in Fig. 9; as well as Fig. S10 in the online supplementary data). The Hylaeochampsidae, Allodaposuchidae (represented by six species), and Gavialoidea formed a set of successive sister groups towards a clade including the other crocodylians in the first analysis, as in some previous studies (e.g., Puértolas-Pascual et al., Reference Puértolas-Pascual, Canudo and Moreno-Azanza2014, Reference Puértolas-Pascual, Blanco, Brochu and Canudo2016; Narváez et al., Reference Narváez, Brochu, Escaso, Pérez-García and Ortega2015, Reference Narváez, Brochu, Escaso, Pérez-García and Ortega2016). In contrast, the Hylaeochampsidae formed, with two basal taxa, a polytomy out of the Crocodylia, and the Allodaposuchidae and Gavialoidea were considered to be two basal clades of the Crocodylia, with undetermined relationships, in the second analysis. The nine North American taxa include: Stangerochampsa mccabei Wu et al., Reference Wu, Brinkman and Russell1996; Albertochampsa langstoni Ericson, Reference Erickson1972; Brachychampsa montana Gilmore, Reference Gilmore1911; Brachychampsa sealeyi Williamson, Reference Williamson1996; Allognathosuchus polyodon (Cope, Reference Cope1872); Allognathosuchus wartheni Case, Reference Case1925; Wannaganosuchus brachymanus Erickson, Reference Erickson1982; Navajosuchus mooki (Simpson, Reference Simpson1930); and Ceratosuchus burdoshi Schmidt, Reference Schmidt1938 (which added to the one German species, Hassiacosuchus haupti Weitel, Reference Weitzel1935) are collectively referred to hereafter as the “Ten Alligatoroid Taxa.” They were all grouped in different clades within the Alligatorinae in the first analysis, with unresolved relationships present for some of these taxa (Fig. 9). In contrast, two of the Ten Alligatoroid Taxa (with no Albertochampsa langstoni) were excluded from the Alligatoridae and formed a clade (Brachychampsa) between the Orientalosuchina and Alligatoridae in the second analysis. Of the other seven, Stangerochampsa formed a polytomy with Protocaiman, the Alligatorinae, and Caimaninae. The remaining six were grouped in the Alligatorinae with an unresolved relationship for some of them within the subfamily (Fig. S10 in the online supplementary data).
The data matrix for the first analysis was modified from that of Li et al. (Reference Li, Wu and Rufolo2019), but the phylogenetic results obtained for the Chinese orientalosuchines in the present study were very different from those of this earlier work. This may have been caused by the addition of Orientalosuchus naduongensis, as well as the inclusion of further information on Dongnanosuchus hsui n. gen. n. sp. (including the Maoming alligator) from the four new skulls described here and the character coding verification undertaken for some of the Chinese crocodylians with a short snout. In Li et al. (Reference Li, Wu and Rufolo2019), the Chinese taxa Jiangxisuchus nankangensis and Eoalligator chunyii were regarded as belonging to the Crocodyloidea rather than Alligatoroidea, as in this study. As for other orientalosuchines, Krabisuchus siamogallicus was considered to be a basal taxon of the Caimaninae and Protoalligator huiningensis a basal taxon of the Alligatoroidea.
Although the second analysis conducted for this study, based on the data matrix derived from that of Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019), also recovered the Orientalosuchina as a monophyletic clade within the Alligatoroidea, the external and internal relationships of the clade, along with the relationships of some other alligatoroids, including the aforementioned Ten Alligatoroid Taxa, are not similar between the two works. In Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019), the Orientalosuchina was grouped together with five of the Ten Alligatoroid Taxa into a single clade, which was further hypothesized to be the sister-group of the Alligatoridae, whereas the remaining North American taxa (Albertochampsa langstoni not being included in the study) were grouped within the Alligatorinae. Furthermore, of the five included orientalosuchines, three had uncertain phylogenetic relationships. This situation stands in contrast to the results of our second analysis in which the Orientalosuchina formed an independent clade just two-step basal to the Alligatoridae and the relationships of all six orientalosuchines were resolved. In addition, Diplocynodon and Leidyosuchus formed a clade in the second analysis as the sister group of the Deinosuchus-Globidonta clade rather than two separate clades. As mentioned earlier, the relationships for the Hylaeochampsidae, Allodaposuchidae, and Gavialoidea, which were resolved in Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019), were undetermined in the second analysis.
