Introduction
The species Spermacoce densiflora DC. and Spermacoce verticillata L., native to Brazil, are popularly known as false-button weeds and belong to the family Rubiaceae. They are sub-shrub species of terrestrial habitat with a perennial life cycle and small seeds, which contributes to their easy dispersion (Nepomuceno et al., Reference Nepomuceno, Souza, Nepomuceno, Miguel, Cabral and Loiola2018).
Both species have a wide distribution in the American continent (Varjão et al., Reference Varjão, Jardim and Conceição2013), being common in Brazilian agricultural areas of the MATOPIBA region (states of Maranhão, Tocantins, Piauí, and Bahia), and becoming a problem weed in this region which is considered one of the largest grain producers in Brazil (Garagorry et al., Reference Garagorry, De Miranda and Magalhães2014). Also, field observations have shown ineffective control with chemicals (Mascarenhas et al., Reference Mascarenhas, Modesto, Dutra and Souza1999; Macedo et al., Reference Macedo, Brandão and Lara2003; Martins et al., Reference Martins, Cabral, Souza and Christoffoleti2009; Marques et al., Reference Marques, Silva, Araujo, Lopes, Corrêa, Freitas and Muniz2010; Santos et al., Reference Santos, Procopio, Silva, Fernandes and Barroso2016, Fadin and Monquero, Reference Fadin and Monquero2019), justifying studies of biology and alternative control methods.
One of the limiting factors to the adequate management of weeds is the lack of knowledge about the germination and establishment of seedlings (Brighenti et al., Reference Brighenti, Castro, Gazziero, Adegas and Voll2003). Germination can be regulated by several environmental factors such as soil salinity, pH, water and oxygen availability, temperature and light (Weller et al., Reference Weller, Florentine and Chauhan2019). Temperature can act directly or indirectly in the germination process, influencing water absorption, the speed of various chemical reactions, and the percentage and speed of germination (Carvalho and Nakagawa, Reference Carvalho and Nakagawa2012; Mahmood et al., Reference Mahmood, Florentine, Chauchan, Maclaren, Palmer and Wright2016).
Moreover, light can affect germination, promoting it in positive photoblastic seeds and inhibiting it in negative photoblastic seeds. Some species are also not affected by light, known as neutral photoblastic species (Silva et al., Reference Silva, Rodrigues and Aguiar2002).
Studies on the biology of weeds are relevant because they provide the basis for the development of appropriate techniques for their management (Ozaki et al., Reference Ozaki, Shimono and Tominaga2018). The intensive use of the same herbicide or herbicides with the same mechanism of action is among the practices commonly employed in the management of weeds and has been selecting tolerant species and resistant weed biotypes to survive in the field (Christoffoleti et al., Reference Christoffoleti, Victoria Filho and Silva1994).
According to Heap (Reference Heap2019), Brazil currently has 51 cases of weed biotypes resistant to different herbicide mechanisms of action, 16 of which have multiple resistance. For example, the weed Conyza sumatrensis has multiple resistance to herbicides with five sites of action.
Another factor that can affect the establishment of a weed is seed depth in the soil (Zhang et al., Reference Zhang, Gao, Dai, Song, Feng and Qiang2019). Seed depth will be influenced by soil disturbance and different tillage practices. Among the forms of soil tillage applied in Brazil is the no-tillage system (FEBRAPDP, 2019). According to data from the 2017 Census of Agriculture, 19% of the soil tillage systems carried out in Brazil adopt no-tillage with straw cover (IBGE, 2020). In this system, vegetative cover (straw) is retained and can contribute to reducing or suppressing the development of weeds, providing direct and indirect effects (e.g., physical barrier, reduced availability of light and allelopathic action), thus hindering germination and emergence (Correia and Durigan, Reference Correia and Durigan2004; Silva Neto et al., Reference Silva Neto, De Pauli, Júnior and Marques2018) and, consequently, reducing the need for herbicides (Vicent-Caboud et al., Reference Vincent-Caboud, Casagrande, David, Ryan, Silva and Peigne2019).
