Introduction
Aphids are phloem-feeding insects that are widely-distributed pests of agricultural ecosystems. In temperate regions, approximately one in four plant species is colonized by at least one species of aphid (Dixon, Reference Dixon1998). A plant can be a host to many aphid species, and aphid species will often colonize a range of plant species (Blackman & Eastop, Reference Blackman and Eastop1984). Aphids feed specifically from the sieve element and cause damage by the direct ingestion of plant nutrients and through aphid-transmitted viruses (Ng & Perry, Reference Ng and Perry2004). Aphids cause substantial losses to crops worldwide and, as a consequence, are routinely the target of insecticide applications, leading to increased costs for producers and possible negative environmental impacts (Oerke & Dehne, Reference Oerke and Dehne2004). Furthermore, widespread insecticide use has seen the selection of insecticide resistance in many aphid species, which commonly leads to a reduction in management options for producers. There is, therefore, considerable scope for the application of genetic resistance to aphids in agriculture.
Genetic resistance to aphids in plants is rarely complete, instead reducing aphid preference for the plant (antixenosis) or aphid growth and development (antibiosis), or increasing the ability of the plant to grow and set seed despite aphid infestation (tolerance), relative to other more susceptible genotypes (Klingauf, Reference Klingauf, Minks and Harrewijn1987). Most aphid resistance genes (R genes) identified to date are restricted in their effectiveness to single aphid species, or even to particular biotypes. For example, while tomato plants containing the Mi-1.2 gene have resistance to root-knot nematode (Vos et al., Reference Vos, Simons, Jesse, Wijbrandi, Heinen, Hogers, Frijters, Groenendijk, Diergaarde, Reijans, Fierens-Onstenk, de Both, Peleman, Peleman, Liharska, Hontelez and Zabeau1998), whitefly (Nombela et al., Reference Nombela, Williamson and Muniz2003) and aphids (Rossi et al., Reference Rossi, Goggin, Milligan, Kaloshian, Ullman and Williamson1998), the resistance to aphids is limited to specific biotypes of potato aphid Macrosiphum euphorbiae (Thomas) (Goggin et al., Reference Goggin, Williamson and Ullman2001). Similarly, biotype-specific resistance is also found in apple against rosy leaf-curling aphid Dysaphis devecta Walker (Roche et al., Reference Roche, van Arkel and van Heusden1997). Other forms of aphid resistance in plants also appear to be restricted to specific aphid species such as melon against cotton–melon aphid Aphis gossypii Glover (Klingler et al., Reference Klingler, Powell, Thompson and Isaacs1998), and wheat or barley against Russian wheat aphid Diuraphis noxia (Mordvilko), greenbug Schizaphis graminum (Rondani), or bird cherry–oat aphid Rhopalosiphum padi (Linnaeus) (Du Toit, Reference Du Toit1987; Webster et al., Reference Webster, Baker and Porter1991; Porter et al., Reference Porter, Burd, Shufran and Webster2000; Weng et al., Reference Weng, Lazar, Michels and Rudd2004).
Individual resistance genes in plants can regulate a number of physiological and biochemical defence responses; any specificity is usually attributed to the presence of a specific elicitor from an invading pathogen or pest (Martin et al., Reference Martin, Bogdanove and Sessa2003; Kaloshian & Walling, Reference Kaloshian and Walling2005). What is responsible for the apparent specificity of aphid resistance genes? Is it possible that a single resistance gene could be effective against a number of aphid species? A better understanding of the mechanisms underlying plant resistance to aphids is required before we can answer these questions. However, our understanding of the fundamental basis, and in particular the molecular basis of aphid defence has lagged behind that of plant defence against pathogens and chewing insects (Kessler & Baldwin, Reference Kessler and Baldwin2002; Ferry et al., Reference Ferry, Edwards, Gatehouse and Gatehouse2004; Edwards & Singh, Reference Edwards and Singh2006). This is partially due to lack of a model plant for study. Research with arabidopsis has played a pivotal role in our understanding of R gene-mediated resistance to a wide range of pathogens (Holt et al., Reference Holt, Hubert and Dangl2003; Thatcher et al., Reference Thatcher, Anderson and Singh2005), but naturally-occurring and simply-inherited aphid resistance has not yet been identified in this model plant species (Cabrera y Poch et al., Reference Cabrera y Poch, Ponz and Fereres1998).
