Introduction
Rising atmospheric CO2 concentration and temperature are two main components of global climate change. Species sensitivities to CO2 and temperature are driven by traits that influence growth, such as photosynthetic metabolism and water use efficiency (Poorter and Navas, Reference Poorter and Navas2003; Morgan et al., Reference Morgan, Pataki and Körner2004b; Ainsworth and Long, Reference Ainsworth and Long2005), or reproduction, such as seed production and seedling recruitment (Morgan et al., Reference Morgan, Mosier, Milchunas, Lecain, Nelson and Parton2004a; Hovenden et al., Reference Hovenden, Wills, Chaplin, Vander Schoor, Williams, Osanai and Newton2008; Williams et al., Reference Williams, Wills, Janes, Vander Schoor, Newton and Hovenden2007). Compared with reproductive responses, the vegetative responses of functional groups to climate change are more consistent and distinctive, with C3 plants usually achieving greater productivity under elevated atmospheric CO2 concentrations due to directly stimulated photosynthesis (Sage and Kubien, Reference Sage and Kubien2003); while C4 plans are more sensitive to temperature and in general have higher thermal optimum than that observed for C3 plants (Sage and Kubien, Reference Sage and Kubien2007).
Seed germination as a critical stage of the life cycle reflects adaptation to local habitats and population dynamics (Paula and Pausas, Reference Paula and Pausas2008), and is strongly dependent on environmental filters (Donohue et al., Reference Donohue, Casas, Burghart, Kovach and Willis2010; Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011; Fraaije et al., Reference Fraaije, ter Braak, Verduyn, Breeman, Verhoeven and Soons2015; Mondoni et al., Reference Mondoni, Pedrini, Bernareggi, Rossi, Abeli, Probert, Ghitti, Bonomi and Orsenigo2015). The contribution of seed traits including germination to plant community dynamics has received increasing attention in recent years (Jimenez-Alfaro et al., Reference Jimenez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016). Understanding seed germination responses to climate change is important for predicting changes in species composition, and therefore in the structure and function of ecosystems. Seed traits, such as seed mass, fill rate, viability and germination have strong adaptive implications for species distribution and abundance under future climates (Hovenden et al., Reference Hovenden, Wills, Chaplin, Vander Schoor, Williams, Osanai and Newton2008).
Effects of CO2 enrichment during plant growth and seed production and the influence on the germination of those seeds have been reported in many species. Newton (Reference Newton1991) observed a trend towards higher seed production per plant and greater individual seed mass but a variable response in seed numbers at elevated CO2. A meta-analysis on 79 species shows that in general, CO2 enrichment resulted in more flowers (+19%), more fruit (+18%), more seeds (+16%), greater individual seed mass (+4%), and greater total seed mass (+25%) (Jablonski et al., Reference Jablonski, Wang and Curtis2002). Thürig et al. (Reference Thürig, Körner and Stöcklin2003) found that elevated CO2 concentrations significantly increased seed number of graminoids, but not that of forbs and legumes, in a natural, nutrient-poor calcareous grassland. In the same study, elevated CO2 concentrations also tended to increase seed mass and shorten the time for germination.
Warming is also expected to influence seed traits. Greater seed viability under artificial warming has been reported in alpine and high-latitude ecosystems (Wookey et al., Reference Wookey, Robinson, Parsons, Welker, Press, Callaghan and Lee1995; Kullman, Reference Kullman2002; Lantz et al., Reference Lantz, Kokelj, Gergel and Henry2009). Positive effects of warming on seed mass have been reported in herbaceous plants in forests (De Frenne et al., Reference De Frenne, Graae and Kolb2010), alpine herbaceous species (Sandvik and Eide, Reference Sandvik and Eide2009), and a native perennial grass (Gao et al., Reference Gao, Wang, Zhang, Dong and Guo2012). However, among 15 species studied in a calcareous grassland, seed mass was not significantly affected by warming after 3 years (Hovenden et al., Reference Hovenden, Wills, Vander Schoor, Chaplin, Williams, Nolan and Newton2007). Parental warming during plant growth and seed development increased subsequent seed germination in shrubs and forbs (Graae et al., Reference Graae, Alsos and Ejrnaes2008), forest tree and herbaceous species (Chidumayo, Reference Chidumayo2008; De Frenne et al., Reference De Frenne, Graae and Kolb2010), and a grass species Themeda triandra (Williams et al., Reference Williams, Wills, Janes, Vander Schoor, Newton and Hovenden2007). It reduced seed germination percentage in a dwarf shrub species in the subarctic (Graae et al., Reference Graae, Alsos and Ejrnaes2008), three savanna woody species in Africa (Chidumayo, Reference Chidumayo2008), and grass species in the Eurasian grassland (Gao et al., Reference Gao, Wang, Zhang, Dong and Guo2012) and Australian temperate grassland (Williams et al., Reference Williams, Wills, Janes, Vander Schoor, Newton and Hovenden2007), but did not affect Leymus chinensis (Gao et al., Reference Gao, Wang, Zhang, Dong and Guo2012) and 20 species in the subarctic (Milbau et al., Reference Milbau, Graae, Shevtsova and Nijs2009).
Despite the high number of studies conducted, most of the previous studies have focused on a single factor in global climate change, either elevated CO2 concentration or parental warming but rarely on the combination of the two. Germination of Austrodanthonia caespitosa, a dominant C3 species from Australia, was affected by an elevated CO2 and warming interaction, such that elevated CO2 or warming reduced germination if applied alone, but not when applied together (Hovenden et al., Reference Hovenden, Wills, Chaplin, Vander Schoor, Williams, Osanai and Newton2008). Information is also limited regarding temperature thresholds for germination such as base temperature and thermal time requirement in changing environments (Jimenez-Alfaro et al., Reference Jimenez-Alfaro, Silveira, Fidelis, Poschlod and Commander2016). Filling these research gaps can facilitate better predictions of ecosystem structure and function under a changing climate.