There is little published information on environmental factors and management practices that affect the germination and emergence of seeds and seedlings of S. densiflora and S. verticillata. This study aimed to evaluate the influence of light, temperature, sowing depth, amount of straw and allelopathic potential of cover crops on the germination and emergence of seeds and seedlings of S. densiflora and S. verticillata.
Materials and methods
Mature seeds of S. densiflora and S. verticillata were collected in Luis Eduardo Magalhães, Bahia, Brazil (772 m altitude: 12°05′58″S and 45°47′54″W), in autumn 2018. Seeds came from areas cultivated with soybean and cotton in crop rotation and were collected randomly from 100 plants of each species. After manual processing, the seeds were stored in plastic containers in a refrigerator at a temperature of 2 to −2°C for 6 months. The experiments were conducted in 2018 and 2019.
Transparent acrylic gerbox boxes (11.0 × 11.0 × 3.0 cm) and 9-cm diameter Petri dishes were used as experimental units in the experiment conducted in the laboratory to evaluate the effect of light, temperature and allelopathic potential of the aqueous extracts of cover crop plants. Each experimental unit consisted of 50 seeds sown on two sheets of blotting filter paper moistened with water or aqueous extracts in an amount equivalent to 2.5 times the mass of the substrate, according to the Rules for Seed Testing (Brasil, 2009).
Plastic pots with a volumetric capacity of 5 L, filled with a clay textured Oxisol and maintained under controlled irrigation were used as experimental units for the experiment conducted in a greenhouse to evaluate the effect of seed burial depth and amount of cover crop straw on the emergence of seedlings of S. densiflora and S. verticillata. Thirty seeds were sown in each experimental unit.
The experimental design was completely randomized, with four replications for each treatment for all experiments. Thus, the sample sizes were 200 and 120 seeds in each treatment for each species in laboratory and greenhouse bioassays, respectively.
Experiment 1: effect of temperature and light on germination
Seeds of S. densiflora and S. verticillata were placed for germination under two light conditions, i.e., presence of light (12-h photoperiod) and absence of light (constant dark), and six temperature treatments, i.e., five constant temperatures (15, 20, 25, 30 and 35°C) and one alternating temperature (20 and 30°C) under a 12-hour dark/light cycle.
The transparent acrylic boxes were covered with electrical tape to exclude light in the constant darkness treatment. Sowing was carried out under green light (Amaral-Baroli and Takaki, Reference Amaral-Baroli and Takaki2001). Each box was wrapped in a 1.0-mm thick transparent plastic bag to avoid the rapid drying of the blotting paper.
After sowing, the boxes were placed in a Biochemical Oxygen Demand (BOD) germination chamber adjusted to the temperature and photoperiod according to each treatment. The number of seeds germinated were counted daily, and these counts were used in calculating the percentage of germination (G%) and germination speed index (GSI) (Maguire, Reference Maguire1962). The criterion for germination was the formation of normal seedlings with the cotyledons fully expanded from the seed tegument. The substrate was re-moistened whenever necessary. The experiment lasted 30 days.
Experiment 2: effect of seed burial depth on seedling emergence
Seeds of S. densiflora and S. verticillata were placed at seven sowing depths (0, 0.5, 1, 2, 4, 6 and 10 cm). The soil surface was homogenized and pressed to standardize the depths and improve the contact of the seed with the soil. The seeds were placed on the soil surface for the 0 cm depth treatment.
Seedling emergence was counted daily until 42 days after sowing (DAS). A seedling was classified as emerged once a fully expanded cotyledon was present. The daily emergence counts were used in calculating the percentage of emergence (E%) and the emergence speed index (ESI) (Maguire, Reference Maguire1962).