Medicago truncatula Gaertn. (barrel medic) is rapidly becoming a valuable legume model system for plant functional genomics (Cook, Reference Cook1999; Oldroyd & Geurts, Reference Oldroyd and Geurts2001; May & Dixon, Reference May and Dixon2004). It is also an important pasture species in Australia (Loi et al., Reference Loi, Nutt, McRobb and Ewing2000), where resistance to aphids has been a primary breeding goal (Nair et al., Reference Nair, Craig, Auricht, Edwards, Robinson, , and Jones2003). Effective resistance against the pasture legume specialists Acyrthosiphon kondoi Shinji (bluegreen aphid) and Therioaphis trifolii f. maculata (Buckton) (spotted alfalfa aphid) have been identified and introgressed successfully into commercial cultivars of M. truncatula (Lake, Reference Lake1993). Medicago truncatula can also be attacked by several other aphid species including T. trifolii (Monell) (spotted clover aphid), Aphis craccivora Koch (cowpea aphid), and Myzus persicae Sulzer (green peach aphid) (Blackman & Eastop, Reference Blackman and Eastop1984). This plant should therefore be an excellent model in which to investigate not only the mechanisms underlying aphid resistance, but also their specificity.
In a previous report we undertook a detailed characterization of the interactions between bluegreen aphid and one pair of closely-related, resistant and susceptible lines of M. truncatula, A17 and Jester (Klingler et al., Reference Klingler, Creasy, Gao, Nair, Calix, Jacob, Edwards and Singh2005). Here, this study is extended to characterize resistance against a second aphid species targeted by the breeding programme in Australia, spotted alfalfa aphid. Most information available on resistance to spotted alfalfa aphid in M. truncatula has resulted from studies focusing entirely on the plant (Ridland & Berg, Reference Ridland and Berg1981; Berlandier et al. Reference Berlandier, Edwards, Nichols, Blake and Matthiessen1999) or on the insect (Lake, Reference Lake1989; Milne, Reference Milne1998). The aim here was to achieve a more comprehensive characterization of spotted alfalfa aphid resistance by looking at both plant and aphid performance.
We also extend the previous study by Klingler et al. (Reference Klingler, Creasy, Gao, Nair, Calix, Jacob, Edwards and Singh2005) by examining resistance in six M. truncatula cultivars. These cultivars represent three pairs of closely-related lines each generated by an initial cross of a parent cultivar to an aphid-resistant accession, followed by two to four generations of recurrent backcrossing with selection for resistance to both bluegreen aphid and spotted alfalfa aphid (Crawford et al., Reference Crawford, Lake and Boyce1989). The aim here was to determine how the phenotypes of aphid resistance in these cultivars varied among the different genetic backgrounds.
The final aim was to determine whether any of the resistance identified was also effective against non-target aphid species. In particular, we were interested in the effectiveness of spotted alfalfa aphid resistance against the closely-related spotted clover aphid. These two aphids are genetically distinct forms of the same species (T. trifolii) (Sunnucks et al., Reference Sunnucks, Driver, Brown, Carver, Hales and Milne1997), which can be distinguished by their ability to survive successfully on red clover (Trifolium pratense L.) and alfalfa-lucerne (M. sativa L.) (Manglitz & Russell, Reference Manglitz and Russell1974).
In pursuing these aims, two different phenotypes of resistance to spotted alfalfa aphid have been characterized, of which one is particularly effective – causing mortality within 24 h. We have also shown that each resistance phenotype has a similar effect on spotted clover aphid, but with notable differences. These results provide an opportunity for further elucidation of the genetic and molecular bases of resistance to various aphid species in this model plant, which is not as easily achieved in other plant–aphid systems under study.
