Introduction
Moth species that occur over large geographical areas due to the historical spread of their primary agricultural host may develop spatial differentiation in female's sex pheromone production and male's behavioral response (Löfstedt, Reference Löfstedt1990; Cardé & Haynes, Reference Cardé, Haynes, Cardé and Millar2004). For instance in the turnip moth, Agrotis segetum (Denis and Schiffermüller), there is substantial variability in the ratio of the five sex pheromone components among world populations, and males tend to prefer those blends that are similar to the ones produced by females from their own population (Löfstedt et al., Reference Löfstedt, Löfqvist, Lanne, Van Der Pers and Hansson1986; Tòth et al., Reference Tòth, Löfstedt, Blair, Cabello, Farag, Hansson, Kovalev, Maini, Nesterov, Pajor, Sazonov, Shamshev, Subchev and Szöcs1992). Significant regional differences in the sex pheromone blend of the tortricid Choristoneura rosaceana (Harris) has been found in apple orchards across North America (El-Sayed et al., Reference El-Sayed, Delisle, DeLury, Gut, Judd, Legrand, Reissig, Roelofs, Unelius and Trimble2003; Stelinski et al., Reference Stelinski, McKenzie, Gut, Issacs and Brunner2007). Intraspecific variation in sex pheromone communication has also been found to be associated with host specialization, as for the Z-and E-tetradecenyl acetate strains of Ostrinia nubilalis (Hübner) (Leppik & Frérot, Reference Leppik and Frérot2012), and the corn and rice strains of Spodoptera frugiperda Walker (Unbehend et al., Reference Unbehend, Hänniger, Meagher, Heckel and Groot2013). Since using the correct sex pheromone blend ratio could affect the success of population monitoring and management with mating disruption-based technologies, it is important to know the exact blend ratio used by geographically separate populations (Cardé & Minks, Reference Cardé and Minks1995).
The oriental fruit moth, Grapholita molesta (Busck), is a widely distributed tortricid fruit pest attacking stone (Prunus spp.) and pome (Malus, Cydonia, and Pyrus spp.) tree fruits in growing regions situated between 20° and 60° latitude in both hemispheres. The center of origin of G. molesta is thought to be in Northwest China from where its current distribution has expanded through international trade and transport of fruit material (Rothschild & Vickers, Reference Rothschild, Vickers, van der Geest and Evenhuis1991; Komai, Reference Komai1999; Zheng et al., Reference Zheng, Peng, Liu, Pan, Dorn and Chen2013). Grapholita molesta was first reported in Australia around 1910 (Bailey, Reference Bailey1979), in Brasil in 1943 (Reis et al., Reference Reis, Nora and Melzer1988), in Chile in 1970 (González, Reference González1980), in Spain and New Zealand in 1976 (Russell, Reference Russell1987; Rubio et al., Reference Rubio, Esteban and Llamas1990), and in South Africa in 1990 (Blomefield & Geertsema, Reference Blomefield and Geertsema1990). Grapholita molesta was first detected in the Eastern United States in 1913, and reported from California in 1942, and it is now found in all peach-growing areas of the USA and Canada (Rothschild & Vickers, Reference Rothschild, Vickers, van der Geest and Evenhuis1991; Bellerose et al., Reference Bellerose, Chouinard and Roy2007).
Grapholita molesta females release a chemical blend from their sex pheromone gland composed of Z-8-dodecenyl acetate (Z8-12:Ac), E-8-dodecenyl acetate (E8-12:Ac), and Z-8-dodecenol (Z8-12:OH), and a blend with a 100:6:10 ratio of these components, respectively, is most attractive to the males (Baker & Cardé, Reference Baker and Cardé1979; Cardé et al., Reference Cardé, Baker and Cardé1979). Synthetic sex pheromones are widely used for population monitoring of G. molesta, and more than 50,000 ha of peach and apple are treated with sex pheromones for mating disruption (Witzgall et al., Reference Witzgall, Kirsch and Cork2010). Interestingly, most commercial lures and mating disruption dispensers used in North America and Europe are loaded with a 93:6:1 blend of the three sex pheromone components (Agrian, 2014). The low ratio of Z8-12:OH used in these dispensers is likely due to studies suggesting that the alcohol is important only for short range behaviors that would be necessary in catching moths with traps, but not in disrupting male searching behaviors for females (Linn & Roelofs, Reference Linn and Roelofs1983).