For comparison with other studies, we used the results of the first phylogenetic analysis for the reasons mentioned earlier. In addition to the studies of Li et al. (Reference Li, Wu and Rufolo2019) and Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019), the phylogenetic analyses of Wang et al. (Reference Wang, Sullivan and Liu2016) and Wu et al. (Reference Wu, Li and Wang2018) also included ‘orientalosuchines’ based on the known taxa available at the time (Krabisuchus siamogallicus, Eoalligator chunyii, Protoalligator huiningensis, and the Maoming alligator, this latter form referred to Dongnanosuchus hsui n. gen. n. sp., here). Neither of the two studies demonstrated a monophyletic clade for those ‘orientalosuchines’ within the Alligatoroidea, and Eoalligator chunyii was in fact included in the Crocodyloidea.
Compared with many other studies on crocodylians that included most alligatoroids and crocodyloids but no Chinese ‘orientalosuchines,’ the phylogenetic results of our first analysis are similar to those of the works that also included all or most of the Ten Alligatoroid Taxa discussed above. In these studies (e.g., Aguilera et al., Reference Aguilera, Riff and Bocquentin-Villanueva2006; Cidade et al., Reference Cidade, Solórzano, Rincón, Riff and Hsiou2017; Souza-Filho et al., Reference Souza-Filho, Souza, Hsiou, Riff, Guilherme, Negri and Cidade2019), Leidyosuchus and Diplocynodon (four species) formed two successive basal clades of the Alligatoroidea. The Ten Alligatoroid Taxa were either grouped in different clades within the Alligatorinae or some of the Ten Alligatoroid Taxa were hypothesized to represent stem groups of the Alligatoridae. This means that none of the Ten Alligatoroid Taxa had a close relationship with any of the Caimaninae, as presented in an early work—one not including Navajosuchus mooki of Brochu (Reference Brochu, Rowe, Brochu and Koshi1999), who provided a comprehensive study on the Alligatoroidea for the first time. In contrast, Cossette and Brochu (Reference Cossette and Brochu2018) considered three of the Ten Alligatoroid Taxa (Brachychampsa, Stangerochampsa, and Albertochampsa) to have no determined relationship to either the Alligatorinae or Caimaninae when they restudied Bottosuchus harlani. These three alligatoroids formed a clade as the sister group of the Caimaninae in Bona et al. (Reference Bona, Ezcurra, Barrios and Blanco2018), which described Protocaiman peligrensis, and in the analysis conducted by Cossette and Brochu (Reference Cossette and Brochu2020) for their re-examination of Deinosuchus, Protocaiman peligrensis was hypothesized as the second basalmost clade of the Caimaninae in Bona et al. (Reference Bona, Ezcurra, Barrios and Blanco2018). Here, it was considered the basalmost clade of the Caimaninae in the first analysis while it was considered as one of the basal alligatorids with an undetermined relationship with the others of the family in the second analysis. Deinosuchus (represented by D. schwimmeri and D. riograndensis) had an undetermined relationship with Leidyosuchus, Diplocynodon (represented by eight species), and a clade including all other alligatoroids in Cossette and Brochu (Reference Cossette and Brochu2020), whereas the genus was considered by our two analyses to be the sister group of the Globidonta, one-step more basal than the Orientalosuchina, as in Rio et al. (Reference Rio, Mannion, Tschopp, Martin and Delfino2020).
Discussion
Dongnanosuchus hsui n. gen. n. sp. is a small-sized crocodyloid with a short snout. It looks similar to Jiangxisuchus nankangensis and Orientalosuchus naduongensis in general appearance and size. However, as argued earlier, morphological differences between Dongnanosuchus hsui n. gen. n. sp. and the other two are also obvious.
In phylogeny, both analyses conducted here recognized the monophyly of the Orientalosuchina. The first analysis grouped Dongnanosuchus hsui n. gen. n. sp., Orientalosuchus naduongensis, and the Jiangxisuchus nankangensis-Eoalligator chunyii clade together in a set of unresolved relationships, supported by two synapomorphies (92-1). In the second analysis, Dongnanosuchus hsui n. gen. n. sp. was considered to be the sister-group of an Orientalosuchus naduongensis-Krabisuchus siamogallicus clade, which was supported by three synapomorphies (characters 97-1, 150-2, and 199-0). Although the relationship of Dongnanosuchus hsui n. gen. n. sp. recognized by the second analysis appears more reasonable in terms of the stratigraphy associated with these fossil taxa, the confidence level of the monophyly of the Orientalosuchina is lower (with a Bremer support value of 1) than that obtained by the first analysis (with a Bremer value of 2). As for other major clades of the Crocodylia, most of them had a very low Bremer support value (1 or 0 in both analyses) for monophyly.. On the other hand, in all previous studies with the Chinese orientalosuchines included, monophyly of the major groups of the Crocodylia were also weakly supported (Wang et al., Reference Wang, Sullivan and Liu2016; Li et al., Reference Li, Wu and Rufolo2019; Massonne et al., Reference Massonne, Vasilyan, Rabi and Böhme2019). It would not be surprising that such relationships demonstrated in our study and others would change when novel taxa and new, more complete specimens of the species currently only known from fragmentary remains are found. As mentioned in Li et al. (Reference Li, Wu and Rufolo2019), there were a number of short-snouted fossil members of the Crocodylia collected from the Upper Cretaceous to Eocene in China before the discovery of Dongnanosuchus hsui n. gen. n. sp.. In addition to those mentioned earlier, they also include Dzungarisuchus manacensis from the upper Eocene of Manas River, Xinjiang and Lianghusuchus hengyangensis Young, Reference Young1948 from the Eocene of Hengyang, Hunan. However, none of them is as well-preserved as Dongnanosuchus hsui n. gen. n. sp. or Jiangxisuchus nankangensis. Additionally, many of the Chinese crocodylians have not been included in phylogenetic studies because their taxonomic status requires revision using modern techniques and methods.