Materials and methods
Plants
Included in this study were three pairs of closely-related lines of M. truncatula (cvs A17 (a Jemalong-derivative)–Jester, Cyprus–Caliph, Borung–Mogul) with varying levels of reported susceptibility (S) or resistance (R) to bluegreen aphid and/or spotted alfalfa aphid. Jemalong is reported to have low resistance to spotted alfalfa aphid, but to be susceptible to bluegreen aphid. Jester was selected from the progeny of two recurrent backcrosses to Jemalong after an initial cross with the aphid-resistant donor line SAD2927 (Hill, Reference Hill2000). The bluegreen aphid resistance in SAD2927 is derived from SA1499, while its spotted alfalfa aphid resistance could be derived from any or all of SA1499, Cyprus or Jemalong itself – all three of which are progenitors of SAD2927 (S. Hughes, personal communication). Based on this pedigree, Jester has ∼91% of its genome derived from Jemalong. A Jemalong derivative, genotype A17, was mostly used in this study. A17 and two other derivatives from Jemalong were monomorphic for 4000 molecular markers (Thoquet et al., Reference Thoquet, Gherardi, Journet, Kereszt, Ane, Prosperi and Huguet2002). Therefore, in this study, A17 and Jemalong were considered equivalent.
Cyprus has been reported to be resistant to spotted alfalfa aphid but not bluegreen aphid (Lake, Reference Lake1993). Caliph was selected from the progeny of two recurrent backcrosses to Cyprus, after an initial cross to a donor parent derived from the cross of SAD 2927 with the spotted alfalfa aphid-resistant line SA10733 (S. Hughes, personal communication). Based on this pedigree, Caliph has ∼89% of its genome derived from Cyprus.
Borung is reported to be highly susceptible to both bluegreen aphid and spotted alfalfa aphid (Lake, Reference Lake1993). Mogul was selected from the progeny of two recurrent backcrosses to Borung after an initial cross to SA10419 as the dual aphid-resistant donor parent (Lake, Reference Lake1993). Mogul therefore has ∼87% of its genome derived from Borung.
Prior to laboratory or glasshouse experiments, seeds were scarified and germinated in the dark on moist filter paper for two days at room temperature.
Aphids
The aphid species tested were the spotted alfalfa aphid T. trifolii f. maculata, the spotted clover aphid T. trifolii, the bluegreen aphid A. kondoi, the cowpea aphid A. craccivora and the green peach aphid M. persicae. Therioaphis trifolii is one of the most damaging aphid pests of alfalfa-lucerne and clover in both the southwestern and eastern USA (Gorz et al., Reference Gorz, Manglitz and Haskins1979; Jimenez et al., Reference Jimenez, Caddel and Berberet1988), and of annual medics and clovers in Australia (Lake, Reference Lake1989; Irwin et al., Reference Irwin, Lloyd and Lowe2001; Nair et al., Reference Nair, Craig, Auricht, Edwards, Robinson, , and Jones2003). Acyrthosiphon kondoi is also an important pest of perennial and annual Medicago spp. in the USA, Australia, and New Zealand (Farrell & Stufkens, Reference Farrell and Stufkens1981; Wellings, Reference Wellings1985; Nair et al., Reference Nair, Craig, Auricht, Edwards, Robinson, , and Jones2003; Zarrabi et al., Reference Zarrabi, Berberet, Payton and Hoard2004). Aphis craccivora is more commonly associated with grain legumes in Australia (Edwards, Reference Edwards2001), but can also cause serious damage to pasture legumes (Gutierrez et al., Reference Gutierrez, Nix, Havenstein and Moore1974; Berlandier et al., Reference Berlandier, Edwards, Nichols, Blake and Matthiessen1999). Myzus persicae is common on non-legume hosts in Australian pastures, and as such is likely to frequently colonize M. truncatula plants. Although M. persicae is unlikely to cause much damage on a non-preferred host like M. truncatula, resistance to this aphid could be important in minimizing the rate of inoculation of plant-infecting viruses.