Pheromone gland composition and male response to different sex pheromone component blends has been studied in G. molesta populations from Australia, Korea, and USA (Beroza et al., Reference Beroza, Gentry, Blythe and Muschik1973; Roelofs & Cardé, Reference Roelofs and Cardé1974; Rothschild & Minks, Reference Rothschild and Minks1977; Baker & Cardé, Reference Baker and Cardé1979, Han et al., Reference Han, Jung, Choi, Lee and Boo2001, Yang et al., Reference Yang, Jung, Han, Boo and Yiem2002). A synthesis of these studies shows that sex pheromone composition is similar across populations, and that males are attracted to a relatively narrow range of E8-12:Ac in the blend, but with slightly different optimal blend ratios reported among populations (Han et al., Reference Han, Jung, Choi, Lee and Boo2001). Some of these studies report traces of the E-isomer in solutions of Z8-12:Ac that vary between undetectable levels to 3%. Since male G. molesta is sensitive to small departures from the optimal Z–E acetate ratio (Baker & Cardé, Reference Baker and Cardé1979; Baker et al., Reference Baker, Meyer and Roelofs1981), comparison of the attractiveness of different ratios among populations in previous studies could be biased by the purity of the sex pheromone blends used in each case. An additional gap in the studies of population variation of sex pheromone blends in G. molesta is that only a few populations from different regions (Australia, Korea, and USA) have been analyzed for their optimal blends.
The objective of our study was to determine if any significant variation exists in the male's sexual response and female's sex pheromone signal of G. molesta across a wider geographical area than previously sampled, including populations from new regions, such as Italy, New Zealand, Spain, Turkey, and Chile, and from regions where populations have previously been analyzed, such as Korea and USA. Efforts were made to reduce the level of variation inherent in such a large study by having all the lures prepared at one laboratory (Spain) and shipped to the other sites. Secondly, to examine the moth's communication system under uniform laboratory conditions a subset of populations was shipped to one laboratory (Spain) for behavioral studies. Finally, to examine whether significant differences in the sexual communication systems of G. molesta might be associated with host selection, populations from both peach and apple were evaluated.
Materials and methods
Sex pheromone blends
The three sex pheromone components of G. molesta, Z8-12:Ac, E8-12:Ac, and Z8-12:OH, were purchased from Pherobank (Wageningen, The Netherlands). The initial purity of each chemical was ≥99%. A stock solution containing a 100:10 ratio of Z8-12:Ac to Z8-12:OH (1.6 μg μl−1 and 0.16 μg μl−1, respectively) was prepared and pipetted into five vials. A different amount of E8-12:Ac was added to each vial to obtain test solutions with varying concentrations (0, 0.08, 0.16, 0.48, and 1.6 μg μl−1) of E8-12:Ac. Gas chromatographic analysis revealed that Z8-12:Ac contained 0.38% E8-12:Ac, and that E8-12:Ac contained 0.24% Z8-12:Ac. Taking this into account, the final blends contained 0.4, 5.4, 10.4, 30.4 and 100.1% of E8-12:Ac relative to Z8-12:Ac. Lures loaded with hexane served as a negative control in field trials. Hexane-rinsed red rubber septa (7.0-mm diameter, Sigma-Aldrich, Madrid, Spain) were labeled with a different letter for each treatment using a permanent marker, and were loaded with 50 μl of the test solutions so that they contained either hexane alone or blends with 80.1 (±0.9) μg of Z8-12:Ac, 8 μg of Z8-12:Ac, and varying amounts (0.3–80.3 μg) of E8-12:Ac. The small variation in the amount of Z8-12:Ac among stock solutions should have had an insignificant effect on male response (Baker et al., Reference Baker, Meyer and Roelofs1981; Varela et al., Reference Varela, Avilla, Anton and Gemeno2011). The solvent was allowed to evaporate and septa were packed in sealed glass bottles and stored at −20 °C until shipping to their destinations via express mail. Septa for Chile and New Zealand were loaded on 12 December 2011 and septa for the other locations were loaded on 6 July 2011. Septa were kept at −20 °C at each location prior to their use. Sampling took place between 21 January 2012 and 28 March 2012 in Chile and New Zealand, and between 6 July 2011 and 1 October 2011 in all Northern hemisphere locations.
