Introduction
The fibre of ruminant diets promotes ruminal health via stimulation of rumination and tamponade, as well as it is used as a substrate for ruminal fermentation (Mertens, Reference Mertens, Hall, Harris and Haskins2000). Feed additive strategies have been evaluated to improve fibre digestibility and feed efficiency. Exogenous fibrolytic enzyme increases the dry matter (DM) and neutral detergent fibre (NDF) in vitro degradation (Gandra et al., Reference Gandra, Miranda, Goes, Takiya, Del Valle, Oliveira, Freitas-Junior, Gandra, Araki and Santos2017; Zayed et al., Reference Zayed, Szumacher-Strabel, El-Fattah, Madkour, Gogulski, Strompfová, Cieślak and El-Bordeny2020), fermentative kinetics (Elghandour et al., Reference Elghandour, Kholif, Hernandez, Mariezcurrena, Lopez, Camacho, Marquez and Salem2016) and beef cattle average daily gain (Tirado-González et al., Reference Tirado-González, Miranda-Romero, Ruíz-Flores, Medina-Cuéllar, Ramírez-Valverde and Tirado-Estrada2018). However, the efficiency of exogenous enzyme is highly variable (Meale et al., Reference Meale, Beauchemin, Hristov, Chaves and Mcallister2014; Abid et al., Reference Abid, Jabri, Beckers, Yaich, Malek, Rekhis and Kamoun2019). The beneficial impact of the exogenous fibrolytic enzymes on fibre digestibility depends on several factors, such as the basal diet composition, enzymatic preparation, methods of application and doses (Mendoza et al., Reference Mendoza, Loera-Corral, Plata-Pérez, Hernández-García and Ramírez-Mella2014; Elghandour et al., Reference Elghandour, Kholif, Hernandez, Mariezcurrena, Lopez, Camacho, Marquez and Salem2016), ruminal retention time and pH, which affects the ruminal activity and enzymatic stability (Meale et al., Reference Meale, Beauchemin, Hristov, Chaves and Mcallister2014).
Microbial feed additive, such as the fungus Aspergillus oryzae, may enhance the utilization of fibre through the improvement of microbial activity (Giraldo et al., Reference Giraldo, Tejido, Ranilla and Carro2008; Sun et al., Reference Sun, Wu, Wang, Wang and Liu2017), due to the mechanical action on the fibre, increasing bacterial access and adherence of microorganisms to fibre fraction, increasing the fibre digestibility (Sosa et al., Reference Sosa, Galindo, Tejido, Díaz, Martínez, Saro, Carro, Ranilla, Ranilla, Carro, Ben Salem and Morand-Fehr2011; Sun et al., Reference Sun, Wu, Wang, Liu and Myung2014). The use of A. oryzae increases the feed intake (Latif et al., Reference Latif, Zahran, Ahmed, Zeweil and Sallam2014), average daily gain (Tricarico et al., Reference Tricarico, Abney, Galyean, Rivera, Hanson, Mcleod and Harmon2007) and milk yield (Kim et al., Reference Kim, Ahn, Chung, Moon, Ha, Seo, Ahn and Lee2006; Sun et al., Reference Sun, Wu, Wang, Wang and Liu2017).
Both additives, fibrolytic enzyme and A. oryzae, have the potential to modulate rumen fermentation and increase fibre digestibility. To the authors’ knowledge, there are no studies evaluating the effect of these additives combination on in vitro digestibility and gas production. It was hypothesized that A. oryzae and exogenous fibrolytic enzyme would synergistically improve gas production and in vitro degradation of different forages. The present study was conducted to evaluate the effect of A. oryzae and fibrolytic enzyme levels on maize and sugarcane silages degradation, fermentative profile and in vitro gas production.
Material and methods
All experimental procedures were in agreement with the Guide for Care and Use of Agricultural Animals in Agricultural Research and Teaching (FASS, 1999), with all animal procedures approved by the University of São Paulo Animal Bioethics Committee (protocol number 7551070817). The experiment was carried out at the Ruminal Fermentation Laboratory (LFR), in the Faculty of Animal Science and Food Engineering (FZEA), the University of São Paulo (Pirassununga, SP, Brazil, 21°57′S, 47°27′E, 630 m a.s.1.).
Treatments and experimental design
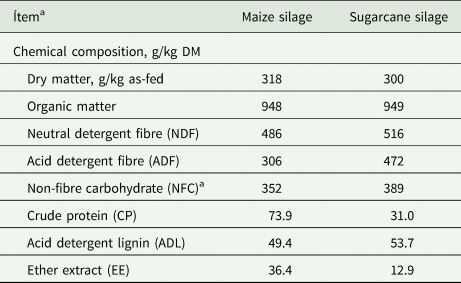
In vitro gas production bioassay was performed as a completely randomized design with three repetitions (inoculum) per treatments. Treatments were obtained from a 4 × 4 × 2 factorial arrangement, in which were evaluated: (1) doses of A. oryzae (A): 0, 20, 60 and 100 mg/l (A0, A1, A2 and A3, respectively); (2) fibrolytic enzyme (E, Fibrozyme Alltech Inc., Nicholasville, KY, USA): 0, 160, 320 and 480 mg/l (E0, E1, E2 and E3, respectively); and (3) roughage: maize and sugarcane silage (Table 1); totalling 32 treatments.
Table 1. Chemical composition of roughages used as substrates in in vitro degradability and gas production bioassay
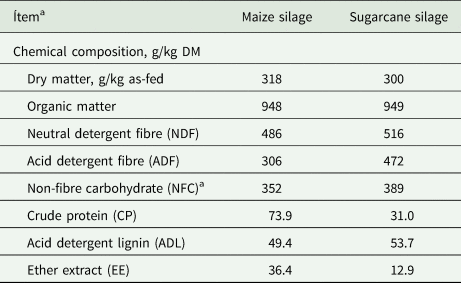
DM, dry matter.
a NFC = 1000 − (NDF + CP + EE + ash) (Balieiro Neto et al., Reference Balieiro Neto, Ferrari Junior, Nogueira, Possenti, Valdinei Tadeu Paulino and Bueno2009).
Aspergillus oryzae (CCT 4359) was cultivated in Petri plates with Sabouraud dextrose agar 4% (Acumidia) and kept in a BOD Incubator (MarqLabor) at 28°C for fungal growth. Then, a morphological analysis was performed by microculture to confirm the cultivated fungus. Large-scale production was carried out at the Department of Food Engineering and Technology of São Paulo State University (UNESP, São José do Rio Preto/SP – Brazil). The fungus was grown in Erlenmeyer (1 litre) containing Sabouraud dextrose agar 4% (Acumidia) and incubated at 28°C to increase the number of viable spores. Subsequently, the vegetative portion along with the spores was collected and incorporated into a nutrient solution containing (NH4)2SO4, MgSO4.7H2O, KH2PO4, FeSO4.7H2O, ZnSO4, e MnSO4. This solution was incorporated into a mixture of sugarcane bagasse and wheat bran (3:1 ratio) and transferred to a bioreactor. The bioreactor was composed of ten jacketed modules, previously autoclaved, made of aluminium. Each module was 20 cm in diameter and 20 cm in length (wide bioreactor), vertically connected. The temperature was monitored at different heights with T-type thermocouples placed between consecutive modules. Water was circulated through the jacket in order to keep the wall temperature constant (Cunha et al., Reference Cunha, Casciatori, Vicente, Garcia and Thoméo2020; Frassatto et al., Reference Frassatto, Casciatori, Thoméo, Gomes, Boscolo and Silva2020).