To address these issues, we used the Prairie Heating and CO2 Enrichment (PHACE) experiment to determine the effects of elevated CO2 and warming and their combination on various seed properties. The PHACE project is located on the mixed-grass prairie of Wyoming, USA. We used thermal time models to identify temperature thresholds during germination, and to link these thresholds to seed properties under climate change conditions. Objectives of this research were: (1) to identify treatment effects on seed fill rate, seed viability, individual seed mass and germination in selected species, including two invasive species; (2) to identify the shifts in germination thresholds of those species as affected by treatments; and (3) to identify seed properties that may affect exotic plant invasion under future climatic conditions. We hypothesized that: (1) seed fill percentage is reduced under parental elevated CO2 concentration and warming, due to the disruption of normal seed development (Spears et al., Reference Spears, Tekrony and Egli1997; Young et al., Reference Young, Wilen and Bonham-Smith2004); (2) parental warming and elevated CO2 would increase and decrease seed viability, respectively, due to altered seed protein content for embryo growth (Andalo et al., Reference Andalo, Godelle, Lefranc, Mousseau and Till-Bottraud1996; Bai et al., Reference Bai, Tischler, Booth and Taylor2003); (3) parental warming, CO2 enrichment, and their combination, which increased growth of several of the studied species (Blumenthal et al., Reference Blumenthal, Resco, Morgan, Williams, Lecain, Hardy, Pendall and Bladyka2013; Reeves et al., Reference Reeves, Blumenthal, Kray and Derner2015), would also increase individual seed mass; and (4) future climatic conditions benefit invasive species over native species at the regeneration stage, mirroring responses of vegetative growth and overall seed production.
Materials and methods
The PHACE experiment
The Prairie Heating and CO2 Enrichment (PHACE) experiment was set up at the United States Department of Agriculture–Agricultural Research Service, High Plains Grasslands Research Station, Cheyenne, WY, USA (41o11′N, 104o54′W) in a northern mixed-grass prairie community (Dijkstra et al., Reference Dijkstra, Blumenthal, Morgan, Pendall, Carrillo and Follett2010). Mean annual precipitation is 384 mm and mean temperatures are 17.5°C in July and –2.5°C in January. The plant community consists of about 55% C3 grasses with Pascopyrum smithii (Rydb.) A. Löve and Hesperostipa comata (Trin. and Rupr.) Barkworth dominating; C4 grasses, almost solely Bouteloua gracilis Lag. ex Griffiths, contribute about 25% of the composition, and sedges, forbs and small shrubs contribute about 20% (Morgan et al., Reference Morgan, LeCain and Pendall2011). The PHACE experiment was initiated in spring 2016. Twenty circular experimental plots (diameter 3.4 m) were established and split into two sections. One half of each plot was retained as the northern mixed prairie plant community, while the other half was designed to study plant invasion. The plant invasion area consisted of three, 80 × 70 cm subplots. The toadflax subplot was planted with 20 Linaria dalmatica (L.) P. Miller seedlings in June 2006 to study its invasion into undisturbed prairie (Blumenthal et al., Reference Blumenthal, Resco, Morgan, Williams, Lecain, Hardy, Pendall and Bladyka2013).
The PHACE experiment used Free Air CO2 Enrichment (FACE) technology to elevate ambient CO2 concentrations (Miglietta et al., Reference Miglietta, Peressotti, Vaccari, Zaldei, Deangelis and Scarascia-Mugnozza2001), and infrared heater arrays to warm the canopy (Kimball et al., Reference Kimball, Conley, Wang, Lin, Luo, Morgan and Smith2008). Two concentrations of atmospheric CO2 were applied starting in April 2006: ambient [385 p.p.m.v. (c)] and elevated [600 p.p.m.v. (C)]. Two temperature regimes were applied starting in April 2007, control (t) and heated (T) (1.5/3.0°C warmer day/night). The atmospheric CO2 application was shut off during the winter and turned on in the spring in each experimental year. The warming treatment ran year round.
Twenty plots were used for the elevated CO2 concentrations and warming treatments, consisting of a full factorial design with five replicates of each of the four combinations [ambient CO2 and ambient temperature (ct); ambient CO2 and warming (cT); elevated CO2 and ambient temperature (Ct); and elevated CO2 and warming (CT)].
Seed collection and germination tests
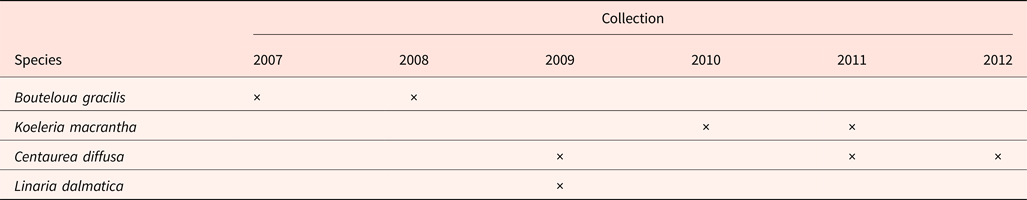
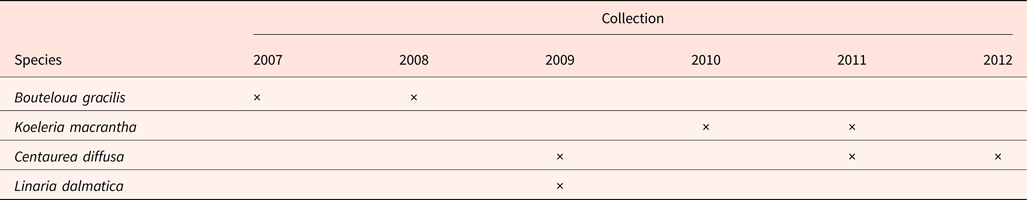
Four species from the mixed-grass prairie were studied (Table 1). Seeds from two native perennial grasses [Bouteloua gracilis and Koeleria macrantha (Ledeb.) Schult] were harvested from the Northern Mixed Prairie area inside each plot. Two invasive species [Centaurea diffusa and Linaria dalmatica] were harvested from the invasive species area of each plot. Because species only produced enough seeds to harvest in some years, the collection years differed by species (summarized in Table 1). Seeds were given at least 4 weeks after-ripening at room temperature (about 20°C) before being stored at 4°C in darkness for approximately 6 months. Seeds were cleaned and the numbers of filled and empty seeds were recorded. Seed fill percentage was then determined using the portion of filled seeds in the total seeds for each treatment. Dry seed mass of 10 to 30 seeds per unit was determined for each species. Seed mass was not recorded for K. macrantha in 2010 and 2011 due to seed limitation.