Field trapping
Field tests were carried out in 2011 and 2012 at eight locations in Europe, Asia, and North and South America (table 1). White delta traps were used in all locations except in the USA where orange traps were deployed. Trap color has previously been shown not to affect G. molesta captures (Myers et al., Reference Myers, Krawczyk and Agnello2009; Zhao et al., Reference Zhao, Rong, Li, Zhang, Kong, Hu, Zhang and Ma2013). Traps were placed in apple, nectarine or peach orchards in 3–5 plots per location. Traps within a plot were placed 20–40 m apart in a transect, and plots were at least 20 m apart from each other. Septa were placed directly on the sticky liner in the trap or were pinned to the inside roof of the trap. Lures were replaced once or twice during each field trial. Traps were checked every 2–10 days and the sticky liners were replaced on each date whenever G. molesta were caught. Traps were rotated to the next transect position on each date. Insects captured in the traps were counted and in some locations they were sexed and the species status identified (Korea and Spain2) according to genital morphology (Horak, Reference Horak1984). Only the catches of male G. molesta were included in the comparisons of sex pheromone blends.
Table 1. Description of G. molesta populations and field and laboratory tests conducted.

1 Field tests were conducted with eight populations from stone fruit and apple orchards. Flight tunnel tests and sex pheromone gland analysis used either laboratory-reared individuals from field-collected populations (France and Spain2) or long-term laboratory colonies (Italy1, USA2, and USA3).
Insects for laboratory tests
Insects from France, Italy2, Spain2, USA2, and USA3 were reared in the laboratory for flight tunnel tests and to analyze sex pheromone composition (table 1). The colonies from France and Spain2 were started new for this study from field-collected insects, whereas insects from Italy2, USA2, and USA3 were long-term laboratory colonies (table 1). Larvae were reared on an agar-based wheat-germ diet (Ivaldi-Sender, Reference Ivaldi-Sender1974). Insects were sexed at the pupal stage and kept in different environmental chambers inside ventilated 2-liter clear plastic cages under a 16:8 L:D photoregime at 23±1 °C. Moths in cages were provided with wicks saturated with a 10% aqueous sugar solution. Newly emerged adults were collected every 1–2 days and used in laboratory assays as 2–5 day-old moths.
Flight tunnel tests
The response of male G. molesta from four populations reared in the laboratory to synthetic sex pheromone blends and to calling females was tested in a flight tunnel (table 1). The flight tunnel consisted of a 150×45×45 cm (length×height×width) glass cage with a sliding door on one side. The floor was solid white, with no visual markings to aid insect flight. A 30-cm-diameter fan at the upwind end of the tunnel, and a 20-cm-diameter exhaust vent at the downwind end created a 0.35 m s−1 wind flow through the tunnel that was vented outside of the building after exiting the tunnel. The air entering the tunnel was unfiltered room air. Temperature inside of the tunnel (averaged for all the tests) was 21.6±0.04 °C (mean±SEM). The flight tunnel was illuminated from above with fluorescent light bulbs producing 150 lux of white light. Tests were carried out during the last 4 h of the photophase and occasionally into the first hour of the scotophase, but in this case the daylight illumination was left on. Males were placed individually in 100-mm-long×30-mm-wide cylindrical metal mosquito screen wire cages with one end permanently closed by rolling it on itself, and the other end covered with a removable aluminum lid. Males were placed in the cages and transferred to the flight tunnel room 30–120 min before the test.
To test the response of males to live females, a few minutes before a test unmated females were placed individually in glass tubes (20 mm diameter×150 mm long) closed with a metal mosquito screen at one end and with an aluminum lid perforated with several 1.5-mm-diameter holes at the other end. Females were kept in a fume hood located outside the flight tunnel room and brought into the flight tunnel only for testing. Only females that were observed calling were used. The tube with the female was placed on an aluminum metal plate at the top of a 25-cm-tall metal-wire platform (0.5-cm-mesh) and aligned with the wind flow so that its metal screen side faced downwind. Three to eight males were tested individually with each female. The order of the populations of females and males tested was randomized on each date.
The response of males from each of the four populations to synthetic sex pheromone blends with different ratios of E8-12:Ac were conducted in the flight tunnel. All blends had 100% Z8-12:Ac (10 ng) and 10% Z8-12:OH, and had one of the following blend ratios of E8-10:Ac: 0.4, 5.4, 10.4, 20.4, 30.4, 60.4, or 100.1%. Treatment solutions were applied in 10 μl loads to 10×15 mm hexane-rinsed filter papers (Whatman® No. 1, Barcelona, Spain) so that the quantity of Z8-12:Ac loaded was 100 ng. The filter paper was held by a 30-mm alligator clip and was placed in a fume hood for 5–10 min to let dry before transferring it to a 20 ml clean vial, where it remained until tested in the flight tunnel 5–180 min later. The glass vial containing the test odor was opened and closed inside the flight tunnel to minimize contamination of the flight tunnel room. The base of the alligator clip was inserted vertically in the slot of a 25-mm binder clip, itself fixed to a 70-mm diameter aluminum metal plate located on top of a metal-wire platform similar to the one used for the females. The filter paper's flat surface was oriented to face the wind flow to obtain a sufficiently turbulent odor plume. Three to five males were flown to each filter paper treatment before changing the paper for another treatment. At the end of a test day a filter paper had been used with 6–12 males, so that filter papers were outside of the glass vial and exposed to the wind flow for a maximum of 24–36 min before being discarded. In a given day only one filter paper was used for each treatment.