Experiment 3: effect of the amount of straw of cover crops
Five cover crop straws were applied at three amounts, corresponding to the average, half and twice the straw produced in the field. The average amount of straw was determined by the company that produces green manure seeds (Pirai Sementes) and observed in production fields. Thus, the amount of straw was 40, 20 and 80 t ha−1 for sunn hemp (Crotalaria juncea) and pearl millet (Pennisetum glaucum), 15, 7.5 and 30 t ha−1 for sorghum (Sorghum bicolor) and hyacinth bean (Dolichos lablab), and 20, 10 and 40 t ha−1 for pigeon pea (Cajanus cajan). The seeds were placed on the soil surface of the pots and the corresponding amount of straw was added over the seeds. Emergence counts were taken until 30 days after setting up the experiment and counts were used in calculating the percentage of emergence (E%) and the ESI (Maguire, Reference Maguire1962).
Experiment 4: allelopathic potential of aqueous extracts of cover crops
The allelopathic potential of the aerial parts of the cover crops sunn hemp, pearl millet, sorghum, hyacinth bean and pigeon pea on the percentage of germination and germination speed of seeds of S. densiflora and S. verticillata was evaluated using different concentrations of aqueous extracts (0, 20, 40, 60 and 100%). Cover crops were sown and at the vegetative stage, before the flowering, were collected from the field to obtain extracts. The aqueous extracts were obtained by drying cover crop plants in an oven at 65°C for 48 h. The dried plant material was then crushed, weighed and immersed for 24 h in distilled water. The crude aqueous extract (100%) consisted of a proportion of 100 mg of dried plant material and 300 mL of distilled water. Subsequently, the extract was filtered through a cloth filter and collected in a Kitasato flask using a vacuum pump coupled to a Büchner funnel with quantitative filter papers. Dilutions were made with distilled water to 60, 40 and 20% (Gatti et al., Reference Gatti, Perez and Lima2004).
The pH and osmotic potential of each extract were measured using a psychrometer. These measurements were performed at a temperature of 25°C and expressed in MPa.
Moreover, tests were carried out with PEG-6000 solution at different concentrations to determine the influence of osmotic potential on the germination of the studied species. The experiment was set up in a BOD germination chamber with 12-h photoperiod and alternating temperature (20–30°C) every 12 h. Germination was counted at 7, 14, 21 and 28 DAS.
Statistical analysis
The experimental design of all experiments was completely randomized, with four replications. An exploratory analysis was carried out to verify whether the premises of the analysis of variance, such as normality, homogeneity and outliers of the data, were being met. The data from experiment 1 were subjected to analysis of variance in a factorial design (light × temperature) and the means were compared by least significant difference (LSD) test at a 5% probability. The data from Experiment 3 were subjected to analysis of variance in a factorial design (cover crop specie × amount of straw of cover crops) and the means were compared by LSD test at a 5% probability. An analysis of variance, followed by the LSD test at a 5% probability, was applied in the other experiments. Statistical analysis was performed using software RStudio® (RStudio Team, 2016).
Results
Experiment 1: effect of temperature and light on germination
A significant effect was observed between the germination of seeds subjected to different temperatures and in the presence of light or darkness (P < 0.05) for both species (Table 1). Although the presence of light promoted the germination of seeds of S. densiflora up to 55% in the treatment with alternate temperature 20–30°C differing from other temperature treatments. For S. verticillata there was a difference within the light treatments at temperatures 15, 25 and 30°C. The highest germination rate was observed at alternating temperature (67% in light treatment and 70% in dark treatment). At the constant temperatures, there was a significantly greater germination of S. verticillata at 15°C than all other constant temperatures with germination decreasing as the temperature increased.
Table 1. Germination (G) and GSI of seeds of Spermacoce densiflora and Spermacoce verticillata under different temperature and light regimes

Note: Means followed by the same lowercase letter in the columns or uppercase letter in rows, for each variable, do not differ from each other by the LSD test at a 5% probability.
The GSI showed no significant difference between the variables. The treatment at 35°C is similar to the maximum temperature, providing the total inhibition of germination above it. The highest percentages of germination and GSI were obtained at the alternating temperature condition.
Experiment 2: effect of seed burial depth on seedling emergence
The percentage of emergence of S. densiflora when the seeds were placed on the soil surface was 75%. Sowing the seeds at a depth of 0.5 cm provided only 13% emergence and at a depth of 6 cm it was 0.8%. There was no emergence from seeds sown at a depth of 10 cm (Figure 1).