Table 1. Seed collections between 2007 and 2012 from the Prairie Heating and CO2 Enrichment experiment plots, located in Cheyenne, Wyoming
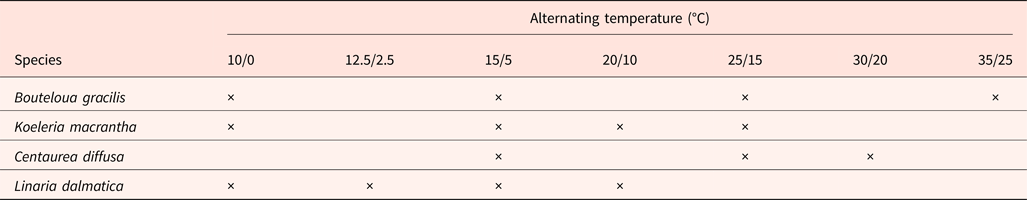
Filled seeds were used for germination and viability tests. Germination tests were conducted using Sanyo growth chambers (Sanyo Versatile Environment Chamber MLR-350H, Sanyo Scientific, USA). One of seven alternating temperatures (12 h/12 h) with a temperature amplitude of 10°C was randomly assigned to each chamber (10/0, 12.5/2.5, 15/5, 20/10, 25/15, 30/20, 35/25°C). Three or four temperature regimes were chosen for the seeds of each species, depending on seed availability (Table 2). Germination tests were conducted under 24 h darkness for all species. A randomized complete block design (RCBD) with three or five replicates was used and replicates were put into growth chambers at 7 day intervals. Each field replicate consisted of a Petri dish with moist filter paper and 25–100 seeds (depending on seed availability) of a single species. Petri dishes were randomly placed in growth chambers using the middle shelves to minimize temperature differences within chambers. Petri dishes were sealed in clear plastic bags to reduce water evaporation and distilled water was added when necessary to keep the filter paper moist. Petri dishes were wrapped by zip-lock bags with two layers of aluminum foil to keep the seeds in darkness. Germination was checked under a green safe light. Seeds were sprayed with 0.05% benomyl solution when growth of microorganisms was observed. Germinated seeds were counted and removed at 1 day intervals. Seeds were considered germinated when the radicle was ≥2 mm. Germination tests were terminated when no seeds germinated for 14 consecutive days.
Table 2. Alternating (12 h/12 h) temperature regimes with temperature amplitude of 10°C for germination tests of seeds from inside the Prairie Heating and CO2 Enrichment experiment plots, located in Cheyenne, Wyoming, determined by availability of seeds

Tetrazolium testing was conducted at the end of the germination test to determine seed viability of the remaining, un-germinated seeds of B. gracilis and K. macrantha. Seeds were stained with 0.1% tetrazolium chloride solution for 24 h at room temperature and then checked under the microscope. Seeds were assumed viable when embryos were stained evenly red (Grabe, Reference Grabe1970). Seeds from C. diffusa and L. dalmatica were considered viable at the end of the germination test if the embryos were firm when pressed (Ren and Bai, Reference Ren and Bai2016). Seed viability was determined by calculating the portion of viable seeds (germinated and non-germinated) in total filled seeds under each temperature regime for a germination test. The maximum viability obtained under all the tested temperature regimes was used as the viability ratio for this species.
Thermal time models were developed according to Qiu et al. (Reference Qiu, Bai, Coulman and Romo2006). A seed population was considered to be composed of subpopulations because of differences in their relative germination rate (Garcia-Huidobro et al., Reference Garcia-Huidobro, Monteith and Squire1982). Germination rates for subpopulations were calculated with the reciprocal of germination time. Base temperature (T b) and thermal time requirements for 50% subpopulation germination (θ50) were calculated for each replicate of each treatment and species. Base temperature and thermal time for the germination of subpopulations were estimated using extrapolation (graphical) methods.
Data analysis
Data on seed mass, seed fill, seed viability, T b and θ50 for B. gracilis, K. macrantha and C. diffusa were analysed as a RCBD with a two-way analysis of variance (ANOVA) using the mixed model procedure in SAS version 9.3 (SAS Institute Inc., USA). For each species, seed mass, seed fill, seed viability, T b and θ50 were taken as the dependent variable, and in each case the main effects and possible interactions of year and climate change treatments were used as independent variables. Replicates and blocks were factored into the model as random effects. When the interaction of year × treatment was significant (P ≤ 0.05), data were analysed within each year for treatment effects. A one-way ANOVA was used to test warming treatment effect on seed mass, seed fill, seed viability, T b and θ50 for L. dalmatica, for which multiple replicates were only available in elevated CO2 plots.
Because different temperature regimes were used for germination, interaction of year, growth chamber temperature, and climate change treatments on seed germination of B. gracilis, K. macrantha and C. diffusa were analysed as a RCBD with a three-way ANOVA using the mixed model procedure in SAS version 9. A two-way ANOVA was conducted to analyse the germination temperature and climate change treatments effects as well as their interaction on germination of L. dalmatica. Treatment means were separated using Tukey's test at P ≤ 0.05.