After 7 days, the bioreactor was opened and a sample was collected for colony-forming unit (CFU) counting, and all fermented content was removed and frozen (−20°C). The culture medium (sugarcane bagasse and wheat bran) overgrown with fungi A. oryzae was preserved by lyophilization. Freeze-drying was performed with a lyophilizer (Semi-Industrial Freeze Dryer LJI010) at an external manifold with a pressure of 0.2 mbar for 20 h (Grzegorczyk et al., Reference Grzegorczyk, Kancelista, Łaba, Piegza and Witkowska2018). The CFU counting was 6 × 108/g of dried A. oryzae.
The doses of the additives used in the present study were defined considering a daily supplementation for cattle with a ruminal capacity of 50 litres. Thus, the doses of A. oryzae were equivalent to 0, 1, 3 and 5 g, and for fibrolytic enzyme were 0, 8, 16 and 24 g.
Animals, substrate and inoculum preparation
Six Holstein cows (Bos taurus taurus) were used as inoculum donors for in vitro assays. Animals were cannulated, kept in free-stall pens with free access to water, receiving a diet with 600 g/kg maize silage and 400 g/kg concentrate for 21 days.
At 6:00 h, before the first feeding, samples of the solid and liquid phases of the ruminal content of each animal were collected separately and individually. The ruminal content was manually filtered with cotton cloth, to obtain solid and liquid fractions. Then, samples of the solid phase were stored in plastic bags, kept in a heated box at 39°C. The liquid phase was stored in pre-warmed thermal bottles, previously flushed with CO2. After sampling, the material was immediately sent to the laboratory. One inoculum was prepared with two donor animals, totalling three repetitions. For each inoculum, equal proportions of liquid and solid were homogenized in a blender for 10 s, previously inflated with CO2, and filtered through three layers of cotton cloth, according to Bueno et al. (Reference Bueno, Cabral Filho, Gobbo, Louvandini, Vitti and Abdalla2005). The inocula were kept in an in vitro incubator at 39°C (TE–150 Tecnal®, Piracicaba, Brazil) and constantly saturated with CO2 until use.
In vitro bioassay and treatments
The fermentative kinetics bioassays were performed according to Theodorou et al. (Reference Theodorou, Williams, Dhanoa, McAllan and France1994) method, adapted by Mauricio et al. (Reference Mauricio, Mould, Dhanoa, Owen, Channa and Theodorou1999) and Bueno et al. (Reference Bueno, Cabral Filho, Gobbo, Louvandini, Vitti and Abdalla2005). Two gas production bioassays were performed simultaneously: a short incubation test (24 h) defined as a methanogenesis bioassay; and another long-term incubation period (96 h) defined as fermentative kinetics bioassay. Blanks were used for each inoculum and all samples were used in triplicate.
For the methanogenesis bioassay, approximately 500 mg of ground sample (1 mm sieve) of roughages were weighed and placed in bags (Ankom, F57), and then placed in fermentation flasks (160 ml). The doses of A and E were added within 100 μl of saline solution per vial, in order to standardize the headspace for gas production in all vials.
After the additives were added to the vials, 25 ml of the ruminal inoculum was diluted with 75 ml of nutrient solution (Menke's buffered medium) as described by Onodera and Henderson (Reference Onodera and Henderson1980), continuously saturated with CO2 and kept at 39°C until use, and immediately transferred to each vial. All vials were sealed with 20 mm butyl rubber septum stoppers (Bellco Glass, Vineland, NY, USA), manually shaken, and kept in a forced-ventilation oven at 39°C.
After incubation for 4, 8, 12, 16 and 24 h, the headspace gas pressure was measured with a pressure transducer and a datalogger (PressDATA 800®, LFR, FZEA-USP, Pirassununga, Brazil), and the values obtained were used to estimate the gas volumes produced, employing the equation defined for the test laboratory conditions: V = p × 6.4278, where V is gas volume (ml) and p is gas pressure (psi) (Santos et al., Reference Santos, Carvalho, Carriero, Magalhães, Batista, Fagundes and Bueno2020). After each pressure reading, a 2 ml sample of the gases produced inside the vials was collected with a syringe to measure the methane concentration. Samples were stored in 10 ml Vacutainer tubes, and cooled until quantitative analyses. After measuring the pressure and collecting the gas samples, the internal pressure of each vial was released at each incubation period with the aid of a syringe, equalizing the vial and atmosphere pressures, in order to not overestimate the gas production of subsequent collection. Then, the vials were shaken for content homogenization before returning to the incubator. At the end of the bioassay (24 h), the vials were opened and bags removed for the determination of in vitro degradation of DM (IVDMD24) and NDF after 24 h of incubation (IVNDF24).
For the fermentative kinetics bioassay, 1 g of ground sample (1 mm sieve) was placed in bags (Ankom, F57), and transferred to fermentation flasks (160 ml), submitted to the same treatments used in the methanogenesis bioassay. Then, 10 ml of the inoculum was diluted in 90 ml of nutrient solution, following the procedures described for the methanogenesis bioassay. The internal gas pressure in the flasks was measured after incubation for 4, 8, 12, 18, 24, 30, 36, 48, 60, 72 and 96 h, and the data were transformed into volume employing the following equation: V = p × 4.6788 (Santos et al., Reference Santos, Carvalho, Carriero, Magalhães, Batista, Fagundes and Bueno2020).
At the end of the bioassay (96 h), each vial was opened and the liquid phase was sampled (2 ml) and transferred to a vessel containing 0.4 ml of formic acid for the determination of short-chain fatty acids (SCFA) and N-ammoniacal concentration (N-NH3). The bags were also removed from the bottles for the determination of in vitro digestibility of DM (IVDMD96) and NDF after 24 h of incubation (IVNDF96).
Laboratory analysis and procedures
The roughage samples were characterized for their chemical composition according to AOAC (2000): DM content (ID 950.15), ash (ID 942.05), crude protein (ID 984.13), ether extract (ID 920.39). The NDF (using amylase, without sodium sulphite) and acid detergent fibre analysis was performed according to Mertens (Reference Mertens2002). Lignin analysis was performed according to Van Soest and Robertson (Reference Van Soest and Robertson1985), whose measurement occurred after 12 M sulphuric acid cellulose hydrolysis in the sample residue.
The samples collected in the methanogenesis bioassay were submitted to methane measurement by gas chromatography (Model 2014; Shimadzu, Tokyo), according to the methodology described by Sallam et al. (Reference Sallam, Bueno, Nasser and Abdalla2010), representing a cumulative 24 h of fermentation. For NH3-N assay, 2 ml of supernatant was mixed with 1 ml of 1 N H2SO4, and analysis was performed using the phenol-hypochlorite method (Broderick and Kang, Reference Broderick and Kang1980). The concentration of SCFA was quantified by gas chromatography, with column Stabilwax, according to Erwin et al. (Reference Erwin, Marco and Emery1961), adapted by Getachew et al. (Reference Getachew, Makkar and Becker2002).