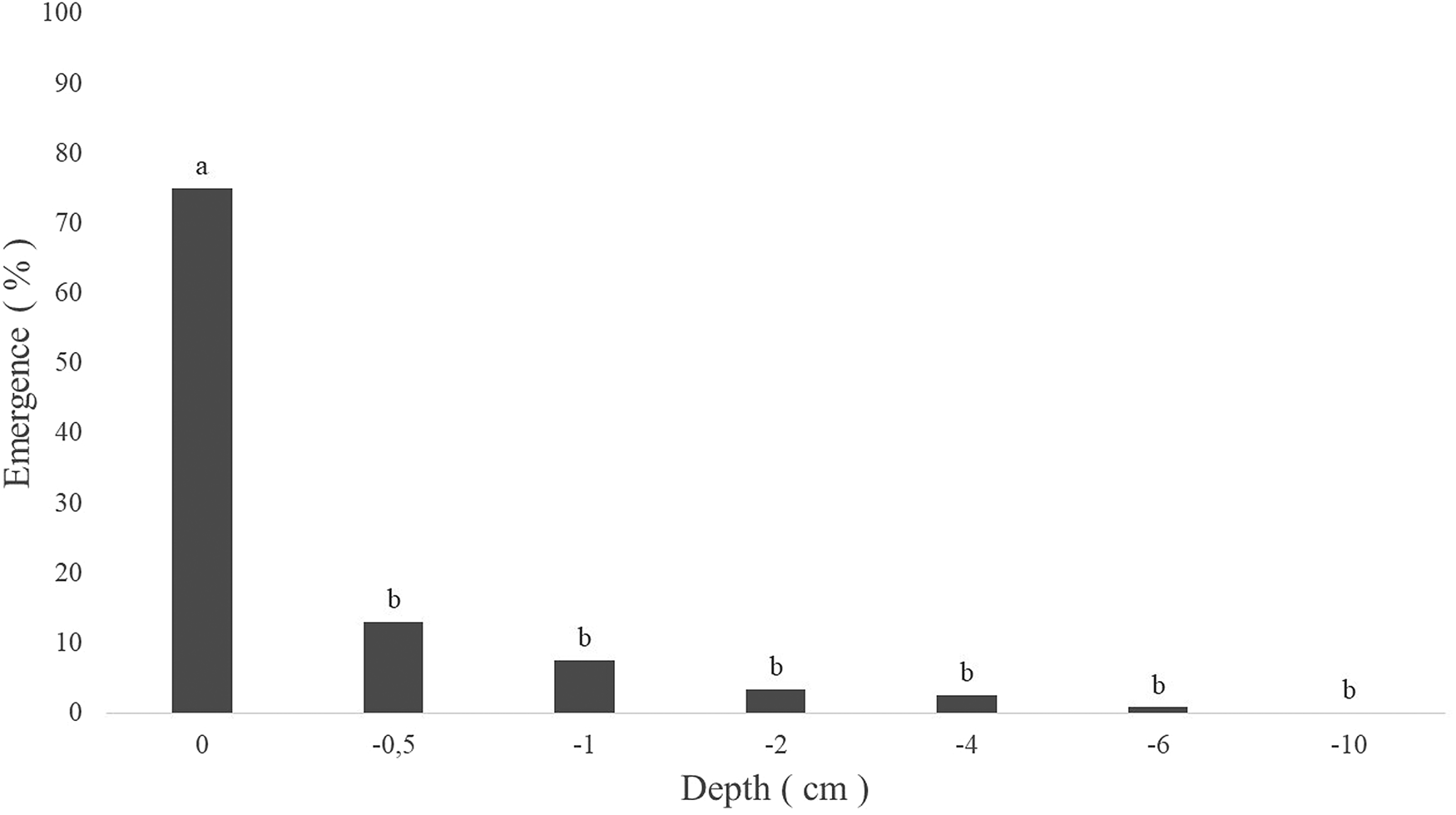
Fig. 1. Emergence after 42 days of seedlings of Spermacoce densiflora from seeds placed at different sowing depths (cm).