Results
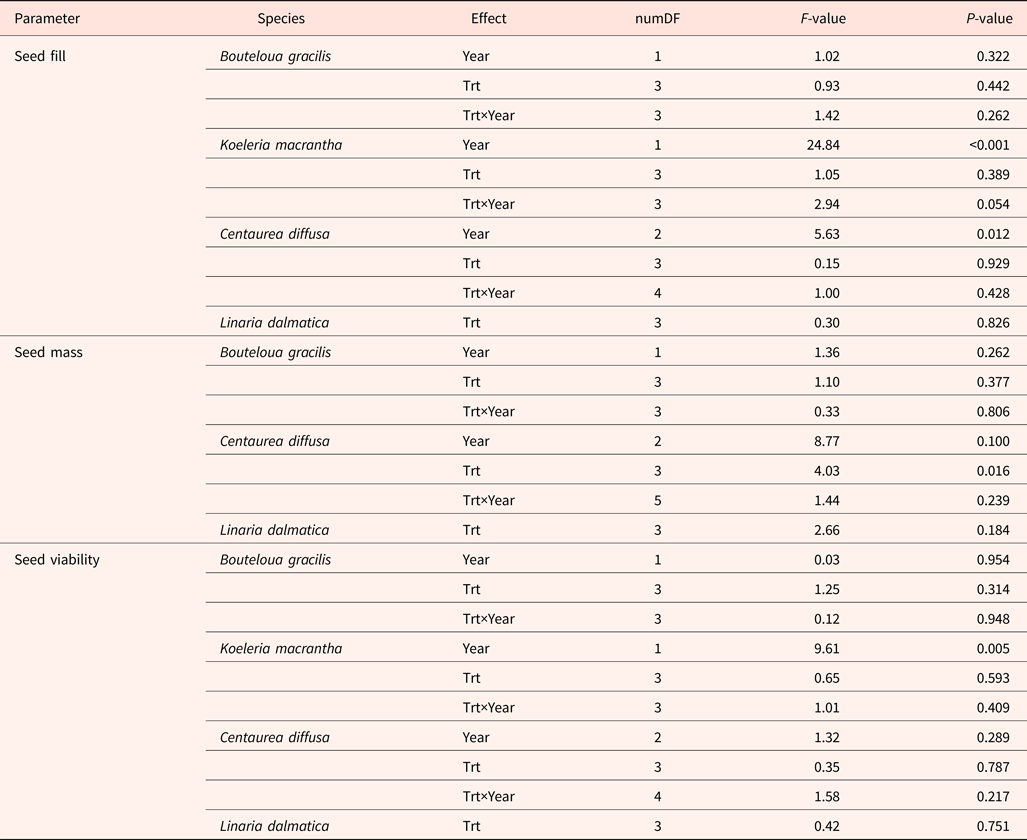
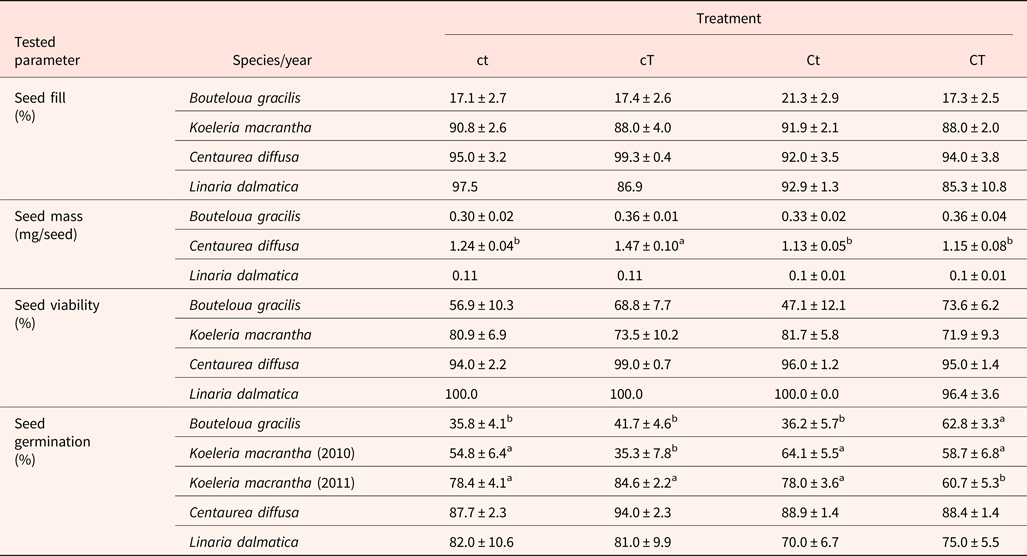
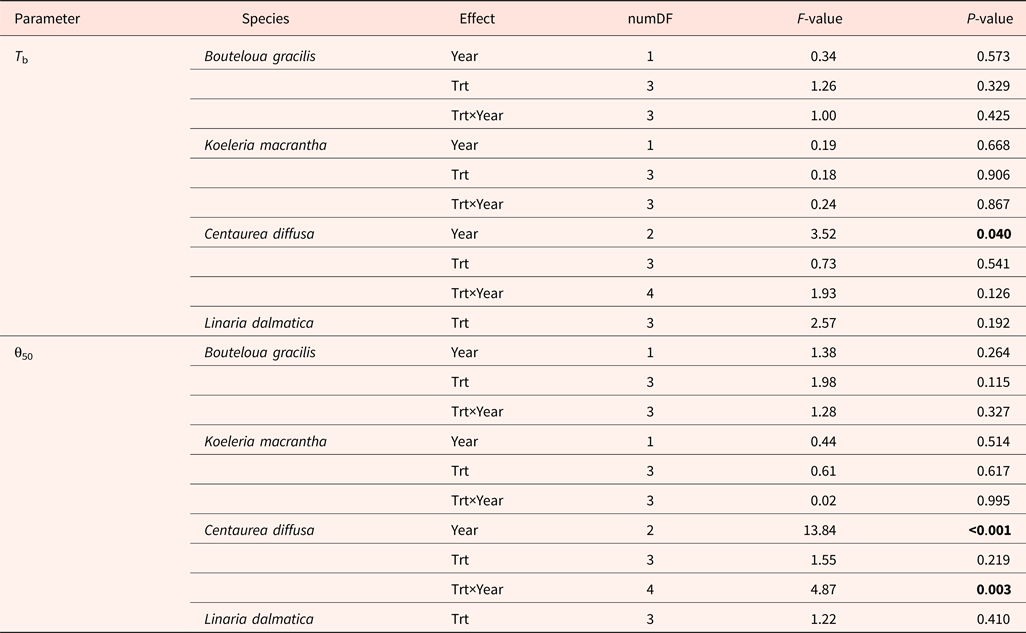
Seed fill of K. macrantha averaged 84.8 and 94.5% in 2010 and 2011, respectively (data not shown). Seed fill of C. diffusa averaged 99.5 and 95.2% in 2011 and 2012, which were significantly higher than that in 2009 (averaged at 86.0%) (data not shown). Seed fill for L. dalmatica and B. gracilis were 90.6 and 18.3%, respectively (Table 5). Seed fill rate was not affected by the interaction of year and climate change treatments (Table 3). Seed fill for K. macrantha and C. diffusa was significantly different between years. There was no significant treatment effect on seed fill in any species (Tables 3 and 5).
