INTRODUCTION
The bigfin reef squid, Sepioteuthis lessoniana, is a neritic squid widely distributed in the Indo-West Pacific region, occurring from Japan to Australia (Jereb & Roper, Reference Jereb and Roper2010). This species is of high commercial value and is harvested with a variety of fishing gear, e.g. lured-hooks, purse seines, jigs, etc., throughout its distributional range. This species is the squid with the highest unit price (~US$15 kg−1) among other squid in Taiwan (Anonymous, 2015). This squid is usually targeted by artisanal fisheries (by hand jigging) and seasonal recreational angling, as well as being a by-catch of neritic trawl fisheries. This species was suggested to be a suitable model animal for studying influences of environmental changes on life-history traits of cephalopods due to its widespread distribution in neritic waters and its ability to be successfully maintained in aquaria (Forsythe et al., Reference Forsythe, Walsh, Turk and Lee2001; Pecl, Reference Pecl2001; Jackson & Moltschaniwskyj, Reference Jackson and Moltschaniwskyj2002; Ikeda & Kobayashi, Reference Ikeda and Kobayashi2010).
Cryptic diversity and the population structure of S. lessoniana throughout its distributional range have been studied, while comprehensive conclusions have not yet been drawn. Based on body colour, egg cases and distribution, three different forms (Red, White and Quacking) of S. lessoniana were proposed in waters of Japan (Segawa et al., Reference Segawa, Hirayana, Okutani, Okutani, O'Dor and Kubodera1993; Izuka et al., Reference Izuka, Segawa, Okutani and Numachi1994, Reference Izuka, Segawa and Okutani1996a, Reference Izuka, Segawa and Okutanib). Low genetic diversity of populations in Japanese waters, but significant separation from those in the East and South China Seas, was suggested (Aoki et al., Reference Aoki, Imai, Naruse and Ikeda2008). In the southern hemisphere, a species complex was suggested in Australian waters based on a genetic analysis (Triantafillos & Adams, Reference Triantafillos and Adams2005). Recently, three cryptic lineages were suggested in tropical waters of the Indo-Pacific region based on a genetic analysis (Cheng et al., Reference Cheng, Anderson, Bergman, Mahardika, Muchlisin, Dang, Calumpong, Mohamed, Sasikumar, Venkatesan and Barber2014). However, studies of S. lessoniana populations around Taiwan are scarce and focused on life-history traits off northern Taiwan (Chen et al., Reference Chen, Chen and Lin2015). It is critical to explore the population structure of this high-value species in the western North Pacific region in the conservation and management context.
Statoliths function as a balance organ in cephalopods and play a similar role as otoliths in teleost fish (Clarke, Reference Clarke1978; Arkhipkin & Bizikov, Reference Arkhipkin and Bizikov2000). Statoliths accumulate due to biomineralization of calcium carbonate and organic matter (Bettencourt & Guerra, Reference Bettencourt and Guerra2000). The frequency of statolith growth increment formation was examined in many species of squid (Clarke, Reference Clarke1978; Jackson, Reference Jackson2004; Arkhipkin, Reference Arkhipkin2005), and most of them were shown to be daily, including in S. lessoniana (Jackson, Reference Jackson1990). The number of statolith increments can be considered to represent the age in days of squids and can further be used for growth rate calculations (Jackson, Reference Jackson1990). Trace elements in the ambient environment are also incorporated into statoliths during the biomineralization process (Campana, Reference Campana1999; Arkhipkin et al., Reference Arkhipkin, Campana, FitzGerald and Thorrold2004). Therefore, with their sequential accumulation and slow metabolism, statoliths are considered as an ideal natural tag recording environment signatures in cephalopods, like otoliths in fishes, which can be used to better understand the population structure and life history (Campana, Reference Campana1999; Thorrold et al., Reference Thorrold, Jones, Hellberg, Burton, Swearer, Neigel, Morgan and Warner2002; Arkhipkin et al., Reference Arkhipkin, Campana, FitzGerald and Thorrold2004; Gillanders, Reference Gillanders2005). Trace elements in statoliths of squid have been examined to investigate spatial and temporal population structures, e.g. Doryteuthis gahi in the South-west Atlantic and coastal Peru (Arkhipkin