After placing the odor stimulus (calling female or treated filter paper) in the upwind platform, the lid of the male cage was removed and placed in the flight tunnel with the open end facing upwind, on top of a metal-wire platform similar to the one used for the odor source and 1.5 m downwind from it. We recorded the number of males that landed on the filter paper or the tube containing the calling female. Each male was given 2 min to respond. At the end of the day the interior of the flight tunnel was cleaned with ethanol and the exhaust fan was left on. All glass and metal utensils were thoroughly washed with acetone and oven-dried at 200 °C.
Sex pheromone gland composition
Gland extracts of 2- to 5-day-old females from the France, Italy2, Spain2, and USA3 populations were collected during the last 3 h of the photophase. The tip of the abdomen containing the sex pheromone gland tissue was excised carefully by pulling it from the abdomen with fine forceps. Abdomen tips were placed in conical bottom vials either individually in 10 or 20 μl hexane or in groups of 5–24 in 10–50 μl of hexane. For quantification of the sex pheromone components the hexane used for extraction contained either 5 or 100 ng of pentadecane (>99% pure, Sigma-Aldrich, Spain). Glands were extracted at room temperature for 30–120 min, and the vials with the extract were stored at −20 °C until analyzed. Prior to analysis the sample was reduced to 1–2 μl via N2 evaporation and the final concentrate was injected in a Hewlett Packard 5890 gas chromatograph connected to a FID detector (Agilent Technologies, Madrid, Spain). The gas chromatograph was equipped with a 30-m-long, 0.25 mm I.D., 0.25-μm film thickness DB-Wax column (Agilent Technologies, Madrid, Spain). The constant helium flow through the column was at 1.0 ml min−1, and the injector and detector temperatures were 250 and 270 °C, respectively. The oven temperature program was increased from 50 to 170 °C at 20 °C min−1, and from 170 to 230 °C at 10 °C min−1, and remained at these temperatures for 10 min. Retention time and quantification were estimated with the injection of synthetic standards and with the internal standard, respectively. The retention times of E8-12:Ac, Z8-12:Ac, and Z8-12:OH were 9.08 min, 9.17.min and 9.78 min, respectively. Between 13 and 27 females were analyzed per population, but the internal standard was used in only eight extracts per population. Therefore, the sample size for quantification was N=8 and the sample size for ratio estimation was N=13–27.
Statistical analyses
Field and flight tunnel data were analyzed with generalized linear mixed models (GLMM) using the package lme4 in R (R development Core Team, 2008). A Poisson distribution with a logarithmic link function was used with the field data, and sex pheromone blend, population, and their interaction were considered as fixed effects while date and plot nested by date were considered as random effects. The logit link function was used to model binary responses (source contact/no source contact) for the flight tunnel tests. The fixed part of the model included sex pheromone blend, population, and their interaction with synthetic sex pheromone, or male and female population for the cross-attraction test with live females. The random part in these models consisted of a random intercept per day. Models were ranked based on a likelihood ratio tests for nested models, or the Akaike Information Criterion for non-nested models. Pearson coefficients and overdispersion estimates were used to further evaluate the fit of the models. Contrasts between pairs of treatments were calculated using the multcomp package of R, and a single-step Bonferroni procedure for correcting for multiple testing was included, setting general type I error at α=0.05. Sex pheromone gland content among populations was analyzed with the Kruskal–Wallis test followed by multiple comparison tests using kruskal.test and kruskalmcp packages, respectively, in R (type I error at α=0.05). The original data and the R codes are available online as supplementary material.