Table 3. ANOVA table (P-value) for seed fill, seed mass and seed viability of Bouteloua gracilis (2007 and 2008), Koeleria macrantha (2010 and 2011), Centaurea diffusa (2009, 2011 and 2012), and Linaria dalmatica (2009), harvested from PHACE plots, located in Cheyenne, Wyoming
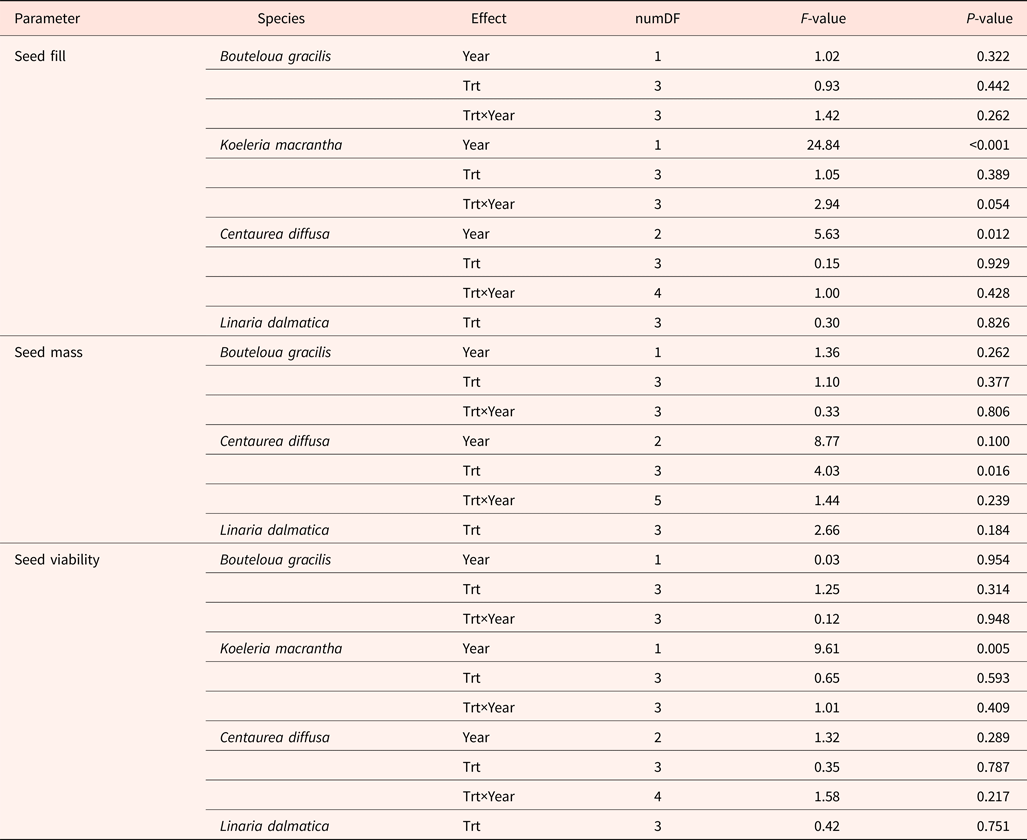
Field treatments (Trt) include ambient CO2, ambient temperature; ambient CO2, warming; elevated CO2, ambient temperature; and elevated CO2, warming.
Individual seed mass varied among species, ranging from 0.1 mg in L. dalmatica, to 1.5 mg in C. diffusa (Table 5). Seed mass was not recorded for K. macrantha (2010 and 2011). There was no significant difference in individual seed mass between climatic change treatments within species, with the exception of C. diffusa. The climatic treatment cT increased seed mass of C. diffusa compared with other treatments. Seed mass of B. gracilis and C. diffusa was not affected by the interaction of year and climatic change treatments (Table 3).
Seed viability of K. macrantha fluctuated between years (Table 3), averaging 67 and 88% in 2010 and 2011 (data not shown). Seed viability was lowest in B. gracilis (62%) and highest in L. dalmatica (99%). Seed viability was not affected by the interaction of year and climatic change treatments, or by treatment (Table 3).