et al., Reference Arkhipkin, Campana, FitzGerald and Thorrold2004); Loligo forbesi in the North Sea and Irish Sea (Wang et al., Reference Wang, Geffen and Nash2012); and Dosidicus gigas of the high seas of the South-east Pacific (off Chile, Peru and Costa Rica; Liu et al., Reference Liu, Chen, Chen, Lu and Qian2011), and in the North-east Pacific (off Washington, California and the Galapagos Islands; Arbuckle & Wormuth, Reference Arbuckle and Wormuth2014). In addition, elemental signatures of statoliths of squid were examined to track natal origins of squid, e.g. Doryteuthis opalescens off California (Warner et al., Reference Warner, Hamilton, Sheehy, Zeidberg, Brady and Caselle2009) and Sepioteuthis australis from south-eastern Tasmania, Australia (Pecl et al., Reference Pecl, Tracey, Danyushevsky, Wotherspoon and Moltschaniwskyj2011). Thus, squid statoliths have been suggested as a suitable tool to understand squid life histories and reconstruct the environmental histories experienced (Arkhipkin, Reference Arkhipkin2005; Arkhipkin & Shcherbich, Reference Arkhipkin and Shcherbich2012).
Sepioteuthis lessoniana is a fisheries resource with high commercial value, although its population structure and stock abundance status are unclear. The population structure and dynamics are essential information for stock assessment and sustainable fisheries. The objective of this study is to investigate the population structure of S. lessoniana around northern Taiwan using life-history traits and elemental signatures of statoliths. The results can help to fill gaps in our understanding of the population structure of S. lessoniana in the North-west Pacific and can possibly be applied to conservation and management measures in the future.
MATERIALS AND METHODS
Sample collection and measurements
Sepioteuthis lessoniana samples were collected from two locations, (1) Ho-ping Island, Keelung (KL) in northern Taiwan, and (2) Ma-gong, Penghu (PH) in the northern Taiwan Strait, between October 2012 and September 2013 (Figure 1). Squid were harvested by recreational jig fishery off northern Taiwan, while mainly as a bycatch of trawling in the northern Taiwan Strait. At least 30 specimens were collected each month, but the actual sample size depended on the harvest of fishermen. Squid samples were stored in a freezer immediately after collection and transported to the laboratory at National Taiwan Ocean University in Keelung.

Fig. 1. Two sampling locations of Sepioteuthis lessoniana around northern Taiwan. (A) Keelung, (B) Penghu.
The squid were thawed, and basic biological information was measured. The dorsal mantle length (ML) and total body weight (BW) were measured to the nearest unit of the instrument, of 1 mm (callipers) and 1 g (scale), respectively. The sexual maturity stage of the squid was determined according to the maturity scale of Boyle & Ngoile (Reference Boyle and Ngoile1993). Statoliths were dissected from the statocysts and washed with milli-Q water and alcohol. One statolith of each pair was stored in liquid paraffin for ageing studies, and the other was kept for chemical analysis.
Statolith processing and reading
The left statolith (wing facing up and lateral dome on the left side) was selected for age determination. After cleaning in xylene and alcohol for ~2 min, the statolith was mounted in resin with hardener (EpoFix kit). After the resin had fully hardened, the statolith was ground on both sides with wet waterproof sandpaper (of 1000, 2400 and 4000 grits), and then polished with a polishing suspension (0.05 µm, Buehler, Lake Bluff, IL, USA) on a polishing cloth. The prepared statoliths were examined under a compound microscope (400×, DM-2500, Leica, Wetzlar, Germany) and photographed with a digital camera. Each frame of images along the longest axis, from the focus to the tip of the lateral dome (statolith radius, SR), was taken and stitched together using the Leica Application Suite. Growth increments of the statolith were determined, and the statolith radius was measured using Image J software. For regions where growth increments were unclear, an extrapolation method was applied to estimate the number of increments. The daily deposition of growth increments in statoliths was previously validated for S. lessoniana (Jackson, Reference Jackson1990). The hatching date was estimated by subtracting the daily age from the date of collection.