The percentage of S. verticillata emergence from seeds sown on the soil surface was 87.5%. Emergence was only 10.8% at a depth of 0.5 cm. The emergence was 1.7% at a depth of 1 cm depth and 0.8% at 2 to 6 cm. There was no emergence of seedlings from a depth of 10 cm (Figure 2).

Fig. 2. Emergence after 42 days of seedlings of Spermacoce verticillata from seeds placed at different sowing depths (cm).
Experiment 3: effect of the amount of straw of cover crops
The presence of straw caused a significant reduction in the emergence for both weed species (P < 0.05) (Table 2). There was no interaction between the cover crop specie and the amount of straw of cover crops. In all treatments, when the seed was placed on the soil surface with no straw, the emergence was significantly higher than the other treatments, with an average emergence of 87.5% for S. densiflora and 86.7% for S. verticillata.
Table 2. Percentage of emergence (E) and ESI of seedlings of Spermacoce densiflora and Spermacoce verticillata subjected to different amounts of straw from the cover crops sunn hemp, pearl millet, sorghum, hyacinth bean and pigeon pea

Note: Means followed by the same letter in each column and for each weed species do not differ significantly from each other by the LSD test at a 5% probability.
a Amounts referring to the control (0), half the recommended (0.5), the recommended (1) and twice (2) the recommended straw level for each cover crop.
The use of half the amount of straw recommended for all cover crop plants significantly suppressed emergence of the seedlings of both weed species compared to the control without straw. There was also a significant difference in the ESI between cover crop straw treatments and the control treatment (P < 0.05), with reduced when straw was added (Table 2).
Experiment 4: allelopathic potential of aqueous extracts of cover crops
No significant difference was detected in the germination of S. densiflora and S. verticillata with the use of different aqueous extracts from the aerial part of the different species of cover plants (sunn hemp, pearl millet, sorghum, hyacinth bean and pigeon pea). The addition of the aqueous solution of each of the cover crops resulted in a significant reduction in germination and GSI of both species compared with the control where no extract was added. However, there was generally no difference in germination or GSI between the different extract concentrations although there is a trend for less germination at higher extract concentrations. The gradual increase in the extract concentrations led to lower percentages of germination and GSI for S. densiflora and S. verticillata. Results also show that the germination of S. verticillata was more greatly reduced by the presence of crop extract than S. densiflora (Table 3).
Table 3. Percentage of germination and GSI of seeds of Spermacoce densiflora and Spermacoce verticillata subjected to different concentrations of aqueous extracts from different cover crops

Note: Means followed by the same letter in each column and for each weed species do not differ significantly from each other by the LSD test at a 5% probability.
The physicochemical characterization of cover crop extracts (Table 4) presented osmotic potential values ranging from −0.23 to −1.24 MPa. The pH values varied from 5.02 to 6.53, with low acidity.
Table 4. pH and osmotic potential of the aqueous extracts from the aerial parts of cover crops measured at concentrations of 20, 40, 60 and 100%

The PEG-6000 solution provided germinations of 22.5, 15 and 0.5% of the seeds of S. densiflora at osmotic potentials of −0.2, −0.4 and −0.8 MPa, respectively (Table 5). No germination was observed for seeds of S. densiflora at osmotic potentials of −1.2 and −1.6 MPa. On the other hand, 27.5% of seeds of S. verticillata germinated at the osmotic potential of −0.2 MPa and 30.5% at−0.4 MPa. No germination was observed at −0.8, −1.2 and −1.6 MPa.
Table 5. Effect of PEG-6000 solutions on the germination (%) of seeds of Spermacoce densiflora and Spermacoce verticillata

Discussion
The results obtained in this study indicate the occurrence of two distinct behaviors in relation to the response to light presence during seed germination: seeds that germinated only in the presence of light and were considered positive photoblastic (S. densiflora) and seeds that were also capable of germinating in continuous darkness, being classified as neutral photoblastic (S. verticilatta) or aphotoblastic.