For in vitro DM degradation (IVDMD) and in vitro NDF degradation (IVNDFD) evaluation, the bags were washed in running water until fully bleached, and transferred to a forced ventilation oven at 55°C and kept for 72 h. Sequentially, they were dried in a non-ventilated oven at 105°C for 45 min. They were then placed in a desiccator and weighed to obtain undigested DM. Subsequently, the bags were destined for NDF analysis in a fibre analyser (Ankom®) at 90°C for 1 h and sequentially washed with hot water and acetone, dried at 60°C for 72 h and then weighed, according to the previous methodology (Detmann et al., Reference Detmann, Paulino, Zervoudakis, Valadares Filho, Euclydes, Lana and Queiroz2001; Casali et al., Reference Casali, Detmann, Valadares Filho, Pereira, Henriques, Freitas and Paulino2008).
The cumulative gas production curves were adjusted by using the model proposed by France et al. (Reference France, Dhanoa and Theodorou1993):
where V t is the accumulated volume (ml) of gases produced after the period of incubation, V f is the final volume or maximum potential for gas production (asymptotic value; ml/g DM), b (1/h) and c (1/2 h) are constant fractional rates, L is lag time, and t is the time (h) of incubation.
Statistical analysis
Data were analysed using SAS® 9.4 software (Statistical Analysis System Inst. Inc., Cary, NC) according to the following statistical model:
with ${\rm e}_{ijkl}\approx N( 0, \;\;\sigma _{\rm e}^2 )$![]() ; where Yijkl is the observed value of the dependent variable; μ is the general mean; Ei is the fixed effect of the fibrolytic enzyme level (i = 1–4); Rj is the fixed effect of roughage (j = 1 and 2); Ak is the fixed effect of A. oryzae level (k = 1–4); E × Rij, E × Aik, R × Ajk and E × R × Aijk are interaction effects between previously defined fixed effects; eijkl is the random residual error (l = 1–3); N stands for Gaussian distribution; and $\sigma _{\rm e}^2$
; where Yijkl is the observed value of the dependent variable; μ is the general mean; Ei is the fixed effect of the fibrolytic enzyme level (i = 1–4); Rj is the fixed effect of roughage (j = 1 and 2); Ak is the fixed effect of A. oryzae level (k = 1–4); E × Rij, E × Aik, R × Ajk and E × R × Aijk are interaction effects between previously defined fixed effects; eijkl is the random residual error (l = 1–3); N stands for Gaussian distribution; and $\sigma _{\rm e}^2$![]() is the residual variance. The degrees of freedom were adjusted using the Kenward–Roger method. Treatment averages were compared with the Fischer means test (LSD; α = 0.05). Interaction effects were declared at P ≤ 0.10. Fibrolytic enzyme and A. oryzae level effects were decomposed using polynomial regression method.
is the residual variance. The degrees of freedom were adjusted using the Kenward–Roger method. Treatment averages were compared with the Fischer means test (LSD; α = 0.05). Interaction effects were declared at P ≤ 0.10. Fibrolytic enzyme and A. oryzae level effects were decomposed using polynomial regression method.
Results
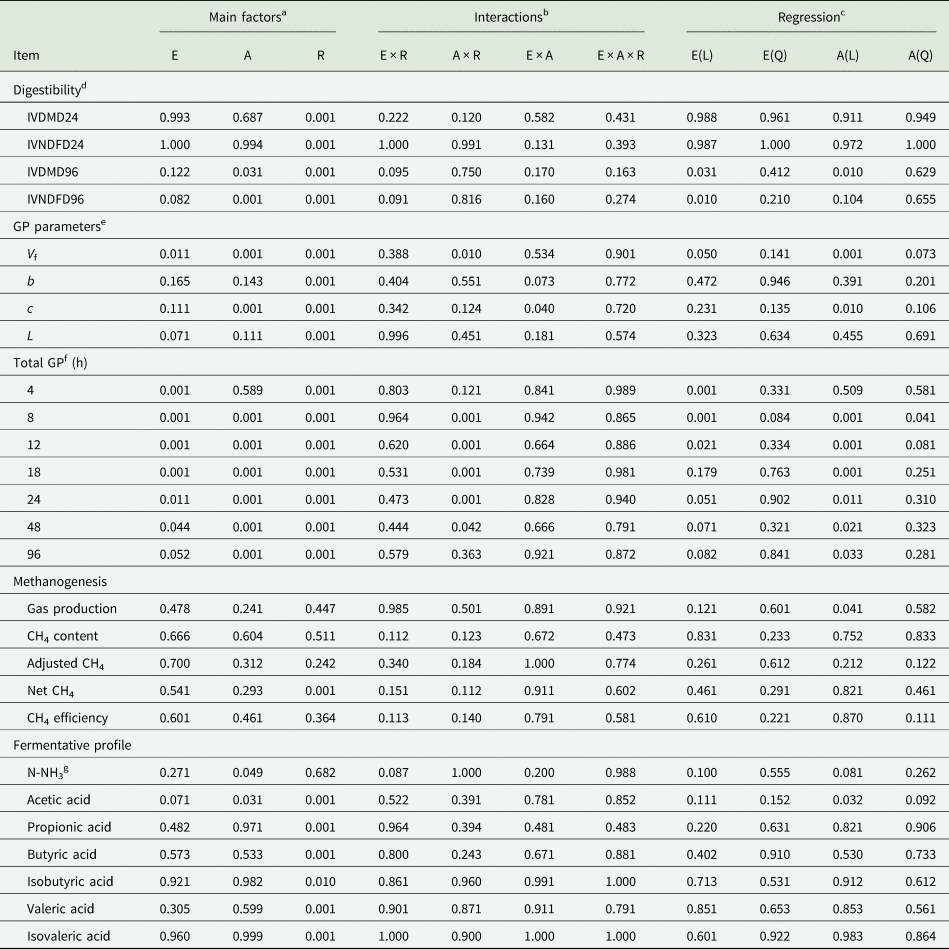
There was no (P ≥ 0.163) three-way interaction among fibrolytic enzyme, A. oryzae and roughage on studied variables (Table 2). There was no (P ≥ 0.131) two-way interaction among fibrolytic enzyme and A. oryzae. As we had a great roughage effect, the results obtained in response to doses of A. oryzae and fibrolytic enzyme were presented for each roughage separately.