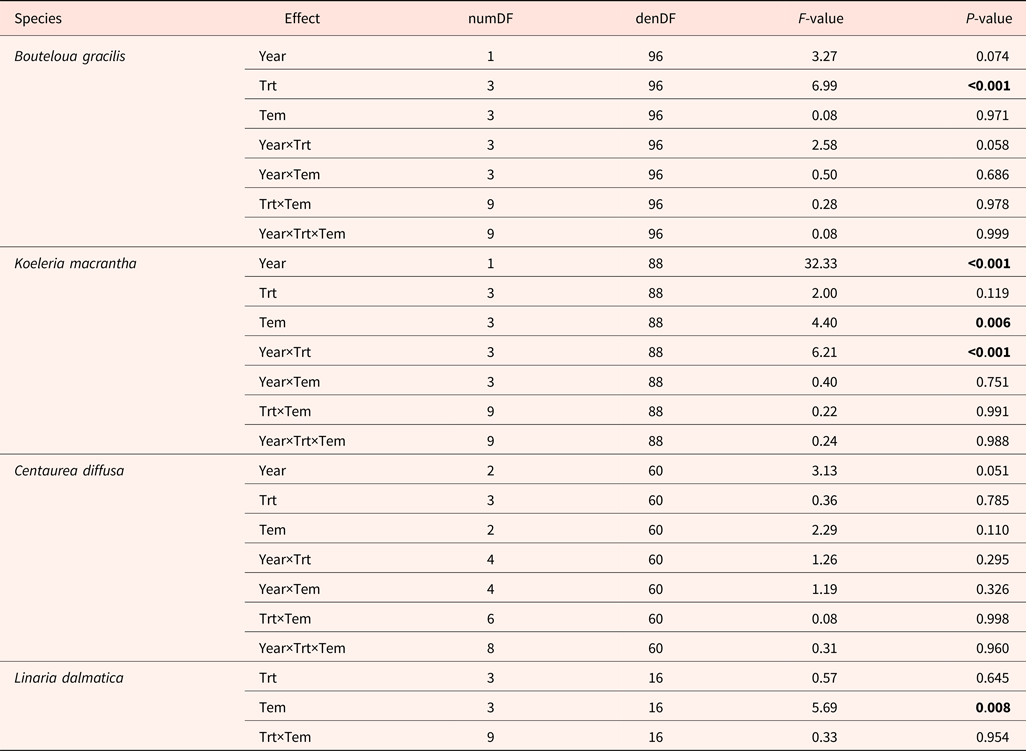
A wide range of temperature regimes was used for the germination tests. High variation in total germination was observed among species, ranging from 44% in B. gracilis to 90% in C. diffusa. We found no interactive effects among year, temperature regime for germination, and climatic change treatment on seed germination (Table 4). High temperatures enhanced and low temperatures reduced germination of K. macrantha and L. dalmatica (data not shown). Germination of B. gracilis and C. diffusa was not affected by germination temperature regime.
Table 4. ANOVA table (P value) for total germination of Bouteloua gracilis (2007 and 2008), Koeleria macrantha (2010 and 2011), Centaurea diffusa (2009, 2011 and 2012) and Linaria dalmatica (2009), harvested from PHACE plots, located in Cheyenne, Wyoming
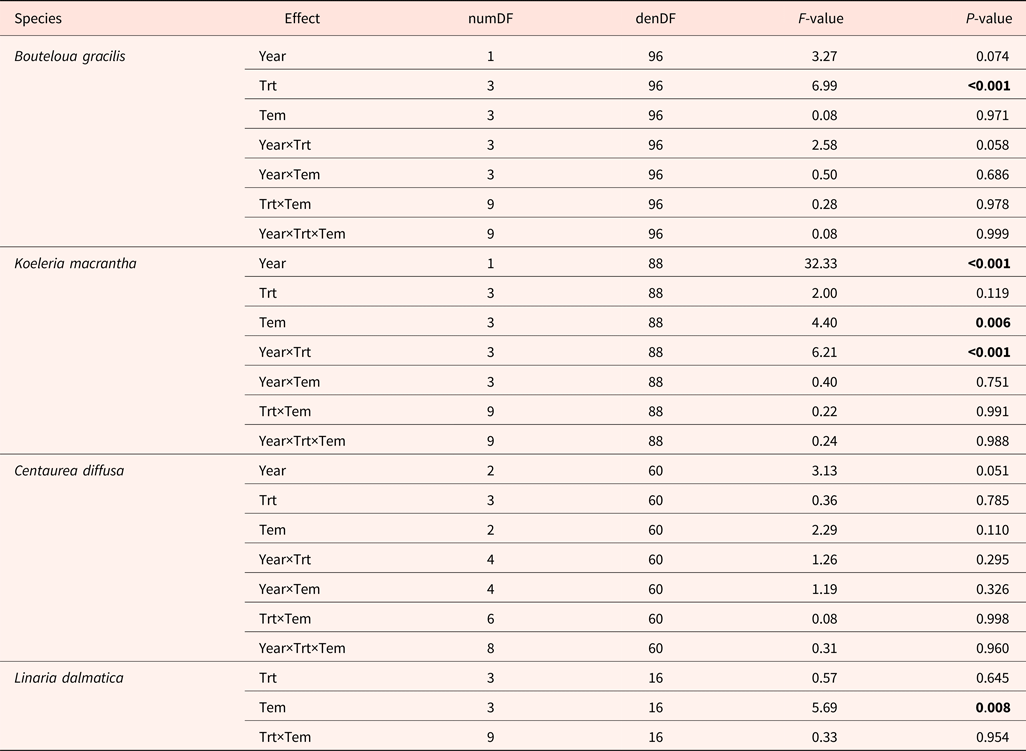
Field treatments (Trt) include ambient CO2, ambient temperature; ambient CO2, warming; elevated CO2, ambient temperature; and elevated CO2, warming; germination temperature treatments (Tem) are shown in Table 2. The bold font signify the P value that less than 0.05, indicating significant treatment effect.
Significant treatment effects on total germination were observed in B. gracilis (Tables 4 and 5). Neither CO2 enrichment nor warming alone affected total germination of B. gracilis, but their combination (CT) significantly increased germination from 36% in the control (ct) to 63% (Table 5). Inconsistent treatment effects were observed between 2010 and 2011 for K. macrantha (interaction P = <0.001). Warming alone significantly reduced germination of K. macrantha compared with the control in 2010 but not in 2011. The combination of CO2 and warming significantly reduced germination of K. macrantha compared with the control in 2011 but not in 2010 (Table 5). None of the treatments significantly affected germination of C. diffusa and L. dalmatica (Tables 4 and 5).