Temperature fluctuation did not alter the seeds sensitivity to light in S. densiflora, since we did not observe seed germination in the darkness in the alternating temperature treatment (20–30°C). Some environmental factors such as extreme and/or alternating temperatures may replace the light requirement for seed germination, since temperature fluctuations interfere with the active production of phytochrome and may induce seed germination in darkness (Pons, Reference Pons and Fenner2000; Probert, Reference Probert and Fenner2000).
In agreement with the results obtained for S. densiflora, the germination of seeds of Digitaria horizontalis was negatively affected by darkness, that is, exhibiting a positive photoblastic character (Mondo et al., Reference Mondo, Carvalho, Dias and Marcos Filho2010). Similar results were observed for seeds of S. verticillata as for the weed Sesbania cannabina which characterizes the species as having neutral photoblastic behavior (Iqbal et al., Reference Iqbal, Manalil, Chauhan and Adkins2019).
The highest percentage of germination occurred in the range of 20–30°C for both species studied. In other species (Chloris barbata, Cynodon dactylon and Cyperus rotundus), the germination process was also dependent on the temperature fluctuation as observed by Loddo et al. (Reference Loddo, Carlesi and Pais da Cunha2019).
Both species showed differences in germination under different constant temperature treatments (Table 1). The non-uniformity of germination in weeds is a strategy that allows these plants to last in the environment (Silva et al., Reference Silva, Rodrigues and Aguiar2002; Cabral et al., Reference Cabral, Miguel and Salas2011). Among the factors associated with germination unevenness are the distribution of seeds in the soil profile, ecophysiological adaptation to the environment, and seed dormancy mechanisms (Brighenti and Oliveira, Reference Brighenti, Oliveira, Oliveira, Constantin and Inoue2011; Carvalho and Nakagawa, Reference Carvalho and Nakagawa2012).
The higher rate of seedling emergence from seeds placed on the soil surface is consistent with the positive photoblastic character of the seeds of S. densiflora. Nosratti et al. (Reference Nosratti, Almaleki and Chauhan2019) observed similar results when studying the effect of sowing depth on the emergence of seedlings of Picnomon acarna, a positive photoblastic weed, with the highest percentage of emergence on the soil surface (94.7%).
Amini et al. (Reference Amini, Mobli and Ghanepour2015) also found a reduction in seedling emergence with an increase in the sowing depth of Lepidium vesicarium, a neutral photoblastic weed, with the highest emergence rate from seed on the soil surface (90%). Nosratti et al. (Reference Nosratti, Amiri, Bagheri and Chauhan2018) also observed that the emergence of Sophora alopecuroides, a neutral photoblastic weed, was greatest (99%) in seeds placed on the soil surface, and the emergence of seedlings decreased with increasing depth.
In general, smaller seeds, with low reserve amounts, have difficulty emerging from increasing sowing depth since this action demands energy expenditure (Schutte et al., Reference Schutte, Tomasek, Davis, Andersson, Benoit, Cirujeda and Murdoch2014). However, emergence does not depend only on reserves. The increase in sowing depth leads to changes in essential factors for overcoming seed dormancy of some species, such as the availability of oxygen, moisture, temperature and soil physical characteristics (Santana et al., Reference Santana, Anastácio, Lima and Mattos2010).
Therefore, a reduction in the emergence of seedlings of S. densiflora and S. verticillata with increasing soil depth may be due to the low amount of reserves in the seeds, as they are small seeds (Varjão et al., Reference Varjão, Jardim and Conceição2013), and changes in essential resources for germination to occur. The results of this experiment suggest that these weeds will be favored under no-tillage systems where most seed remain on the soil surface. Tillage that buries seed to 10 cm or more is likely to prevent emergence. However, seed burial can result in increased persistence of seeds.
Seeds sown on the soil surface showed the highest ESI because there is higher light availability under this condition, with no need to promote energy expenditure to overcome the soil layer on the seedling. In addition, the increase in sowing depth promotes a higher concentration of carbon dioxide near seeds, impairing the emergence process (Guedes et al., Reference Guedes, Alves, Gonçalves, Viana, Moura and Costa2010).