Aphids of each species were obtained from colonies initiated from single aphid clones collected in South Australia or Western Australia and were reared on alfalfa-lucerne Medicago sativa for bluegreen aphid and spotted alfalfa aphid, arrowleaf clover Trifolium vesiculosum L. for spotted clover aphid, faba bean Vicia faba L. for cowpea aphid, and radish Raphanus sativus L. for green peach aphid with 14 h light (23°C)/10 h dark (20°C) under high pressure sodium lamps and fluorescent light at 280 μE m−2 s−1. Aphids were transferred to experimental plants with a fine paintbrush. Single clones were used for each aphid species to minimize within-treatment variability, but additional clones have been tested and produced similar results in separate experiments with the same plant genotypes (data not shown) – with the exception of spotted clover aphid and cowpea aphid, for which only single clones were available for testing.
Plant damage experiments
The experiments were conducted in glasshouses at SARDI (Adelaide, South Australia) and at CSIRO (Floreat, Western Australia). For each aphid species, six replicate plants of each M. truncatula line were randomly arranged and were grown in an aphid screening chamber. After two weeks, each plant was infested with two apterous adult aphids. The damage of each plant was visually assessed three weeks after infestation and scored on a 1 to 5 scale (1: no visual damage; 2: plants slightly stunted, no leaf discoloration; 3: leaf yellowing; 4: heavily stunted, vein chlorosis (T. trifolii only); 5: a dead plant) (Nair et al., Reference Nair, Craig, Auricht, Edwards, Robinson, , and Jones2003).
Aphid performance
The survival and growth rate of each aphid species were measured after 3 days on individual plants of each M. truncatula line with six replicates for each aphid species–plant line combination. Plants were grown in individual 0.9 l pots in a growth chamber with 16 h light:8 h dark under fluorescent light at 100 to 120 μE m−2 s−1, and a constant temperature of 22°C. Three weeks after sowing, a cohort of eight pre-weighed, early-instar nymphs was placed on each plant. Individual plants and nymphs were caged. Three days after the infestation, the number and weight of surviving aphids on each plant were recorded. The mean relative growth rate (MRGR) of surviving nymphs was calculated as the per diem difference between the logarithms of the initial mean weight of aphids placed on the plant (Worig) and the final, mean weight of living aphids removed from the plant (Wsur) (Leather & Dixon, Reference Leather and Dixon1984; Edwards, Reference Edwards2001):
Whenever there was 100% aphid mortality during this three day experiment, a follow-up experiment was conducted during which survivorship was recorded at day one and day two after infestation.
Statistical analysis
Plant damage, aphid survival and aphid MRGR were analysed independently by one-way ANOVA. Survivorship data were normalized using arcsine-square root transformation. Post hoc means comparisons were conducted using Duncan's new multiple range. All statistical analyses were performed using GenStat 6.2 (Lawes Agricultural Trust, Rothamsted Experimental Station).
Results
Spotted alfalfa aphid
The six M. truncatula cultivars varied dramatically in susceptibility or resistance to spotted alfalfa aphid in terms of both plant tolerance and aphid performance. On reportedly-susceptible plants, damage symptoms developed quickly, appearing as yellowing patches or leaf chlorosis surrounding the aphid infestation sites and/or vein chlorosis on uninfested developing leaves. At three weeks, most Borung plants were dead while A17 and Mogul plants were only showing yellowing on the old leaves. Vein chlorosis on uninfested developing leaves was always observed on Borung, but appeared only occasionally on A17. There was no evidence of plant damage on Jester, Cyprus and Caliph. The damage scores on the six cultivars at three weeks are shown in table 1.
Table 1. Mean damage score (1: no visual damage to 5: dead plant) for three pairs of closely-related Medicago truncatula lines infested with five aphid species.
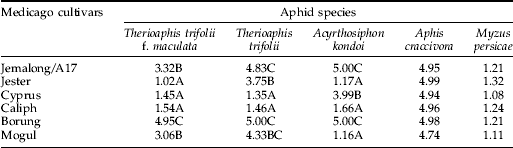
Means within columns followed by the same letter are not significantly different.