Table 2. ANOVA table showing P values (probability of non-significant effects) for the main treatment factors (roughage, fibrolytic enzyme and Aspergillus oryzae), and their interactions on digestibility, gas production parameters, total gas production, methanogenesis and fermentative profile
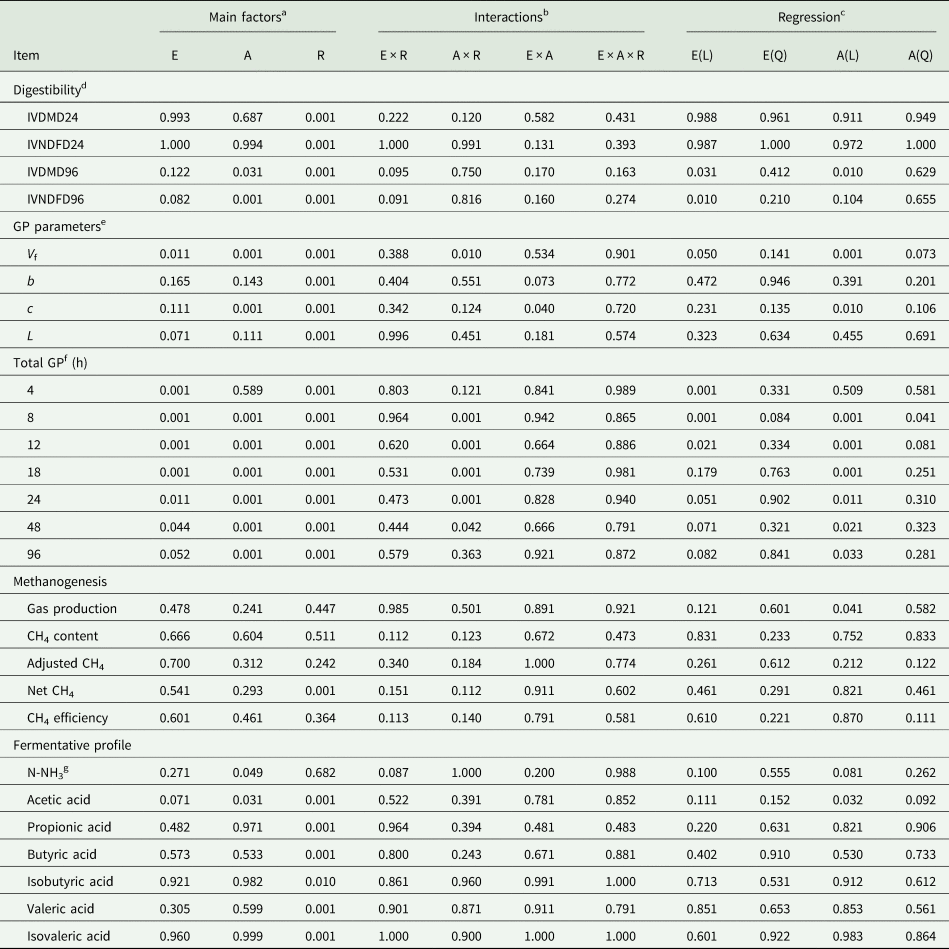
a E: fibrolytic enzyme; A: A. oryzae; R: roughage.
b Two-way and three-way interaction among main factors.
c L: linear; Q: quadratic.
d IVDMD: in vitro dry matter digestibility (24 and 96 h of incubation); IVNDFD: in vitro NDF digestibility (24 and 96 h).
e Gas production parameters, obtained by the model of France et al. (Reference France, Dhanoa and Theodorou1993); V f: potential for gas production; b and c: constants fractional rates; L: lag time.
f Cumulative gas production at different times of incubation. CH4, methane.
g N-NH3: ammoniacal nitrogen.
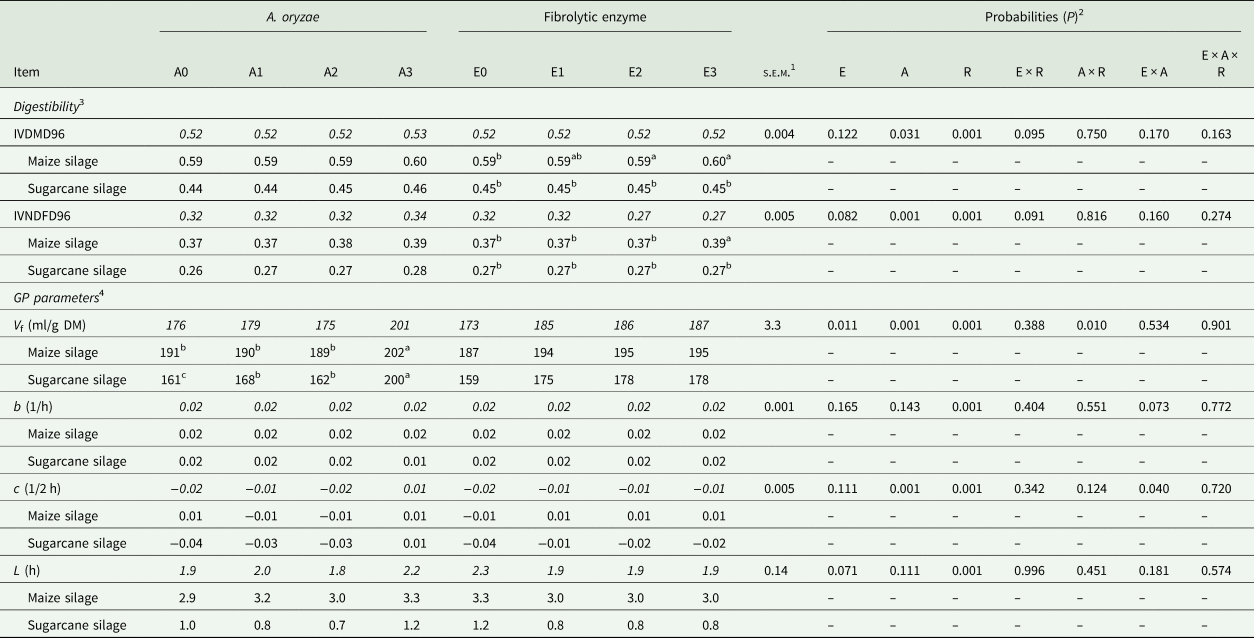
For the digestibility parameters, it was observed that the inclusion of A. oryzae increased the IVDMD96 independently of the roughage (P = 0.031) and the effect was linear (P = 0.010) as the dose increased (0.516, 0.518, 0.519 and 0.526 for A0, A1, A2 and A3, respectively; Table 3). Likewise, increasing doses of A. oryzae promoted an increase in IVNDFD96, regardless of the roughage (P = 0.001), whose highest value was also for the highest dose of A. oryzae (0.316, 0.319, 0.324 and 0.335 for A0, A1, A2 and A3, respectively). For the degradability parameters obtained from the France model, there was a significant two-way interaction between A. oryzae and roughage (P = 0.010; Table 3), where the effect of treatment with increasing doses of A. oryzae over the V f was different according to the roughage source. In sugarcane silage, A. oryzae increased the potential for gas production when compared to the control (P = 0.001), whereas the higher dosage resulted in the highest observed value. In maize silage, this effect was significant only in the highest dose of A. oryzae.