Elemental analysis
Concentrations of trace elements in statoliths of S. lessoniana were analysed by solution-based inductively coupled plasma mass spectrometry (ICP-MS, Thermo X-Series 2, Waltham, MA, USA). Squid were divided into four groups, two locations (Keelung and Penghu) with two seasonal cohorts (spring and autumn), and 25 samples were examined in each group. Statolith samples were digested in ultrapure nitric acid (70%), and then diluted with Milli-Q water in factors of 10, 100, 1000 and 10,000, for subsequent analyses. Precision estimates (relative standard deviation) were as follows: Mg/Ca = 0.5%, Mn/Ca = 3.6%, Sr/Ca = 0.3%, Ba/Ca = 0.6% and Pb/Ca = 1.5%. Most of the values for trace elements (except Cd) were within an acceptable range (<10%).
Statistical analysis
A principal component analysis (PCA) was conducted to examine variations in five life-history traits (BW, ML, number of increments, statolith radius and gonadosomatic index (GSI)) and variations in eight elemental concentration ratios (element/Ca ratios) in statoliths between cohorts and locations for the squid. Scores of the first two PCs of each group were used to analyse the similarity in life-history traits and elemental signatures in statoliths between groups using a cluster analysis with Euclidean distances and an unweighted pair-group average method. All statistical analyses were performed with SPSS Statistics version 20.0 (SPSS, Chicago, IL, USA).
RESULTS
Life-history traits
In total, 574 squid (286 females and 288 males) from KL and 375 squid (169 females and 206 males) from PH were collected between October 2012 and September 2013 (Table 1). The ML ranged 30–399 mm and BW ranged 4–2565 g for KL samples and 82–302 mm and 59–1155 g, respectively, for PH samples. For squid in KL, the smallest individual occurred in June 2013 (30 mm, 4 g), while the largest one occurred in March 2013 (399 mm, 2565 g), both were males. For squid in PH, the smallest individual occurred in October 2012 (a female of 82 mm, 59 g), while the largest one occurred in January 2013 (a male of 302 mm, 1155 g). The MLs and BWs of squid significantly differed between these two locations (N = 949, F = 80.122, P < 0.001).
Table 1. Summary information for Sepioteuthis lessoniana samples around northern Taiwan between October 2012 and September 2013.
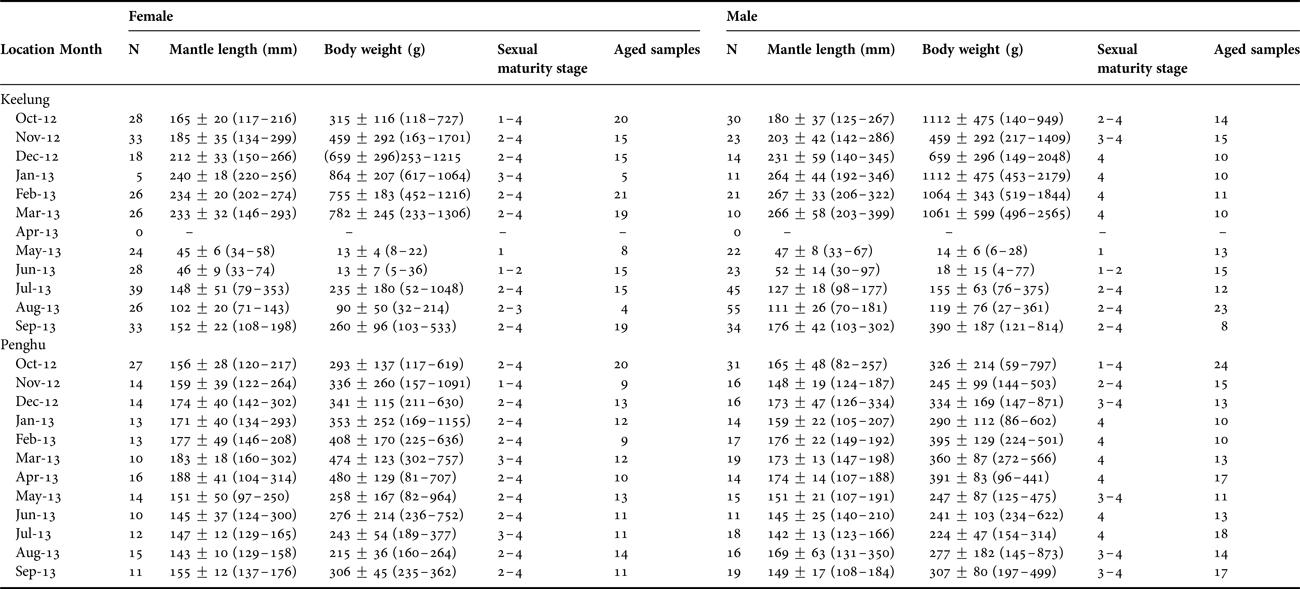
N: total sample size; Mean ± standard deviation and range in the parentheses for mantle length and body weight.