Table 5. Seed fill, seed mass, seed viability and total germination for Bouteloua gracilis (2007 and 2008), Koeleria macrantha (2010 and 2011), Centaurea diffusa (2009, 2011 and 2012) and Linaria dalmatica (2009), harvested from PHACE plots, located in Cheyenne, Wyoming
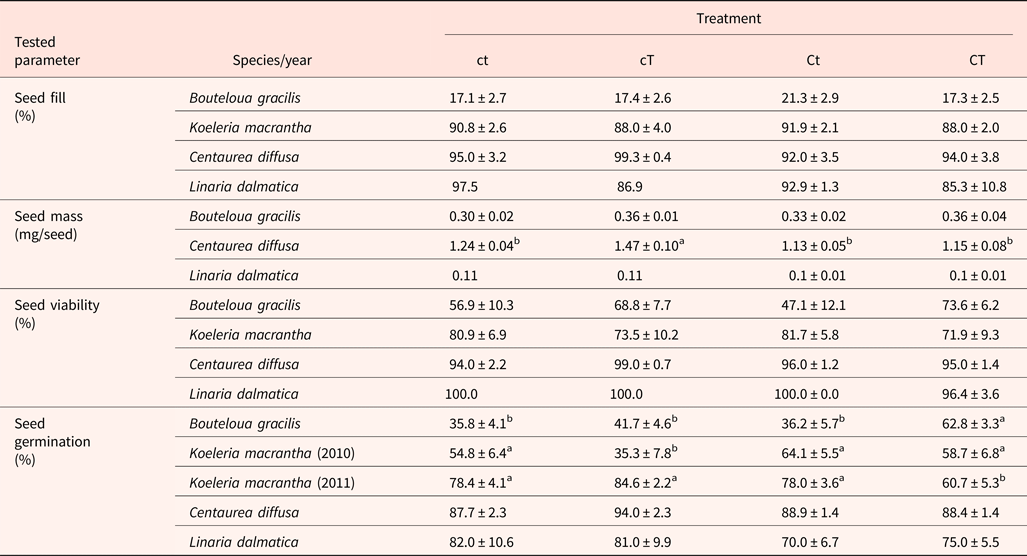
Treatments include ambient CO2, ambient temperature (ct); ambient CO2, warming (cT); elevated CO2 concentrations, ambient temperature (Ct); and elevated CO2, warming (CT).
Germination data were not significantly affected by the interaction between treatments and temperatures (Table 4) and was averaged across different temperature regimes.
The values are means ± standard error. Mean comparisons were done using Tukey's test. Different superscript letters indicate significant differences at α = 0.05.
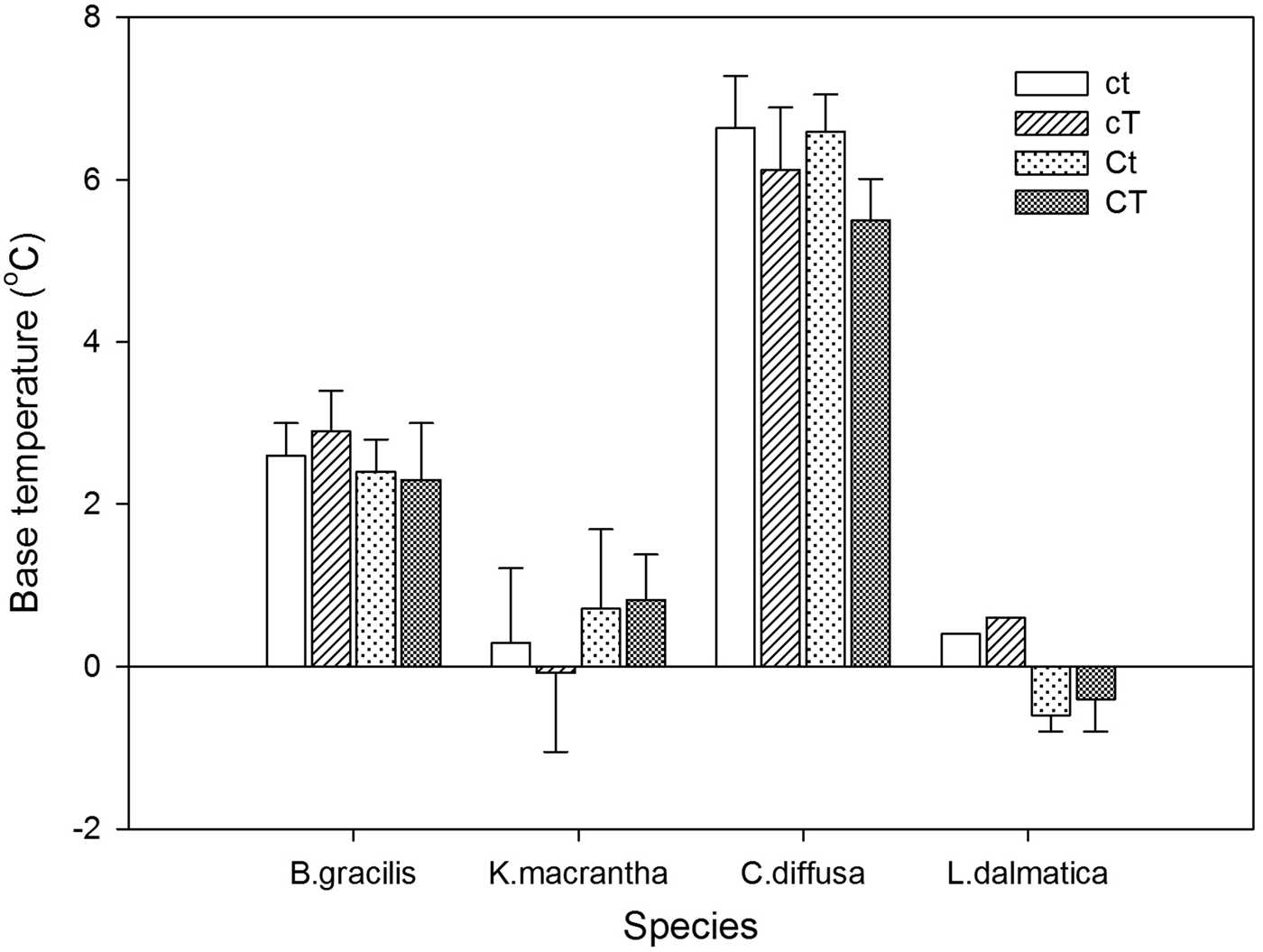
Base temperatures (T b) varied among species, with the lowest value in L. dalmatica (0°C) and the highest value in C. diffusa (6.2°C) (Fig. 1). T b was not affected by the interaction of year and climate change treatments (Table 6). Climate change treatments had no effect on T b.