Results
Field tests
The total number of moths captured during field studies at each location ranged between 208 in USA1 to 6549 in Chile. Analyses of genital morphology found that all of the moths from Spain2 were G. molesta, and 3.1% of the captures in Korea were Grapholita dimorpha Komai, and another 1.9% was an undetermined tortricid species. Females constituted 0.0, 0.8, 1.4, and 2.0% of total captures in USA1, Chile, Spain2, and Italy1, respectively, with no conspicuous difference in the sex ratio among treatments.
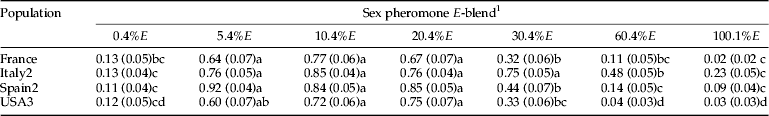
A clear separation in the mean total number of male moth catches was found among blends in nearly every population (table 2, figure S1, and tables S1–S3). The 5.4% E-blend captured significantly more males than any other blend. The 10.4% E-blend was the second most attractive and caught more males than the 0.4%E, 30.4%E, and 100.1% E-blends with all populations, except in Turkey where moth catches with the 10% E-blend did not differ from the catches with the 0.4% E-blend. In Chile, Italy1, Korea, New Zealand, Spain2, and Turkey the 0.4% E-blend attracted more males than the 30.4% E-blend, whereas in Spain1 and USA1 these two blends attracted similar number of males. Traps baited with hexane lure captured few moths (0.26% of all males caught). The 0.4% E-blend always attracted significantly more males than hexane, except with USA1. Traps baited with the 100.1% E-blend outperformed traps with the hexane lure in only the population from Chile. In comparison, traps baited with the 30.4% E-blend outperformed the hexane lure in four populations. The mean separations in statistical tests among E-blends in these field trials were similar in both the three apple and five stone fruit orchards (table 2).
Table 2. Expected mean (SEM) number of G. molesta male captures in pheromone traps as predicted by the estimated parameters of a GLMM model.

Row means followed by a different letter were significantly different, P<0.05, Tukey's test.
1 Pheromone traps were baited with hexane or with sex pheromone blends containing different percentages of the E8-12:Ac isomer relative to a constant load of 80 μg of Z8-12:Ac (100%) and 8 μg of Z8-12:OH on rubber septa. Data and further statistical results are provided in the supplementary data, tables S1–S3.
Male response to calling females
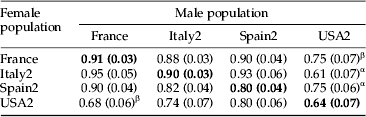
The percentage of male G. molesta flying upwind to calling females ranged between 61 and 95% in flight tunnel assays (table 3, tables S4–S6). There were no significant preferences for male to female from the same population relative to pairs from different populations, except for males from the French population that were more attracted to their own females than to females from USA2 (long-term laboratory colony). Males from the USA2 population were more responsive to France females than to their own females. Italy2 and Spain2 females attracted fewer males from the USA2 than males from their own population. In general, USA2 adults were both less responsive and attractive in the flight tunnel than the other populations (table 3).
Table 3. Expected mean (SEM) probabilities of inter-population landing of G. molesta males near females in flight tunnel bioassays as predicted by the estimated parameters of a GLMM model.
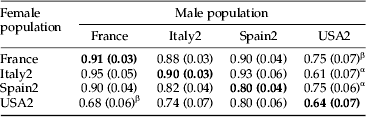
The diagonal bold line represents the within-population level of male to female attraction in the flight tunnel. Within a female population (row) when males from another population responded different from males from their own (in bold), the former population was labeled with an ‘α’ (P<0.05, Tukey's test). Within a male population (i.e. column) when males responded different to females from another population than to their own (in bold), the former population was labeled a ‘β’. Data and further statistical results are provided in the supplemental data, tables S4–S6.
Male response to synthetic blends
Significant differences in the response of males to sex pheromone blends containing different ratios of E8-12:Ac were found in flight tunnel assays with the four populations tested (table 4, tables S7–S9). Male response to the 5.4%E, 10%E, and 20.4% E-blends were not significantly different among populations. In addition, the breadth of non-significantly different E-blends was greater in some populations. For example, the male's response for Italy2 to the 30.4% E-blend was also similar to these three E-blends. With the USA3 population the response of males to the 5.4% E-blend was not different from its response to the 30.4% E-blend. Male responses to the other E-blends were significantly lower than to these most attractive blends with each population.
Table 4. Expected mean (SEM) probabilities of G. molesta males landing near a synthetic sex pheromone source with variable percentages of E8-12:Ac in the blend in a flight tunnel experiment as predicted by the estimated parameters of a GLMM model.