Monthly ML compositions of squid in KL showed an apparent growth pattern, i.e. the ML increased from May to February of the following year. However, monthly ML compositions of squid in PH showed less variability during the study period, although slight increases were noted between December and March and between June and September (Figure 2, Table 1). ML-BW relationships of squid were described using power equations, and a significant difference was found between the two locations for each sex (Figure 3; ANCOVA, female: F 1, 463 = 23.303, P < 0.05; male: F 1, 495 = 38.494, P < 0.05).

Fig. 2. Monthly composition in mantle length of Sepioteuthis lessoniana around northern Taiwan. (A) Keelung, (B) Penghu.

Fig. 3. Scatterplot of mantle length and body weight for Sepioteuthis lessoniana around northern Taiwan. (A) female, (B) male.
Growth increments of 298 squid (153 females and 145 males) in KL and 316 squid (141 females and 175 males) in PH were successfully determined using the statolith microstructure (Table 1). Age ranges of squid in KL (52–183 days for females and 63–175 days for males) were wider than those of squid in PH (95–169 days for females and 77–186 days for males; Figure 4). Much-older squid were noted in PH than in KL, particularly for those aged older than 150 days.

Fig. 4. Frequency distribution of age composition for (A) female and (B) male Sepioteuthis lessoniana around northern Taiwan.
Hatching dates of squid were estimated by subtracting the daily age from the date of collection. Squid in KL hatched almost year-round, except in December and January, with peaks in March to May and July to October. Squid in PH hatched year-round with peaks in March to April and July to October (Figure 5). Based on the hatching date distribution, two seasonal cohorts of squid were identified in each location, i.e. a spring cohort (SC, hatching in March to May) and an autumn cohort (AC, hatching in July to October).

Fig. 5. Frequency distribution of back-calculated hatching dates of Sepioteuthis lessoniana around northern Taiwan. (A) Keelung, (B) Penghu.
The spawning season was between July and the following March for squid in KL, while it spanned over the entire year for those in PH (Figure 6). For each sexual maturity stage, females were slightly older than males (Figure 7). The SC in KL matured (sexual maturity stage 4) at around 115 days, while the AC in KL matured at around 125 days. The SC in PH matured at around 130 days, while the AC in PH matured at around 125 days. However, fewer samples of juvenile stages, particularly for the SC in PH, were noted.

Fig. 6. Percentage of female and male spawners by month from the total spawners in Keelung (KL) and Penghu (PH).

Fig. 7. Average number of increments (age in day, black circles or grey squares) and standard deviation (lines) by maturity stage in females and males for Septioteuthis lessoniana around northern Taiwan. Sample sizes of less than 3 were disregarded.
Elemental signatures
In total, 187 statolith subsamples (at sexual maturity stages 3 and 4) were used for elemental analyses. Elemental concentrations of statoliths were expressed as ratios to Ca which was the most abundant element in statoliths. Sr was the second most abundant trace element, with ratios to Ca of 0.8–11.7 mmol mol−1. Concentrations of other elements were in the order of K, Fe, Zn, Cu, Ba and Pb.
Concentration ratios of six elements (Mg, Fe, Cu, Zn, Sr and Ba) significantly differed between the two locations. Squid in PH possessed higher elemental concentrations than those in KL, except for Zn (Table 2).
Table 2. Concentration ratio of elements to calcium (mean ± 2 SE) within statoliths for Sepioteuthis lessoniana from two locations and two cohorts around northern Taiwan.