Table 3. Digestibility coefficient of dry matter and neutral detergent fibre, at 96 h of incubation, and gas production parameters, in response to doses of A. oryzae and fibrolytic enzyme, with different roughage sources
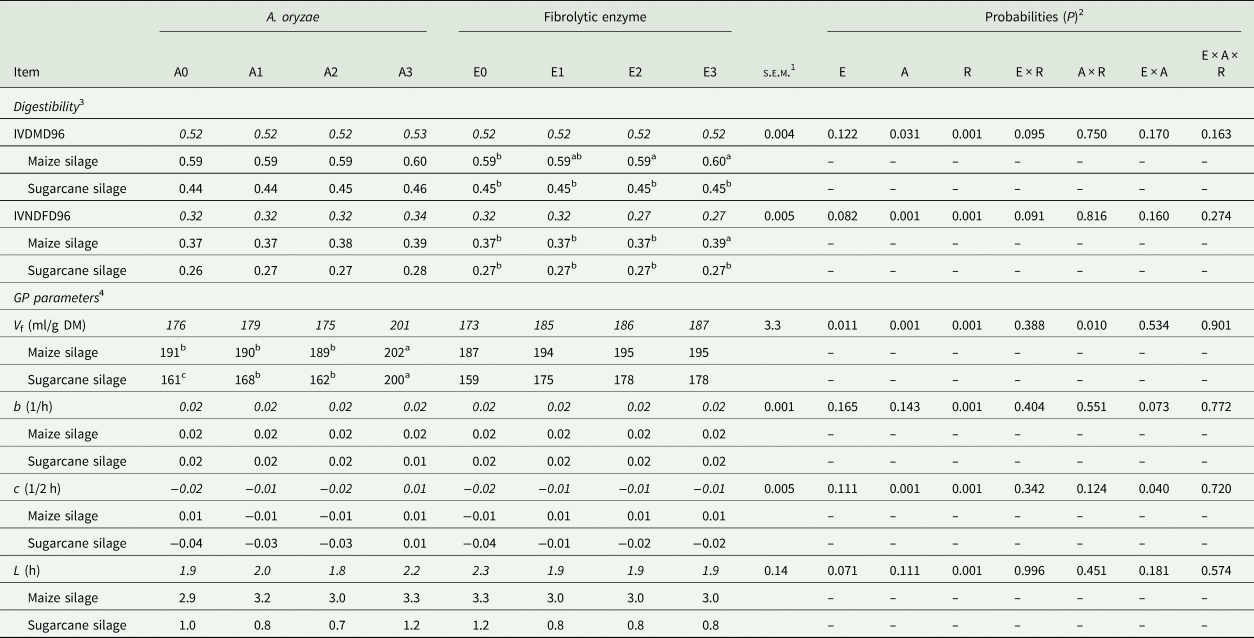
Means within a row with different superscript letters differ.
Means highlighted in italics are the combination of roughage X additive.
1 Standard error mean.
2 E: fibrolytic enzyme; A: A. oryzae; R: roughage.
3 IVDMD: in vitro dry matter digestibility coefficient (96 h of incubation); IVNDFD: in vitro NDF digestibility coefficient (96 h).
4 Gas production parameters, obtained by the model of France et al. (Reference France, Dhanoa and Theodorou1993); V f: potential for gas production; b and c: constants fractional rates; L: lag time.
There was a trend of a significant two-way interaction among fibrolytic enzyme and roughage source for IVDMD96 and IVDFD96 (P = 0.095 and P = 0.091, respectively; Table 4), where the inclusion of fibrolytic enzyme increased the IVDMD96 only when the substrate was the maize silage, with no effect on sugarcane silage (P = 0.952). Similarly, there was an increase in IVNDFD96 only in response to the highest dose of fibrolytic enzyme when the substrate was the maize silage. Furthermore, there was a linear increase in V f in response to increasing enzyme doses (P = 0.011), regardless of the roughage (173.1, 184.8, 186.4 and 186.7 ml/g of DM, for E0, E1, E2 and E3, respectively). In addition, there was a trend of reduction of lag time in response to enzymatic treatment in both roughages (2.23, 1.90, 1.90 and 1.90 h for E0, E1, E2 and E3, respectively; P = 0.071).
Table 4. Cumulative gas production (GP expressed in ml/g DM) in response to doses of A. oryzae and fibrolytic enzyme

Means within a row with different superscript letters differ.
Means highlighted in italics are the combination of roughage X additive.
1 Standard error mean.
2 E: fibrolytic enzyme; A: A. oryzae; R: roughage.
For the cumulative gas production, it was observed that treatment with increasing doses of A. oryzae also increased gas production, but this effect was different according to the roughage (Table 4). In sugarcane silage, A. oryzae increased the gas production when compared to the control (P = 0.001), whereas the higher dosage resulted in the highest observed gas production value in all evaluated times, however there was no effect on gas production after 4 h of incubation. In maize silage, this effect was significant only in the highest dose of A. oryzae. Similarly, it was observed that enzymatic treatment increased gas production in both roughages (P < 0.051, Table 4).
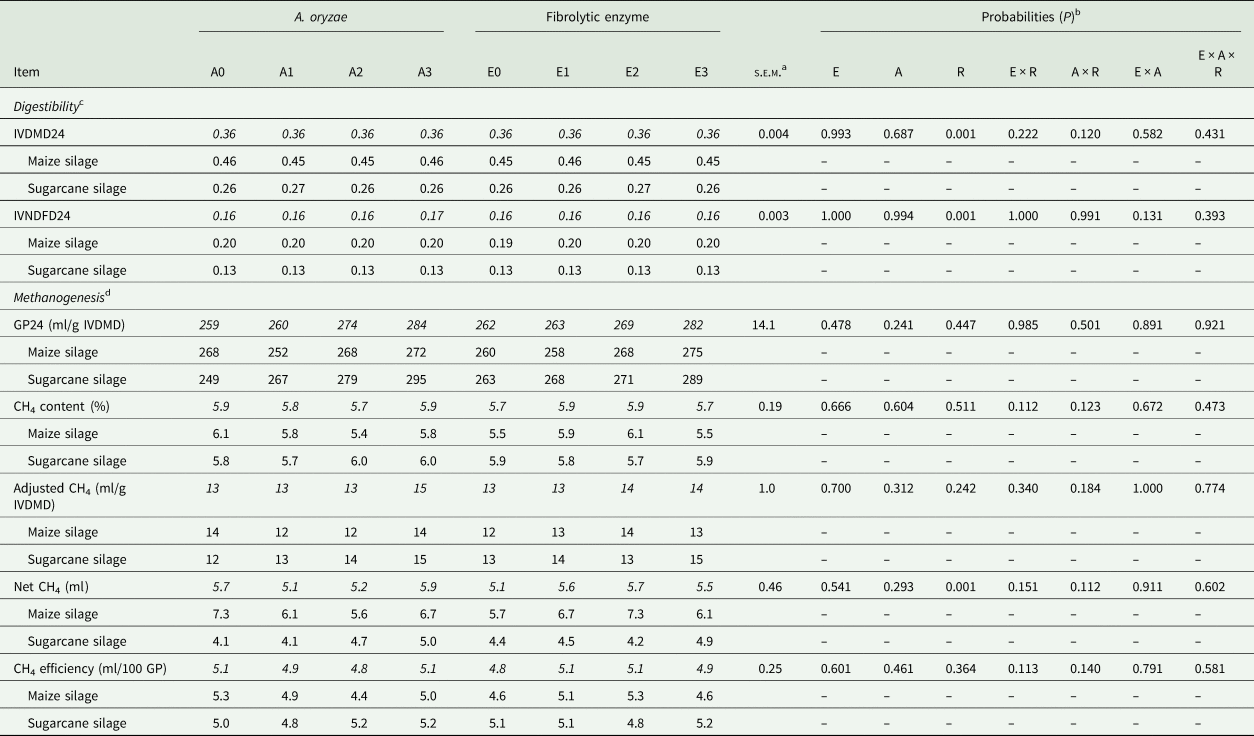
In the methanogenesis bioassay, there was no significant effect of treatments on methane production parameters (P > 0.241; Table 5). Similarly, there was no two-way interaction effect (P ≥ 0.120) among treatments on IVDMD24 and IVNDFD24. Although there was no effect of enzyme and A. oryzae on IVD24 of DM and NDF, the degradation was higher (P = 0.001; Table 2) in maize than sugarcane silage (Table 5; 0.454 v. 0.262 for IVDMD; and 0.196 v. 0.127 for IVNDFD, respectively).