As indicated by both phylogenetic analyses conducted here, the Orientalosuchina represents an independent clade with no close relationship to any of the aforementioned Ten Alligatoroid Taxa—it deviated from the mainline of the Alligatoroidea after Deinosuchus in North America during the late Cretaceous. This indicates that the Orientalosuchina split from the Alligatoridae (see Fig. 9) or a later alligatoroid clade (see Fig. S10 in the online supplementary data) and dispersed from North America to Asia via Beringia through a divergence event as early as the Campanian. Such a dispersal event is consistent with the Late Cretaceous age of the basal alligatorids of North America (such as Stangerochampsa mccabei and Brachychampsa montana), but conflicts with the evidence for the Late Cretaceous dinosaur dispersal from Asia to North America, including tyrannosauroids, hadrosaurids, and ceratopsians (Loewen et al., Reference Loewen, Irmis, Sertich, Currie and Sampson2013; Farke et al., Reference Farke, Henn, Woodward and Xu2014; Prieto-Márquez et al., Reference Prieto-Márquez, Fondevilla, Sellés, Wagner and Galobart2019). In contrast, Massonne et al. (Reference Massonne, Vasilyan, Rabi and Böhme2019), who were the first to recognize the Asian Orientalosuchina, concluded that the Orientalosuchina formed a sub-lineage with five of the Ten Alligatoroid Taxa; it dispersed to Asia from North America after at least four divergence events occurred within the sub-lineage during the Late Cretaceous. This would mean that the Orientalosuchina had no close relationship with the Alligatoridae in terms of either phylogeny or dispersal pattern. However, as discussed earlier, the phylogenetic results obtained here are only weakly supported, as in previous studies; the origin and dispersal hypotheses of the Orientalosuchina and the later alligatoroid clades as reflected by these results need to be refined by future studies in order to verify the patterns observed in our data.
Conclusions
New discoveries of specimens representing the Maoming alligatoroid played a key role in the establishment of a new taxon, Dongnanosuchus hsui n. gen. n. sp. Our phylogenetic analyses incorporating the new species and other new alligatoroids confirm the monophyly of the Orientalosuchina. The current phylogenetic results support the North American origin of the Orientalosuchina, but suggest that the clade is the sister-group of the Alligatoridae or later alligatoroid clade and dispersed into Asia after a divergence event occurred in the main lineage rather than a sub-lineage of the Alligatoroidea during the Late Cretaceous. Fossil sampling for new taxa and better specimens of species currently only known from fragmentary material will be fundamental for establishing a stable phylogenetic pattern for the Alligatoroidea as well as for improving hypotheses on the early history and dispersal routes of alligatoroid clades between continents.
Acknowledgments
We are grateful to W.-C. Hsu who allowed us to study the specimens stored in the Darwin Museum, Keelung (Jilong), Taiwan, China. X.-C. Wu thanks the staff of the Geological Department of the National Museum of Natural Science (NMNS), Taichung for their hospitality during his visit as well as the collections crew of the Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, China for their gracious help during his examination of specimens held there. J.E. Martin and M. Delfino kindly provided us copies of their works for reference. We appreciate T. Massonne and two anonymous reviewers as well as the editor, H.-D. Sues, who carefully examined the manuscript, offering critical comments and suggestions that led to its great improvement. This work was supported by research grants from the NMNS (to H.-Y. Shan), from the Canadian Museum of Nature, Ottawa, Canada (RCP09 to X.-C. Wu), and from Tokyo Gakugei University, Japan (to T. Sato). We appreciated the assistance of the Willi Hennig Society through providing access to the software TNT 1.5.
Data availability statement
Data available from the Zenodo Digital Repository: http://doi.org/10.5281/zenodo.5041226?