SC, spring cohort; AC, autumn cohort.
For squid in KL, Mg and Pb concentrations of the SC were higher than those of the AC, while Fe, Cu, Zn, Sr and Ba concentrations of the AC were higher than those of the SC. For squid in PH, K, Fe and Zn concentrations of the SC were higher than those of the AC, while Cu, Sr and Ba concentrations of the AC were higher than those of the SC (Table 2).
Spatiotemporal variations in life-history traits and elemental signatures
Spatiotemporal variations in life-history traits (BW, ML number of increments (NI), statolith radius (SR), and GSI) were analysed using a PCA. The first two factors, PC1 and PC2, respectively explained 64.4% and 18.4% of the total variance (Table 3). PC1 was positively correlated with four variables (BW, ML, NI and SR), while PC2 was positively correlated with the GSI. Four squid groups (two location and two cohorts) were identified in the PC1 and PC2 bi-plot. The AC in KL was in the positive direction along PC1, while the SC in KL was in the opposite direction of PC1. The SC and AC in PH almost overlapped along PC1, while they could be divided along PC2 (Figure 8A).

Fig. 8. Principal component analysis plot of (A) five life-history traits, and (B) eight elemental concentration ratios of Sepioteuthis lessoniana around northern Taiwan. (KL_SC, spring cohort in Keelung; KL_AC, autumn cohort in Keelung; PH_SC, spring cohort in Penghu; PH_AC, autumn cohort in Penghu.)
Table 3. Principal component analysis results of five life-history traits for Sepioteuthis lessoniana populations around northern Taiwan (N = 280).

BW, body weight; ML, mantle length; SR, statolith radius; NI, number of increments; GSI, gonadosomatic index.
The first two factors, PC1 and PC2, respectively explained 38.3% and 17.9% of the total variance in elemental concentrations (Table 4). PC1 was positively correlated with all elemental concentrations, particularly Sr and Ba. PC2 was positively correlated with four elemental concentrations, particularly Mg and K. The four squid groups could be identified along PC1 in the bi-plot. The ACs in KL and PH were in the positive direction of PC1, while the SCs in KL and PH were in the negative direction of PC1 (Figure 8B).
Table 4. Principal component analysis results of eight elemental concentration ratios within statoliths for Sepioteuthis lessoniana populations around northern Taiwan (N = 187).

Dendrograms from the cluster analysis illustrated the similarity in life-history traits and elemental signatures of squid between the two cohorts in the two locations (Figure 9). Distinct similarity patterns were found in life-history traits and elemental signatures of the squid groups. Variations in life-history traits of squid between locations were greater than those of squid between seasonal cohorts (Figure 9A). In contrast, variations in elemental signatures of squid between seasonal cohorts were greater than those of squid between the two locations (Figure 9B).

Fig. 9. Clustering analysis by (A) life-history traits, and (B) elemental concentration ratios of Sepioteuthis lessoniana around northern Taiwan. (KL_SC, spring cohort in Keelung; KL_AC, autumn cohort in Keelung; PH_SC, spring cohort in Penghu; PH_AC, autumn cohort in Penghu.)
DISCUSSION
This study used life-history traits and statolith elemental signatures to explore the population structure of Sepioteuthis lessoniana around northern Taiwan. The results showed greater variations in life-history traits between populations in the two locations, with greater variations in the statolith element signatures between the two seasonal cohorts. These approaches can uncover the latent population structure of squid in a geographic and/or temporal local scale, which is difficult to detect by genetic approaches (Aoki et al., Reference Aoki, Imai, Naruse and Ikeda2008; Cheng et al., Reference Cheng, Anderson, Bergman, Mahardika, Muchlisin, Dang, Calumpong, Mohamed, Sasikumar, Venkatesan and Barber2014). Such a population structure might be critical in the context of fisheries management, particularly for commercially important species.
Variations in life-history traits
Different life-history traits for the two S. lessoniana populations (KL and PH) were found in this study. In addition, a significant difference between seasonal cohorts at KL was noted. Life-history traits of squid may respond to oceanographic conditions, particularly seawater temperature, in the two locations (Pecl, Reference Pecl2001; Jackson & Moltschaniwskyj, Reference Jackson and Moltschaniwskyj2002; Moreno et al., Reference Moreno, Azevedo, Pereira and Pierce2007).