Table 5. Effect of A. oryzae and fibrolytic enzyme on digestibility and methane emission evaluated by in vitro methanogenesis bioassay
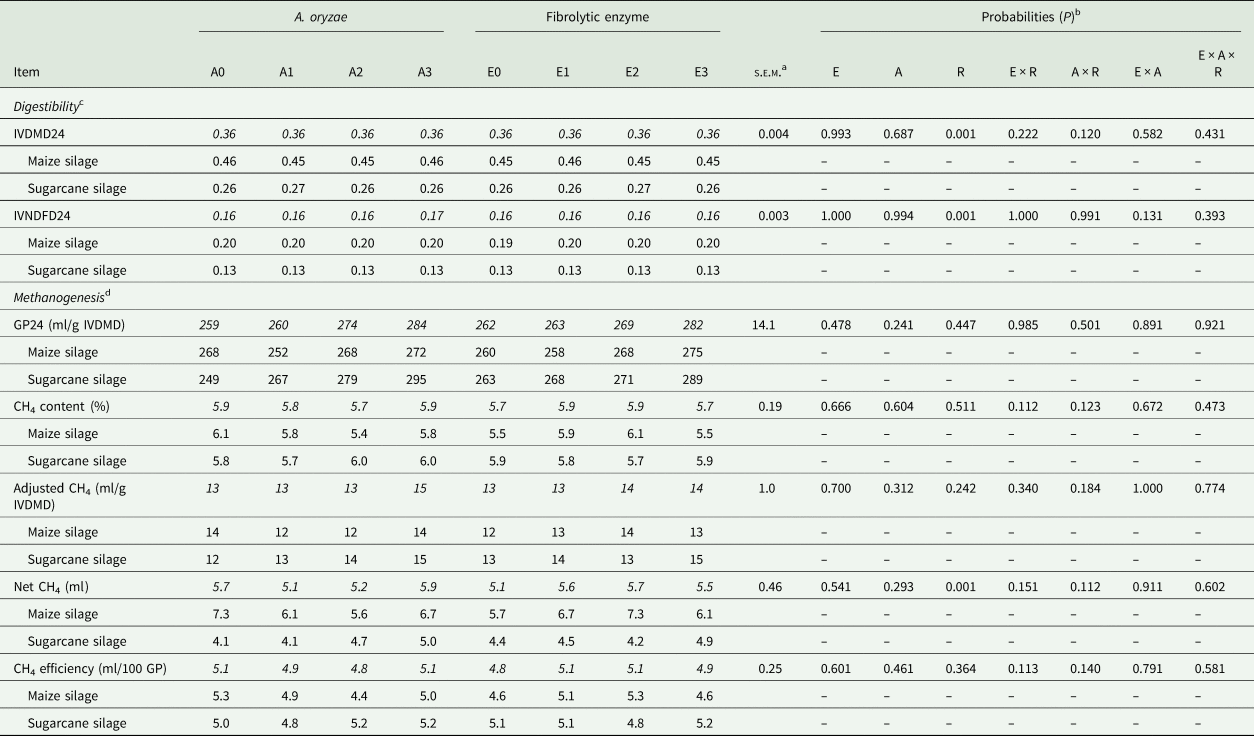
Means highlighted in italics are the combination of roughage X additive.
a Standard error of the mean.
b E: fibrolytic enzyme; A: A. oryzae; R: roughage.
c IVDMD: in vitro dry matter digestibility coefficient (24 h of incubation); IVNDFD: in vitro NDF digestibility coefficient (24 h).
d GP24: gas production per gram of degraded dry matter, after 24 h of incubation; methane (CH4) efficiency = [100 × (adjusted CH4/GP)].
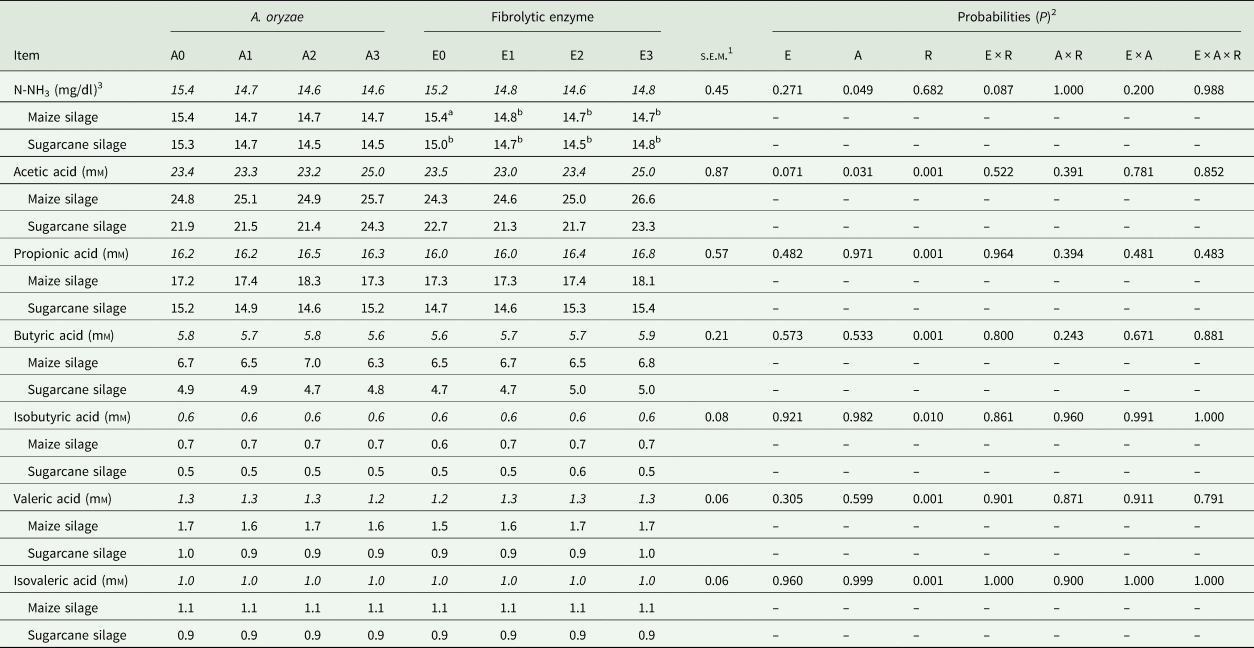
A reduction in N-NH3 concentration was observed in response to A. oryzae treatment (P = 0.049; Table 6), regardless of roughage (15.4, 14.7, 14.6 and 14.64 mg/dl, for A0, A1, A2 and A3, respectively). In addition, the highest dose of A. oryzae (A3) increased the concentration of acetic acid, independently of the roughage (23.38, 23.36, 23.18 and 25.02, for A0, A1, A2 and A3, respectively; P = 0.031). No significant effect of the A. oryzae on the other fermentative parameters was observed (P > 0.305).