The performance of spotted alfalfa aphid over three days reflected the plant damage at three weeks (table 1 and fig. 1). Survivorship was significantly different (P<0.001) among the six cultivars (fig. 1A). While approximately 90% of nymphs survived on Borung and 70% on its closely-related line Mogul, only about 40% of spotted alfalfa aphids survived on A17. On Jester, Cyprus and Caliph, all the aphids were dead within three days. The trend of MRGR for each cultivar was consistent with the survivorship (fig. 1A, B). On Jester, Cyprus and Caliph, less than 20% of nymphs survived day one (fig. 1A).

Fig. 1. Survivorship at day 1, day 2 and day 3 (A) and mean relative growth rate over 3 days (B) of Therioaphis trifolii f. maculata nymphs on six Medicago truncatula cultivars. Values are mean and standard error of six replicates. Means labelled with the same letter(s) are not significantly different (P>0.05). ND: mean relative growth rate not determined, as no aphids survived the 3-day bioassay. For survivorship, only day 3 (


Spotted clover aphid
Spotted clover aphid responded to the six M. truncatula cultivars in a similar fashion to spotted alfalfa aphid, but with notable differences. In the glasshouse experiment, damage symptoms appeared more quickly on A17 and Borung as yellowing patches or leaf chlorosis surrounding the aphid infestation site (table 1). Unlike spotted alfalfa aphid, vein chlorosis was not observed after spotted clover aphid feeding in this experiment. Some vein chlorosis has been observed previously on Borung under similar conditions of spotted clover aphid feeding, but much less pronounced than that caused by spotted alfalfa aphid feeding (J. Klingler, personal communication). Although damage symptoms were also observed on Jester and Mogul, they were much less pronounced than those observed on their closely-related lines of A17 and Borung, respectively (table 1).
As was observed for spotted alfalfa aphid, survivorship and MRGRs of spotted clover aphid differed significantly (P<0.001) among the six cultivars (fig. 2A,B) and most mortality had occurred by day one (fig. 2A).

Fig. 2. Survivorship at day 1, day 2 and day 3 (A) and mean relative growth rate over 3 days (B) of Therioaphis trifolii nymphs on six Medicago truncatula cultivars. Values are mean and standard error of six replicates. Means labelled with the same letter(s) are not significantly different (P>0.05). ND: mean relative growth rate not determined, as no aphids survived the 3-day bioassay. For survivorship, only day 3 (


Bluegreen aphid
For all six cultivars, the plant damage results in the glasshouse were consistent with available field data (Lake, Reference Lake1993; Hill, Reference Hill2000). When the six M. truncatula cultivars were infested with bluegreen aphid, the aphids quickly spread across the whole plant in the case of A17, Cyprus and Borung. For these cultivars, plants rapidly developed damage symptoms, such as yellowing and distortion of developing leaflets. After three weeks, these cultivars showed severe stunting, sometimes resulting in plant death (table 1). In contrast, Jester, Caliph and Mogul showed very little damage over the same period of time following bluegreen aphid infestation.
After three days of infestation, about 90 to 100% of aphids survived without significant differences (P>0.05) among the six cultivars (fig. 3A). However, there was a significant difference (P<0.001) in MRGR with significantly lower growth rates on Jester, Caliph and Mogul than on A17, Cyprus and Borung, respectively (fig. 3B). These overall results on the three pairs of closely-related lines with bluegreen aphid were consistent with the previous results on the A17 and Jester pair with bluegreen aphid infestation (Klingler et al., Reference Klingler, Creasy, Gao, Nair, Calix, Jacob, Edwards and Singh2005).

Fig. 3. Survivorship (A) and mean relative growth rate (B) of Acyrthosiphon kondoi nymphs on six Medicago truncatula cultivars over 3 days. Values are mean and standard error of six replicates. Means labelled with the same letter(s) are not significantly different (P>0.05).
Cowpea aphid and green peach aphid
In contrast to what was found for bluegreen aphid, spotted alfalfa aphid and spotted clover aphid, the six M. truncatula cultivars did not differ significantly in their susceptibility or resistance to cowpea aphid or green peach aphid.
In glasshouse tests, all six cultivars were intolerant of feeding by cowpea aphid. Following infestation, aphids developed rapidly on all plants. Plants showed severe yellowing, wilting and stunting. The degrees of damage were not significantly different (P>0.05) between the six cultivars (table 1). Neither aphid survivorship nor MRGRs were significantly different (P>0.05) among the six lines (fig. 4A,B).