Waters around northern Taiwan are dominated by several ocean currents and are modified by seasonal monsoons and the regional bottom topography (Tang et al., Reference Tang, Tai and Yang2000; Jan et al., Reference Jan, Wang, Chern and Chao2002, Reference Jan, Sheu and Kuo2006). The oceanographic conditions off northern Taiwan are significantly influenced by the seasonal migration of the Kuroshio Current (Tang et al., Reference Tang, Tai and Yang2000). In contrast, the oceanographic conditions of the Taiwan Strait are strongly influenced by the monsoon systems (Jan et al., Reference Jan, Wang, Chern and Chao2002, Reference Jan, Sheu and Kuo2006). Waters off Keelung are influenced by both the China Coastal Current and Kuroshio Current in summer and Kuroshio Current alone in winter, while Penghu waters are surrounded by the South China Sea Warm Current in summer but by the China Coastal Current and Kuroshio Branch Current in winter. The distinct life-history traits of S. lessoniana between the two locations also reflect the seasonal variability of oceanographic conditions, particularly physical parameters such as seawater temperature, which is impacted by the specific dominant current.
Squid growth patterns are influenced by ambient water temperatures (Forsythe, Reference Forsythe, Okutani, O'Dor and Kubodera1993, Reference Forsythe2004), as well as food availability (Jackson & Moltschaniwskyj, Reference Jackson and Moltschaniwskyj2001). Temperatures fluctuated seasonally both in KL and PH waters, while KL has a wider range and an even lower temperature in winter (17.5– 30.2 °C) off northern Taiwan, resulting in more significant differences in life-history traits between seasonal cohorts than PH (ranging between 21.0–29.4 °C). However, fewer small-sized individuals in PH samples might be a result of gear selectivity, trawling in PH and recreational jigging in KL, or a specific life-cycle stage for squid in PH. Further studies are necessary in the future to clarify these possibilities.
Variations in elemental signatures
Concentrations of statolith trace elements of squid between locations were less variable than those between seasonal cohorts. Concentrations of statolith trace elements are supposedly influenced by the marine environment, particularly chemical parameters (Yatsu et al., Reference Yatsu, Mochioka, Morishita and Toh1998). These results imply that chemical features of the marine environments are similar in the two locations in the same season. Different water masses carry different trace elements concentrations, especially during seasonal transition when the distribution ranges of water masses were strongly influenced by monsoons. Similar oceanographic conditions (chemical parameters) in KL and PH waters could be expected during peak hatching months (season) for squid: the Kuroshio Current and China Coastal Current in KL waters, and the China Coastal Current and Kuroshio Branch Current in PH waters during spring; and the China Coastal Current and Kuroshio Current in KL waters, and the China Coastal Current and South China Sea Warm Current in PH waters during autumn. However, significant seasonal differences in concentrations of trace elements in statoliths were found for squid in the two locations. This suggests significant seasonal variations in marine environments (chemical parameters) in both locations which are impacted by dominant currents and the seasonal monsoon system: from the Kuroshio Current in winter to the China Coastal Current in summer in KL waters, and from the Kuroshio Branch Current in winter to the South China Sean Warm Current in summer in PH waters.