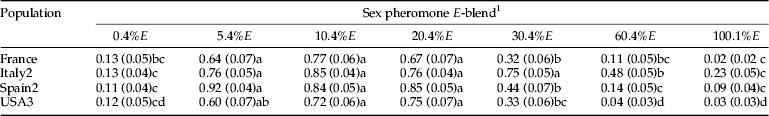
Row means followed by a different letter were significantly different, P<0.05, Tukey's test.
1 Moths were flown to treated filter paper all loaded (10 μl) with 100% Z8-12:Ac (100 ng) and 10% Z8-12:OH, and blends with one of the following ratios of E8-10:Ac: 0.4, 5.4, 10.4, 20.4, 30.4, 60.4, or 100.1%. Probabilities were obtained from the estimated parameters of a GLMM model. Data and further statistical results are provided in the supplemental data, tables S7–S9.
Pheromone gland composition
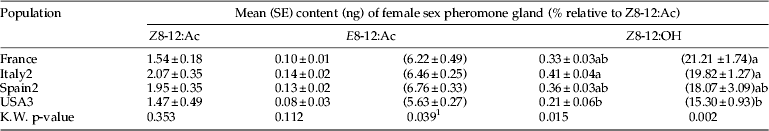
Female gland extracts from the three field-collected European populations had similar quantities and ratios of the three sex pheromone components (table 5). The percentage of Z8-12:OH in the blend was close to 20%. However, the long-term laboratory population from California (USA3) had lower percentages of the two minor components relative to Z8-12:Ac in their blend than some populations. Female moths from USA3 also had significantly less Z8-12:OH than females from the other long-term laboratory population, Italy2.
Table 5. Mean (SEM) quantity and percentages of the three sex pheromone components in gland extracts of female G. molesta.
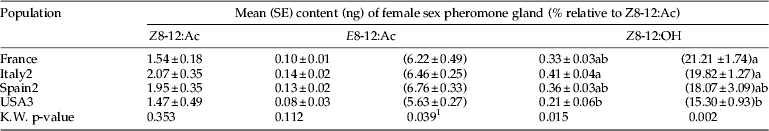
Column means followed by a different letter were significantly different, P<0.05, multiple comparison after Kruskal–Wallis test.
1 The overall Kruskal–Wallis test was significant but there were no significant mean differences among treatment pairs.
Discussion
The response of male G. molesta from eight worldwide populations in field and flight tunnel studies to synthetic sex pheromone and virgin females was found to be quite similar. The principal difference found in these studies was between long-term laboratory colonies from the USA and field-collected populations from either apple or peach. We did observe that under our controlled laboratory conditions males responded in the flight tunnel optimally to a broader range in the percentage of the E-isomer than under field conditions.
Other examples of species such as G. molesta that have low intraspecific geographical variation in sex pheromone communication are rare (e.g. Haynes & Baker, Reference Haynes and Baker1988; Haynes & Hunt, Reference Haynes and Hunt1990; Wu et al., Reference Wu, Wu, Chen, Xu, Liu, Mao, Quo and Du2012; Du et al., Reference Du, Li, Chen, Lin, Wang and Qin2013), and in contrast with a larger number of species (moths, insect, and other animals) that show geographical population differences (reviewed in all of the following: Löfstedt, Reference Löfstedt1990; El-Sayed et al., Reference El-Sayed, Delisle, DeLury, Gut, Judd, Legrand, Reissig, Roelofs, Unelius and Trimble2003; Cardé & Haynes, Reference Cardé, Haynes, Cardé and Millar2004; Smadja & Butlin, Reference Smadja and Butlin2009). This pattern may be caused, at least in part, by an underrepresentation of negative results (i.e. lack of significant geographical variation) in the scientific literature. However, it could also indicate that species with geographical population variation in their sex pheromone communication channel are more numerous than those with uniform sex pheromones. The lack of any substantial geographical variability with G. molesta could be explained by its relatively recent spread (<100 years) over much of its geographic range (Rothschild & Vickers, Reference Rothschild, Vickers, van der Geest and Evenhuis1991), and its limited dispersal capability (Ellis & Hull, Reference Ellis and Hull2012). However, a recent genetic study found a weak but significant global pattern of isolation-by-distance on a continental scale in G. molesta (Kirk et al., Reference Kirk, Dorn and Mazzi2013). Perhaps more extensive studies, including populations from all regions within its geographical expansion: Australia, Brazil, Canada, China, Eastern Europe, Japan, and South Africa, would find different results.