Moreover, temperature variations on the soil surface are greater than those in other depths (Cardoso et al., Reference Cardoso, Alves, Bruno, Alves and Silva2008), improving germination conditions, as verified for Experiment 1 (Table 1).
The presence of cover crop straw significantly reduced the emergence of both weed species, regardless of the type of cover crop. Even the treatment with less straw in the soil generated enough mulch to reduce the presence of S. densiflora and S. verticillata. In the recommended amount of straw (treatment 1) the weed suppression was effective. Successful weed suppression using cover crop has been reported for annual crops such as rice (Oryza sativa) preceded by pigeon pea as a cover crop (Roder et al., Reference Roder, Maniphone and Keoboulapha1998), corn by using Lupinus albus, Lathyrus sativos, Triticum aestivum, Avena strigosa, Raphanus sativus (Martins et al., Reference Martins, Gonçalves and Silva Junior2016) and soybean by using as cover crop–sorghum hybrid (S. bicolor cv. Bicolor) with Sudan-grass (S. bicolor cv. Sudanense).
The application of straw on the soil surface promotes a physical barrier to be overcome by seedlings, which tend to consume their energy reserves before reaching the surface and starting photosynthesis (Correia et al., Reference Correia, Durigan and Klink2006). In addition, the presence of vegetation cover on the soil may have interfered with the emergence of weeds by preventing photons from reaching the seeds, thus affecting their development (Sage and Kubien, Reference Sage and Kubien2003). These results are consistent with the positive photoblastic behavior of S. densiflora, the need for alternating temperatures for the germination of seeds of S. densiflora and S. verticillata, and the small seed size of both species.
According to Adegas et al. (Reference Adegas, Vargas, Gazziero and Karam2017), the presence of straw on the soil surface reduces the thermal amplitude and the incidence of light at long wavelengths. The authors also reported that the presence of straw on small seeds reduces the chances of seedlings emerging, as they have no reserves that guarantee their survival until they have access to light. It may probably explain the higher seedling emergence in the control treatment.
Alberguini and Yamashita (Reference Alberguini and Yamashita2010) used sugarcane, corn and brachiaria grass straw and observed a significant reduction in the emergence of seedlings of Vernonia ferruginea compared to the absence of straw. Borges et al. (Reference Borges, Freita, Mateus, Sá and Alves2014) worked with different amounts of the cover plants S. bicolor, Pennisetum americanum, Sorghum sudanense and Urochloa ruziziensis and found a reduction in weed infestation regardless of the amounts used. Araldi et al. (Reference Araldi, Yamashita, Carvalho, Campos, Roque and Dallacort2015) worked with three types of straw (sunn hemp, corn and brachiaria grass) and four amounts (0, 2, 4 and 8 t ha−1) on the emergence of C. juncea and observed that the presence of straw from 2 t ha−1 reduced its emergence, with an emergence lower than 1% under the highest amount.
The species S. densiflora and S. verticillata showed significance with the use of half of the recommended amount of all the cover crops studied, with a higher ESI than the other treatments. Generally, the use of twice the recommendation showed less or no difference from the treatment with the recommended amount for the used cover crops.
The presence of straw hampers the emergence of seedlings of S. densiflora and S. verticillata by delaying and even preventing germination from occurring. Trezzi et al. (Reference Trezzi, Vidal, Mattei, Silva, Carnieleto, Gustmann and Machado2006) evaluated the effects of different levels of sorghum, corn and oat straw on the control of Euphorbia heterophylla and also observed that the presence of straw on the soil surface reduced the emergence speed of this weed.
Another possible effect of the straw of cover plants is its allelopathic potential. The release of chemical substances tends to be slow when the straw is placed on the soil, in contrast to what occurs when it is incorporated into the soil, and the intensity of the allelochemical effect depends on its concentration (Pereira et al., Reference Pereira, Teixeira, Souza, Silva and Martins2011).
Cover crop extractions significantly inhibited the germination of both weeds. The different concentrations of extract had little impact on weed suppression, however there was a trend for greater suppression at higher extract concentration. Hoffmann et al. (Reference Hoffmann, Das Neves, Bastos and Da Luz Wallau2007) found similar results when evaluating the GSI of seeds of Lactuca sativa and Bidens pilosa in different concentrations of aqueous extracts of Nerium oleander. The authors observed that the increased concentration of extracts promoted lower GSI values.