Fig. 4. Survivorship and mean relative growth rate of Aphis craccivora nymphs (A, B) and Myzus persicae nymphs (C, D) on six Medicago truncatula cultivars over 3 days. Values are mean and standard error of six replicates.
In the case of green peach aphid, infestation in the glasshouse experiment did not reveal any evidence of aphid feeding damage to any of the plants (table 1). When aphid performance was measured, only 30 to 60% of nymphs survived. There was a non-significant trend towards higher survivorship and MRGR on A17 and Jester than on the other four cultivars (fig. 4C,D), and there was also a trend towards reduced MRGR on the bluegreen aphid resistant lines Caliph and Mogul (fig. 4D). Biologically-relevant differences in the performance of green peach aphid may have been masked by high variability within lines.
Discussion
Medicago truncatula is becoming a pre-eminent legume model system for plant–microbe interactions and functional genomics. In the present study we characterized in detail three pairs of closely-related lines of M. truncatula for their susceptibility/resistance to five aphid species. In particular, we have identified two clearly different phenotypes of resistance to spotted alfalfa aphid. Both mechanisms also provide a similar, but somewhat less effective resistance against the spotted clover aphid. The resistance phenotype observed in Cyprus, Caliph, and Jester appears to be novel and highly effective; on all three lines, most nymphs died within 24 h, regardless of the developmental stage of the aphids. A second, less effective resistance phenotype was observed in A17 and Mogul, on which spotted alfalfa aphid mortality rates were more moderate (40–80%) and growth rates were suppressed. Similar spotted alfalfa aphid nymphal mortality rates to those we observed on A17 and Mogul have been reported on M. truncatula cultivar Sephi and on M. littoralis line Z-243 which, like Mogul, derive their spotted alfalfa aphid resistance from SA10419 (Lake, Reference Lake1989). Reported mortality rates of spotted alfalfa aphid on resistant lucerne-alfalfa are also around 50–80% after 24 h (Ruggle & Gutierrez, Reference Ruggle and Gutierrez1995). This suggests that the high rates of spotted alfalfa aphid and spotted clover aphid mortality on Cyprus, Caliph, and Jester may be quite unusual, and as such should be studied further. Of particular interest is whether the two resistance phenotypes are controlled by independent genes or the same gene in different genetic backgrounds.
The similarity of the spotted alfalfa aphid resistance phenotype between Jester and Cyprus–Caliph may be explained by the fact that Jester does contain some Cyprus genetic material in its pedigree (Hill, Reference Hill2000). Jester is also likely to contain components of the spotted alfalfa aphid resistance observed in its recurrent parent Jemalong (=A17). It is interesting that unlike in Cyprus and Caliph, spotted alfalfa aphid resistance in Jester is not as effective against spotted clover aphid. This may indicate that genetic background can have a strong influence on the specificity of an aphid resistance mechanism. In this case the specificity of resistance to spotted alfalfa aphid in Jester may be derived from A17, as it is the only progenitor of Jester that exhibits a similar differential response to spotted alfalfa aphid and spotted clover aphid.
It is striking that the resistance mechanism(s) in A17 and Jester appears to be affecting two conspecific aphids (spotted alfalfa aphid, spotted clover aphid) in very different ways. In some systems, the specificity of aphid resistance is thought to be mediated by plant recognition of aphid feeding (Goggin et al., Reference Goggin, Williamson and Ullman2001; Klingler et al., Reference Klingler, Creasy, Gao, Nair, Calix, Jacob, Edwards and Singh2005), possibly in response to a factor in the aphid saliva. The interaction between Jester and spotted alfalfa aphid vs. spotted clover aphid may provide an exciting opportunity to investigate the mechanism by which plants respond differentially to the feeding of closely-related aphids. Such research may point to the mechanisms used by spotted alfalfa aphid biotypes to become virulent against particular M. sativa resistance genes (Nielson & Kuehl, Reference Nielson and Kuehl1982).