Our study confirms previous trapping studies in Australia, Korea, and USA where the optimal proportion of E8-12:Ac relative to Z8-12:Ac is close to 5–6%, and where suboptimal percentages of E8-12:Ac result in gradual decreases in male response (Beroza et al., Reference Beroza, Gentry, Blythe and Muschik1973; Roelofs & Cardé, Reference Roelofs and Cardé1974; Baker & Cardé, Reference Baker and Cardé1979; Yang et al., Reference Yang, Jung, Han, Boo and Yiem2002). Also our study found a similar ratio of the two acetate components as a previous study analyzing the female's sex pheromone gland (Yang et al., Reference Yang, Jung, Han, Boo and Yiem2002). However, female moths from a population collected from Eastern Canada had a somewhat lower proportion of E8-12:Ac, 2.9% relative to Z8-12:Ac (El-Sayed & Trimble, Reference El-Sayed and Trimble2002). Our laboratory results also differed from some of the findings in earlier reports. For example, blends containing a 20% or higher ratio of E8-12:Ac had very low levels of attraction (Roelofs & Cardé, Reference Roelofs and Cardé1974; Baker & Cardé, Reference Baker and Cardé1979; Baker et al., Reference Baker, Meyer and Roelofs1981; Linn & Roelofs, Reference Linn and Roelofs1981). In contrast, we found that with males from the two long-term laboratory populations the 30.4% E-blend performed as well as the 5.4% E-blend. Laboratory rearing can clearly impose selection pressure to create lab-adapted populations which may differ widely from wild populations in their responsiveness to sexual and host cues (Boller, Reference Boller1972). Male G. molesta reared in the laboratory for 20 years were found to possess significantly fewer sensilla trichodea on antennae than wild males (Nagy & George Reference Nagy and George1981). Other factors, such as temperature, lure emission rates, and the presence of background host plant volatiles could also have contributed to these differences (Baker et al., Reference Baker, Cardé and Miller1980; Linn & Roelofs, Reference Linn and Roelofs1991; Varela et al., Reference Varela, Avilla, Anton and Gemeno2011).
Characterization of the behavioral role of Z8-12OH in the sex pheromone blend has varied among both laboratory and field studies of G. molesta since it was first reported to be part of the active sex pheromone blend (Cardé et al., Reference Cardé, Baker and Roelofs1975). Studies have shown a significant effect of Z8-12:OH on male behaviors between blends lacking it and blends containing 3%, but no difference among blends containing from 3 to 100% of Z8-12:OH (Baker & Cardé, Reference Baker and Cardé1979; Linn & Roelofs, Reference Linn and Roelofs1983). Other studies have found that Z8-12:OH is not necessary for attraction (Roelofs & Cardé, Reference Roelofs and Cardé1974; Yang et al., Reference Yang, Jung, Han, Boo and Yiem2002), and that its proportion in the blend can vary widely without affecting male response (Linn & Roelofs, Reference Linn and Roelofs1983). The proportion of Z8-12:OH in the sex pheromone blend appears to be more variable than the acetate blend among worldwide populations. For example, Lacey & Sanders (Reference Lacey and Sanders1992) in Australia recovered only trace amounts of Z8-12:OH in female's effluvia. The population from Eastern Canada had only 5.8% of Z8-12:OH in the female's sex pheromone gland (El-Sayed & Trimble, Reference El-Sayed and Trimble2002), or only a third of what was found in our study and also previously in Korea (Yang et al., Reference Yang, Jung, Han, Boo and Yiem2002). In comparison, both Cardé et al. (Reference Cardé, Baker and Cardé1979) and Baker et al. (Reference Baker, Cardé and Miller1980) found ≥30% of Z8-12:OH relative to the amount of Z8-12:Ac in the effluvia released by females in their laboratory-reared colonies in New York. The variability in the ratio of Z8-12:OH was low among our four populations. Further tests are needed to consider how the much larger differences found between the proportions of Z8-12:OH among G. molesta female populations worldwide could influence differences in male response among populations.