Table 6. Fermentative profile in response to the treatment with Aspergillus oryzae and fibrolytic enzyme
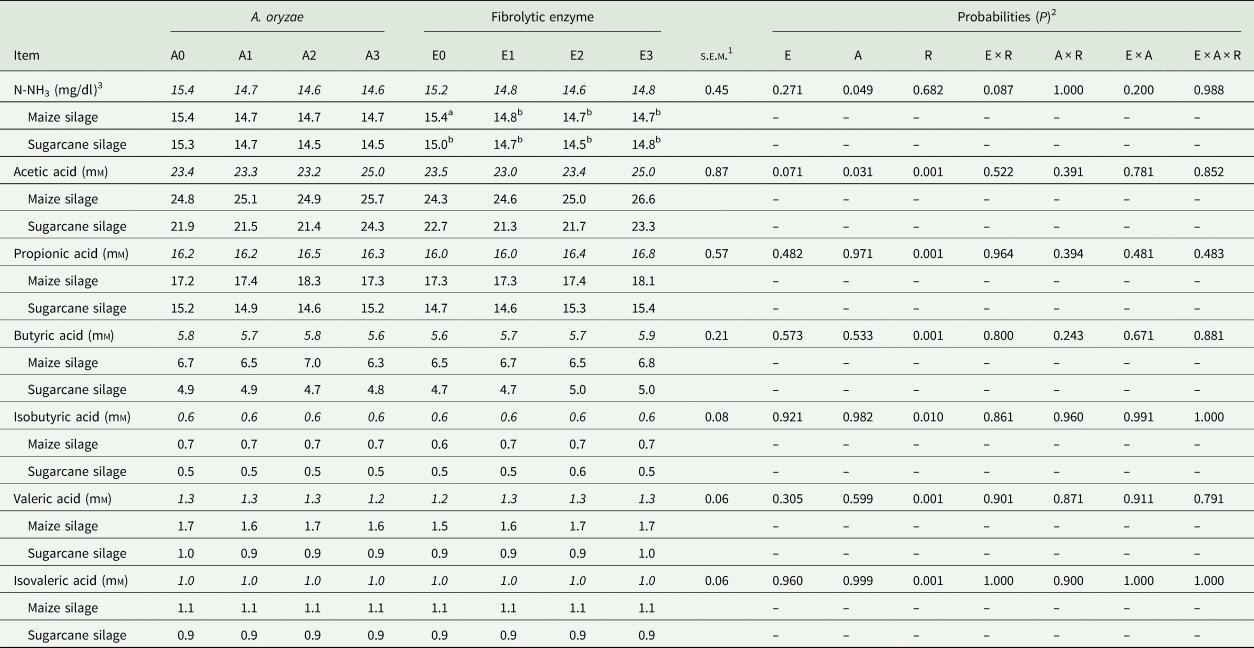
Means within a row with different superscript letters differ.
Means highlighted in italics are the combination of roughage X additive.
1 Standard error mean.
2 E: fibrolytic enzyme; A: A. oryzae; R: roughage.
3 N-NH3: ammoniacal nitrogen.
There was a trend (P = 0.087; Table 6) for N-NH3 concentration reduction in response to fibrolytic enzyme treatment, however this effect was significant only for maize silage. In addition, there was a trend of increase in acetic acid concentration in response to the enzymatic treatment (P = 0.071), where the highest dose (E3) presented the highest value for this variable, regardless of the roughage (23.6, 22.9, 23.4 and 25.0 μm, for E0, E1, E2 and E3, respectively).
Discussion
The results of the current study indicate that live cultures of A. oryzae and fibrolytic enzyme are able to modulate the in vitro ruminal fermentation, decreasing N-NH3 and improving acetic acid concentration and in vitro degradation of DM and NDF. However, there is no synergic effect among them, and the positive response to these additives was dependent on doses and type of roughage used. To our knowledge, this is the first study on the use of live cultures of A. oryzae in combination with fibrolytic enzyme in the modulation of in vitro ruminal fermentation, although previous studies have used these additives separately (Meale et al., Reference Meale, Beauchemin, Hristov, Chaves and Mcallister2014; Sun et al., Reference Sun, Wu, Wang, Liu and Myung2014).
The potential for gas production in an in vitro assay is directly related to the chemical composition, especially fibrous content and structural polysaccharides (Musco et al., Reference Musco, Koura, Tudisco, Awadjihe, Adjolohoun, Cutrignelli, Mollica, Houinato, Infascelli and Calabro2016). In this context, the degradability characteristics of roughage should be considered when evaluating the effect of additives on ruminal fibre digestibility. In the current study, additives increased gas production at all incubation times, regardless of roughage. This result observed for the A. oryzae is similar to others reported in the literature (Morgavi et al., Reference Morgavi, Beauchemin, Nsereko, Rode, McAllister and Wang2004; Sun et al., Reference Sun, Wu, Wang, Liu and Myung2014), where the microbial additive may increase fibrolytic activity by stimulating the growth of the rumen microorganisms. Aspergillus oryzae has a physical action on fibre due to the growth of mycelium and hyphae through the roughage, increasing bacterial access and colonization of the fibre fraction, increasing fibre digestibility (Giraldo et al., Reference Giraldo, Tejido, Ranilla and Carro2008). Furthermore, fungi can produce a range of enzymes, fibrolytic and amylolytic, and phytohormones that stimulate the activities of microorganisms, which can also promote improvements in fibre digestibility (Sher et al., Reference Sher, Faheem, Ghani, Mehmood, Rehman and Bokhari2017; Zayed, Reference Zayed2018). Similarly, the addition of fibrolytic enzyme may increase fibrolytic activity by stimulating the growth of ruminal microorganisms through the release of cell wall compounds (López-Aguirre et al., Reference López-Aguirre, Hernández-Meléndez, Rojo, Sánchez-Dávila, López-Villalobos, Salem Abdel-Fattah, Vázquez-Armijo, Ruíz and Joaquin2016).
Despite the increase in gas production observed in all incubation times, A. oryzae increased the in vitro NDF and DM digestibility, regardless of the type of roughage. The possible explanation for this could be the chemical composition of roughage, since the proportion of rumen potentially digestible NDF may affect the response to the addition of fibrolytic enzyme (Mendoza et al., Reference Mendoza, Loera-Corral, Plata-Pérez, Hernández-García and Ramírez-Mella2014). Mendoza et al. (Reference Mendoza, Loera-Corral, Plata-Pérez, Hernández-García and Ramírez-Mella2014) describe that the addition of exogenous enzymes promotes improvements in the digestibility parameters of roughages that present a high proportion of potentially digestible fibre fraction. In the current study, the fibrolytic enzyme presents high xylanase activity, which may have acted in the most digestible fractions of sugarcane, altering or weakening the cell wall structure, without reflecting on the significant effect on in vitro NDF digestibility (Giraldo et al., Reference Giraldo, Tejido, Ranilla and Carro2008; Sakita et al., Reference Sakita, Bompadre, Dineshkumar, Lima, Abdalla Filho, Campioni, Oliva Neto, Bremer Neto, Louvandini and Abdalla2020).