The present results confirm previous reports that M. truncatula is generally a more suitable host for spotted clover aphid than for spotted alfalfa aphid (Milne, Reference Milne1998). Interestingly, systemic vein chlorosis in this study was only ever observed in response to spotted alfalfa aphid feeding, never in response to spotted clover aphid feeding, suggesting that this symptom may not always be a good indicator of host suitability. Resistance and vein chlorosis symptoms are also not necessarily correlated for Russian wheat aphid, Diuraphis noxia, on wheat (Assad et al., Reference Assad, Behrabi, Pakniyat and Nematollahy2004). Further investigations on the differential performance of spotted alfalfa aphid and spotted clover aphid on resistant and susceptible M. truncatula genotypes could also provide valuable insight into the mechanisms used by these aphids to feed successfully on host plants.
In contrast to the results for spotted alfalfa aphid and spotted clover aphid, there was no suggestion that bluegreen aphid resistance was affected by genetic background. While the MRGRs were significantly lower on the resistant lines than on their susceptible counterpart, the survivorship did not differ significantly between susceptible and resistant plants. This result contrasts to the resistance we have described against spotted alfalfa aphid and spotted clover aphid. Of the three pairs of closely-related lines used in this study, at least two of the resistant lines are thought to share the same resistance gene, AKR, derived from SA1499 (Klingler et al., Reference Klingler, Creasy, Gao, Nair, Calix, Jacob, Edwards and Singh2005). Bluegreen aphid survival and growth, and the tolerance of the plant to bluegreen aphid feeding were similarly affected in the three resistant lines. These results are consistent with the hypothesis that the same gene is mediating resistance in all three resistant lines.
In the present study, both cowpea aphid and green peach aphid showed no difference in performance among the lines, and neither species exhibited growth rates as high as has been reported on optimal hosts (Edwards, Reference Edwards2001). Despite low growth rates, cowpea aphid caused high levels of damage, suggesting that M. truncatula may be particularly intolerant to feeding by this aphid species. Our data on both the plant tolerance and aphid performance confirm that the M. truncatula cultivars are non-preferred host for green peach aphid though the aphids performed better on the A17 and Jester pair. The results for cowpea aphid and green peach aphid indicate that there is some target species-specificity in the resistance mechanisms to bluegreen aphid, spotted alfalfa aphid and spotted clover aphid, which is typical of genetic resistance mechanisms against aphids (Klingler et al., Reference Klingler, Powell, Thompson and Isaacs1998; Porter et al., Reference Porter, Burd, Shufran and Webster2000; Bournoville et al., Reference Bournoville, Carre, Landre, Aupinel, Grimaud and Epardaud2003).
Medicago truncatula has rapidly developed into a valuable model system for plant functional genomics. The value of M. truncatula as a model for studying aphid defence has already been demonstrated in studies with bluegreen aphid (Klingler et al., Reference Klingler, Creasy, Gao, Nair, Calix, Jacob, Edwards and Singh2005). Transcription profiling suggests that the octadecanoid pathway is involved in this resistance (Gao et al., Reference Gao, Anderson, Klingler, Nair, Edwards and Singh2006). The identification of resistance with varying effectiveness against spotted alfalfa aphid and spotted clover aphid in this study indicates that M. truncatula should also be useful for studying the mechanisms of defence against these aphid pests – and more importantly, for comparing and contrasting effective plant defences against different aphid species. A better understanding of aphid resistance mechanisms in M. truncatula should improve our capacity to develop durable resistance to multiple aphid species across legume and non-legume crops.
Acknowledgements
The authors thank Louisa Bell and Steve Robinson for technical support, John Klingler, Paul De Barro and Judith Lichtenzveig for helpful comments on an earlier version of this manuscript, and other members in the Edwards laboratory (CSIRO Entomology), Floreat WA for constructive discussion. LG was supported by a CSIRO postdoctoral fellowship. The Australian Medicago Genetic Resource Centre, SARDI is acknowledged for the supply of germplasm for the study. The aphid–plant interaction work in the authors' research groups is supported in part by the Grains Research and Development Corporation (GRDC) and the Department of Education, Science and Training (DEST) in Australia.