Vargas et al. (Reference Vargas, Passos and Karam2018) evaluated the effect of aqueous extracts from the aerial parts of 13 cover crops, including sunn hemp, millet, sorghum and pigeonpea, on the germination of seeds of S. verticillata and observed that sunn hemp was among the cover crops to provide higher suppressive effect on the germination of this weed.
The percentage of seed germination, of both species was numerically higher in the experiment with PEG-6000 between potentials of −0.2 and −0.4 MPa than the percentage of germination obtained under different aqueous extracts from the aerial parts of cover crops with similar osmotic potential. Therefore, the reduction in the percentage and germination speed is due to a synergistic effect between the reduction of the osmotic potential and the presence of secondary metabolites with allelopathic activity.
Fonseca and Perez (Reference Fonseca and Perez2003) obtained similar results when evaluating the germinative behavior of seeds of Adenanthera pavonina subjected to PEG-6000 solutions. The authors observed an absence of germination at osmotic potentials below −0.4 MPa. Gatti et al. (Reference Gatti, Perez and Lima2004) analyzed the effects of aqueous leaf extracts of Aristolochia esperanzae at concentrations of 25, 50, 75 and 100% on the germination of L. sativa L. and Raphanus sativus L. and observed the germination of these species only under solutions with osmotic potential higher than −0.4 MPa.
The reduction in the percentage of germination and lower GSI values of weeds at a 100% concentration may be due to the effect of the water potential of these solutions and not to the allelopathic activity of the aqueous extracts from the aerial parts of cover plants.
The data shown in Tables 2, 3 and 4 for S. densiflora allow observing that an increase in the amount of straw led to a reduction in the percentage of emergence, which can be mainly attributed to a physical effect relative to the sunn hemp and a joint effect between the physical, allelopathic and water restriction effect when using straw of millet, sorghum, hyacinth bean and pigeonpea. In this case, the water and allelopathic potential effects of pigeonpea were more expressive. The use of sunn hemp straw for S. verticillata may have contributed to the suppression of seedlings mainly due to an allelopathic effect. The reduction in seedling emergence from the use of sorghum, hyacinthbean and pigeonpea can be mainly attributed to the effect of reducing the water and allelopathic potentials, with sorghum showing a more evident allelopathic effect in the control of this weed.
Conclusions
S. densiflora is photoblastic positive because there was no germination in the darkness. On the other hand, the germination of S. verticillata showed that this species is photoblastic neutral. The highest percentage of germination and GSI for both species were obtained under alternating temperature. The seedling emergence of both species was maximum when placed on the soil surface. However, seedling emergence presented a significant decrease from a depth of 0.5 cm, while the emergence speed rate was zero at a depth of 10 cm for both species. The presence of straw contributed to reducing the percentage and speed of the emergence of seedlings of S. densiflora and S. verticillata, while the use of twice its recommended amount had no difference from the treatment with the recommended amount for all cover crops. Moreover, the increase in the concentration of aqueous extracts from cover crops was accompanied by lower percentages of germination and GSI for S. densiflora and S. verticillata.
The increased incidence of S. densiflora and S. verticillata in the northeast of Brazil may be associated with little knowledge about the weed biology, linked to the choice of use only a chemical control and the inadequate management of the seed bank. In this context, in a conventional cultivation system, these weeds can become difficult to control. Measures such as soil preparation, providing seed burial at depths greater than 0.5 cm followed by cover crops can provide growers with an effective method for managing these weeds. However, more studies are needed to understand the physiological and environmental behavior of S. densiflora and S. verticillata, since the results presented in this work can be combined with different types of controls, thus expanding the possibilities of integrated and more sustainable management of S. densiflora and S. verticillata.
Acknowledgement
We thank CAPES for the financial support through the research productivity grant for the first author.
Conflict of interest
No potential conflict of interest has been declared.