Statolith trace elements can provide substantial information to reconstruct a cephalopod's environmental history (Ikeda et al., Reference Ikeda, Arai, Kidokoro and Sakamoto2003). Elemental signatures such as Sr/Ca and Ba/Ca in statoliths are considered thermometers for recording ambient water temperatures and productivity of environments experienced by the organisms respectively (Arkhipkin et al., Reference Arkhipkin, Campana, FitzGerald and Thorrold2004; Zumholz et al., Reference Zumholz, Klügel, Hansteen and Piatkowski2007b). In general, the Sr/Ca ratio of a squid statolith is negatively correlated with the ambient water temperature (Ikeda et al., Reference Ikeda, Arai, Sakamoto, Kidokoro and Yoshida1996; Arkhipkin et al., Reference Arkhipkin, Campana, FitzGerald and Thorrold2004), although effects on salinity and food intake were also found (Ikeda et al., Reference Ikeda, Yatsu, Arai and Sakamoto2002; Zumholz et al., Reference Zumholz, Hansteen, Klügel and Piatkowski2006, Reference Zumholz, Hansteen, Piatkowski and Croot2007a). Other trace elemental constituents in cephalopod statoliths are not well understood, but their relation to ambient water chemistry is believed to be similar as studies in fish otoliths. Our data suggested that the concentration ratios of five elements (Mg, Fe, Cu, Sr and Ba) were significantly higher for squid in PH than those for squid in KL (Table 2). The Sr/Ca and Ba/Ca concentrations could be verified by the temperature which occurs in cold seasons, with slight differences in minimum temperatures between locations. The different concentrations of Mg/Ca Fe/Ca and Cu/Ca might due to the different water masses that squid experienced. Statoliths collected from the two locations showed different concentrations in these elements demonstrating abiotic difference in squid habitats (Table 2). In addition, concentrations of Fe, Cu and Sr are substantial elements illustrating variability of squid between the two locations, while those of Sr and Ba are substantial elements illustrating the variability of squid between seasonal cohorts (Table 2). A higher Sr/Ca ratio in PH waters and in the autumn cohort in both locations was noted (Table 2). This may be a plausible result of the whole statolith analysis applied in this study, which represents an overall concentration of trace elements in statoliths throughout the life history of a squid. A more detailed approach such as LA-ICPMS is necessary for detecting statolith trace elements at specific life-history stages, which could characterize specific marine environments of the surrounding waters experienced by the squid.
Sepioteuthis lessoniana populations around North Taiwan
The population structure of S. lessoniana around northern Taiwan was examined by life-history traits and statolith trace element concentrations in this study. Life-history traits of squid between the two locations were more variable than those between seasonal cohorts, while concentrations of trace elements in statolith of squid between the seasonal cohorts were more variable than those between the two locations (Figure 9). This implies seasonal variability in oceanographic conditions experienced by squid between the two locations.
Squid in KL and PH waters may undertake different migration routes, one in the southern East China Sea (off northern Taiwan), and another in the northern Taiwan Strait (as shown by data on life-history traits). Each population experiences seasonal variations in marine environments that are influenced by the dominant ocean currents and the seasonal monsoon (Tang et al., Reference Tang, Tai and Yang2000; Jan et al., Reference Jan, Wang, Chern and Chao2002). However, for the same season, less variation in marine environments was found between the two locations (as shown by statolith elemental signatures). This suggests that the marine environments in the two locations are similar in the same season, or the same squid cohort in the two locations may experience similar marine environments during their life history. However, this study examined the whole statolith of squid which could mask substantial life-history events during migration periods of squid (Liu et al., Reference Liu, Chen, Chen and Tian2013, Reference Liu, Chen and Chen2015).
CONCLUSION
Life-history traits and statolith trace elements of S. lessoniana populations around northern Taiwan were examined in this study. Variations in life-history traits of squid between the two locations were greater than those of squid between seasonal cohorts. Nevertheless, variations in statolith trace elements of squid were greater between seasonal cohorts than those of squid between the two locations. Life-history traits and statolith trace elements of squid may be influenced by hydrographic conditions of the marine environments in the two locations, when responding to variability in physical and chemical parameters. This study provides novel information on the population structure of S. lessoniana around northern Taiwan, which can be impacted by seasonal variations in dominant oceanic currents and monsoon systems. Further studies by genetic analysis and/or trace elements on specific sites within statoliths are needed to improve our understanding of the population structure and possible movement and migration routes of squid around Taiwan.
ACKNOWLEDGEMENTS
We thank the fishermen of Ho-ping Island and Ma-gong fishing port for assistance in collecting squid samples. Constructive comments from two anonymous reviewers are greatly appreciated.
FINANCIAL SUPPORT
This study was partially funded by the Ministry of Science and Technology, Republic of China (Taiwan), through grants NSC 102-2313-B-019-007 and MOST 103-2313-B-019-013 to CSC, and NSC 102-2621-B-019-006-MY3 to CHW.