Some recent studies have demonstrated that intraspecific variability in moth sex pheromone communication is associated with host specialization, i.e., the Z and E strains of O. nubilalis (Leppik & Frérot, Reference Leppik and Frérot2012), and the corn and rice strains of S. frugiperda (Unbehend et al., Reference Unbehend, Hänniger, Meagher, Heckel and Groot2013). Grapholita molesta is associated with several hosts in the family Rosaceae, and it typically attacks stone fruit (peach and nectarine) shoots (the preferred hosts and larval feeding tissue) early in the season and then moves to pome fruit (apple and pear) later where it feeds on shoots and fruits (Rothschild & Vickers, Reference Rothschild, Vickers, van der Geest and Evenhuis1991). Major fruit growing regions are usually characterized as a large continuous interplanting of cultivars of both host groups, a factor that could limit host-race formation. In our study, no obvious differences were found in the response of G. molesta males to sex pheromone blends for populations sampled in stone fruit and pome fruit. However, all field populations were studied and collected from mixed-crop production areas.
Host-races might be more likely to occur in large areas where only one of these two hosts occur, such as in Brazil where apple is commonly attacked by G. molesta in regions widely separated from the country's major peach production (Reis et al., Reference Reis, Nora and Melzer1988). While, host races have not been described for G. molesta, a recent study using genetic markers in China found significant differences in the genetic structures of populations collected from apple and pear late in the season compared with peach populations (Zheng et al., Reference Zheng, Peng, Liu, Pan, Dorn and Chen2013). In contrast, Kirk et al. (Reference Kirk, Dorn and Mazzi2013) found no evidence of differences in the genetic structure between populations from different hosts. Future comparisons of the sex pheromone blend of G. molesta from different hosts in countries, such as Brazil or China would provide more useful data on the potential for divergent evolution in sexual signaling in this moth pest.
Another important aspect likely affecting the apparently limited plasticity of the isomer ratio in the blend of G. molesta is the strong degree of stabilizing selection imposed by the sympatric olethreutinae tortricid species attacking Prunus, Malus, and Pyrus tree species using the same isomers but in different ratios. The maintenance of reproductive isolation among sympatric tortricid species has been shown to be due to the specificity of their individual mating chemical signals and not due to temporal isolation (reviewed in Roelofs & Brown, Reference Roelofs and Brown1982). For example, Grapholita prunivora (Walsh) in North America and Grapholita funebrana (Treltschke) in Europe have overlapping distributions with G. molesta, and their sex pheromone blends have been identified as including Z8-12:Ac with 2.2 and 3.0% of the E-isomer, respectively (Roelofs & Cardé Reference Roelofs and Cardé1974; Guerin et al., Reference Guerin, Arn, Buser, Charmillot, Toth and Sziraki1986). E8-12:Ac and Z8-12:Ac (70:30 ratio) are also the sex pheromone blend of the false codling moth, Cryptophlebia leucotrata (Meyrick) which overlaps with the distribution of G. molesta in orchards in South Africa (Persoons et al., Reference Persoons, Ritter and Nooyes1977). Furthermore, the role of secondary minor components in sex pheromone blends defines species specificity; e.g. Z8-12:OH inhibits male G. funebrana and G. prunivora; and components from these species’ sex pheromone blends, such as Z6-12:Ac and Z10-14:OH inhibits male G. molesta (Baker & Cardé Reference Baker and Cardé1979; Guerin et al., Reference Guerin, Arn, Buser, Charmillot, Toth and Sziraki1986; Tòth et al., Reference Tòth, Sziráki, Szöcs and Sáringer1991). Greater documentation and understanding of interspecies tortricid communication systems present in both the native range of G. molesta in China and across the worldwide commercial plantings of suitable hosts likely surrounded by different complexes of native moth species and hosts could be instructive on measuring the rate of environmentally imposed selection.
Supplementary Material
The supplementary material for this article can be found at http://www.journals.cambridge.org/BER
Acknowledgements
We would like to thank the financial support of MEC, Spain (AGL2010-17486) to C.G., D.B, and L.A.E., and the Chilean grant ‘Conicyt MEC 80120005’ to C.G. In Girona, Spain, Cesca Alcalá with RTA conducted the field experiments and Maria Carbó obtained the research sites. In Turkey, the research assistant Bilgi Pehlevan (Uludag University, Turkey) was very helpful in these studies. In Korea the work was supported by the Basic Science Research Program through the National Research Foundation of Korea. In Italy, Matteo Anaclerio and Manuela Cigolini (Catholic University) were very helpful. Help with the studies conducted in Oregon was provided by Rick Hilton at Oregon State University, Medford, OR. Laboratory colonies from Pennsylvania were provided by Greg Krawczyk, Penn State University, Biglerville, PA, and from California by personnel at the USDA, ARS Laboratory, Parlier, CA. Jean-Claude Tournié, of Prestagro, collected insects in France and shipped them to Spain.