As mentioned earlier, in the current study, our initial approach was that A. oryzae and exogenous fibrolytic enzyme would synergistically improve gas production and in vitro fibre degradation. That approach was based on the fact that the xylanases can hydrolyse xylan, increasing cellulose accessibility to the cellulase enzymes through increasing fibre swelling and fibre porosity (Gonçalves et al., Reference Gonçalves, Takasugia, Jiaa, Moria, Nodab, Tanakac, Ichinosed and Kamiyaa2015). When using these additives together, the presence of a diversity of enzymes together could reach a variety of substrates, increasing the fibre digestibility (Srinivas et al., Reference Srinivas, Chaturvedi, Malik and Asgar2008). So, as mentioned earlier, A. oryzae can produce a range of enzymes, including cellulose, and the supplementation with fibrolytic enzyme would substantially increase fibre degradability, once that xylan is one of the major mechanisms that limited the accessibility of the cellulase enzymes to the cellulose. However, in the current study, there is no synergic effect among them. A possible explanation would be related to the type of exogenous enzyme used. The supplementation of cellulases with xylanase can increase the rate and extent of cellulose hydrolysis (Hu et al., Reference Hu, Arantes and Saddler2011). However, it appears that the type of interaction between xylanase and cellulase enzymes is dependent on several factors, such as enzyme ratio and total enzyme loading (Jeoh et al., Reference Jeoh, Wilson and Walker2006; Zerva et al., Reference Zerva, Pentari, Grisel, Berrin and Topakas2020). Addressing this, Hu et al. (Reference Hu, Arantes and Saddler2011) observed a strong synergistic effect at low cellulase loading and when a high xylanase to cellulase ratio was used. Furthermore, Zayed et al. (Reference Zayed, Szumacher-Strabel, El-Fattah, Madkour, Gogulski, Strompfová, Cieślak and El-Bordeny2020) in an in vitro study reported that the simultaneous use of inoculants containing fungal and bacterial strains, as a source of exogenous cellulolytic enzymes, increased the fibre digestibility of rice straw.
In this context, other factors may influence the results when using fibrolytic enzyme in order to modulate rumen fermentation, such as dose–response effect, as well as the types of enzymes. The main fibrolytic enzymes are cellulases and xylanases, which act mainly in the degradation of cellulose and hemicellulose, respectively. Considering the high xylanase activity of the exogenous enzyme used in the present study, this could explain the result observed for IVDMD96 in response to the enzymatic treatment, which was significant only for maize silage, due to the fact that sugarcane silage has a lower hemicellulose content, justifying the lesser effect of the fibrolytic enzyme on this roughage when compared to maize silage. López-Aguirre et al. (Reference López-Aguirre, Hernández-Meléndez, Rojo, Sánchez-Dávila, López-Villalobos, Salem Abdel-Fattah, Vázquez-Armijo, Ruíz and Joaquin2016), when evaluating different types of fibrolytic enzymes (cellulase, xylanase and the combination of both) at different dosages, observed that cellulase presented the best fermentative kinetics parameters for gas production, since the highest dose of fibrolytic enzyme with xylanase activity reduced gas production at all incubation times. Thus, the high dosage of xylanase may have affected the binding of enzymes to receptors present in the substrate, and consequently reducing the binding of fibrolytic microorganisms to fibre (Beauchemin et al., Reference Beauchemin, Morgavi, McAllister, Yang, Rode, Garnsworthy and Wiseman2001). However, Togtokhbayar et al. (Reference Togtokhbayar, Cerrillo, Rodriguez, Elghadour, Salem, Urankhaich, Jigjidpurev, Odongo and Kholif2015) described that the addition of fibrolytic enzyme with high xylanase activity at different doses in an in vitro assay using wheat straw as a substrate promoted increased gas production at all evaluated incubation times. These results demonstrate that the chemical composition of roughage affects exogenous enzyme responses, as well as the dose and type of enzyme used.
There are indications that enzymes produced by fungi, such as the A. oryzae, may act on the degradation of lignocellulosic materials, but a longer ruminal retention time is required for the effective action of fibrolytic microorganisms (Kumar et al., Reference Kumar, Barrett, Delwiche and Stroeve2009). Thus, it is assumed that the addition of exogenous fibrolytic enzymes may promote lag time reduction, since enzymes can degrade substrate complex carbohydrates to simple forms at an early stage of fermentation, thus allowing rapid growth of bacterial population, and consequent colonization and fibre fermentation (López-Aguirre et al., Reference López-Aguirre, Hernández-Meléndez, Rojo, Sánchez-Dávila, López-Villalobos, Salem Abdel-Fattah, Vázquez-Armijo, Ruíz and Joaquin2016). In the current study, both additives increased the V f. However, for A. oryzae, this effect was dependent on the roughage source. In addition, A. oryzae showed higher lag time, in both roughages, when compared to the fibrolytic enzyme (3.15 v. 2.96 h for maize silage, and 0.89 v. 0.84 h for sugarcane silage), which indicates that A. oryzae needs more time for its development to promote significant improvements in fibre digestibility. Moreover, the lack of results from the A. oryzae on gas production after 4 h of incubation may reinforce this hypothesis.
In this context, a way to potentiate the effect of the fungus would be to increase the number of viable spores, by increasing the doses of A. oryzae, which could mitigate the effect of lag time. It is assumed that the increase in the number of viable spores would result in greater adherence to the fibrous particle, increasing the surface area available for fibrolytic activity and, consequently, enhancing the fibre digestibility over the incubation time (Sjaastad et al., Reference Sjaastad, Hove and Sand2010). This could justify the better results of gas production potential, as well as for digestibility parameters, for treatments with the highest dose of A. oryzae.
In the current study, A. oryzae reduced ammonia-N content regardless of roughage, whereas fibrolytic enzyme reduced ammonia-N only in maize silage. Moreover, either enzyme or A. oryzae increased acetate concentration. Acetate production is related to the action of fibrolytic microorganisms that ferment carbohydrates to acetate. These microorganisms use ammonia-N as the source of nitrogen (Russell and Wilson, Reference Russell and Wilson1996), which may justify the effects of additives in both variables. Thus, the additives stimulated the fibrolytic microbiota, increasing the in vitro NDF digestibility, and increasing the acetate as a final fermentation product. In addition, this result can be correlated to the increase in gas production with the A. oryzae treatment, and the highest values were observed at the highest A. oryzae dose, which may be explained by the higher number of spores in this treatment, resulting in a higher fibre degradation.
Conclusion
The additives did not have a synergistic effect on gas production and digestibility, contrary to the initial hypothesis, that the additives would synergistically enhance the fibre digestibility. However, the results of the current study indicate that doses of A. oryzae and fibrolytic enzyme are able to modulate the in vitro ruminal fermentation, decreasing N-NH3 and improving acetic acid concentration and in vitro degradation of DM and NDF.
Financial support
This study was funded by São Paulo Research Foundation – FAPESP (Number 2017/11537-6).
Conflict of interest
None.
Ethical standards
All applicable international, national and/or institutional guidelines for the care and use of animals were followed (University of São Paulo Animal Bioethics Committee, protocol number 7551070817).