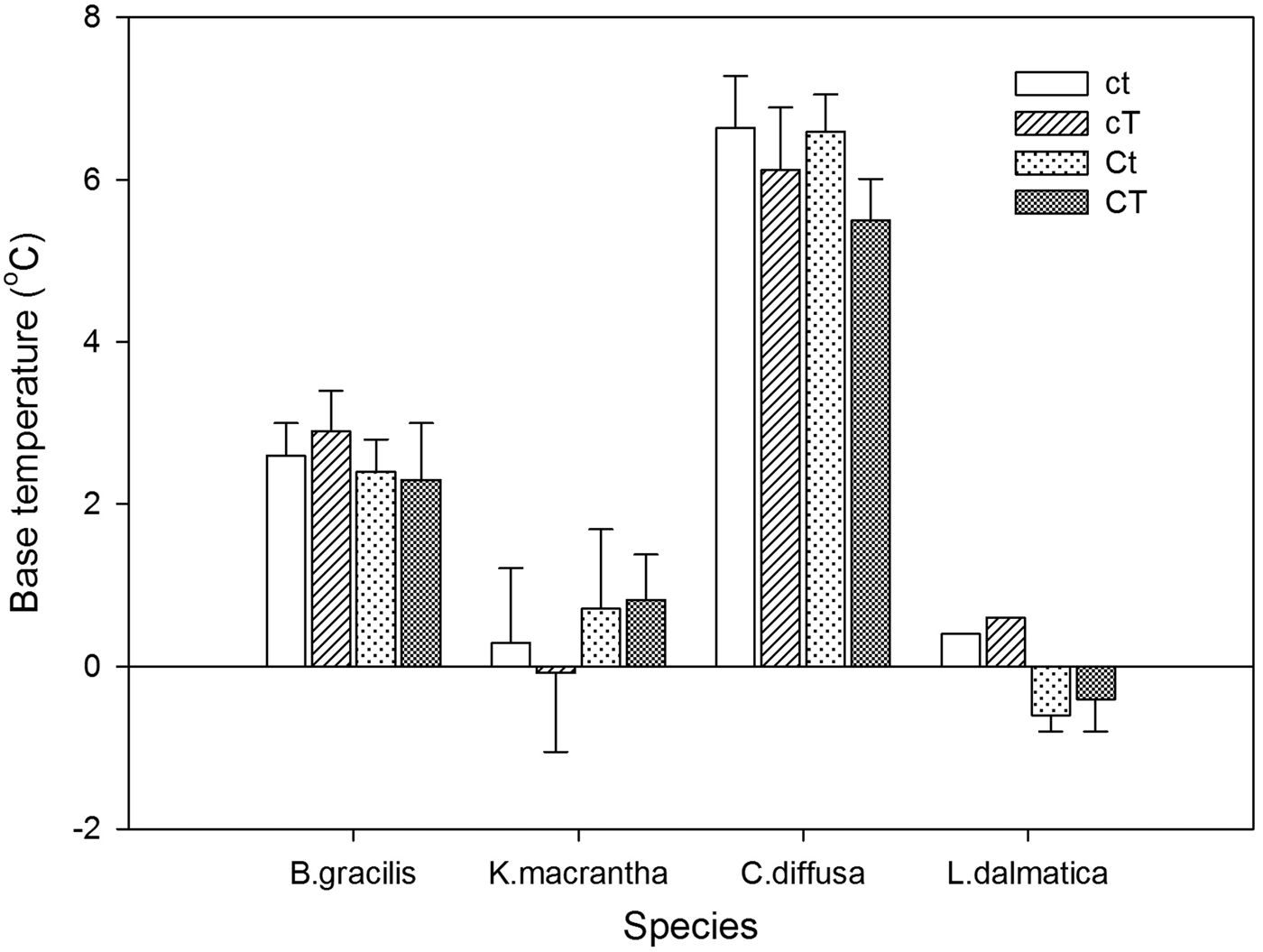
Figure 1. Base temperature (T b, °C) for Bouteloua gracilis (2007 and 2008), K. macrantha (2010 and 2011), Centaurea diffusa (2009, 2011 and 2012), and Linaria dalmatica (2009) from PHACE plots, located in Cheyenne, Wyoming. Treatments include ambient CO2, ambient temperature (ct); ambient CO2, warming (cT); elevated CO2 concentrations, ambient temperature (Ct); and elevated CO2 concentrations, warming (CT). The bars are means ± standard error. Mean comparisons were done using Tukey's test at P ≤ 0.05.
Table 6. ANOVA table for base temperature (T b) and thermal time requirement for 50% subpopulation germination (θ50) of Bouteloua gracilis (2007 and 2008), Koeleria macrantha (2010 and 2011), Centaurea diffusa (2009, 2011 and 2012) and Linaria dalmatica (2009), harvested from PHACE plots, located in Cheyenne, Wyoming
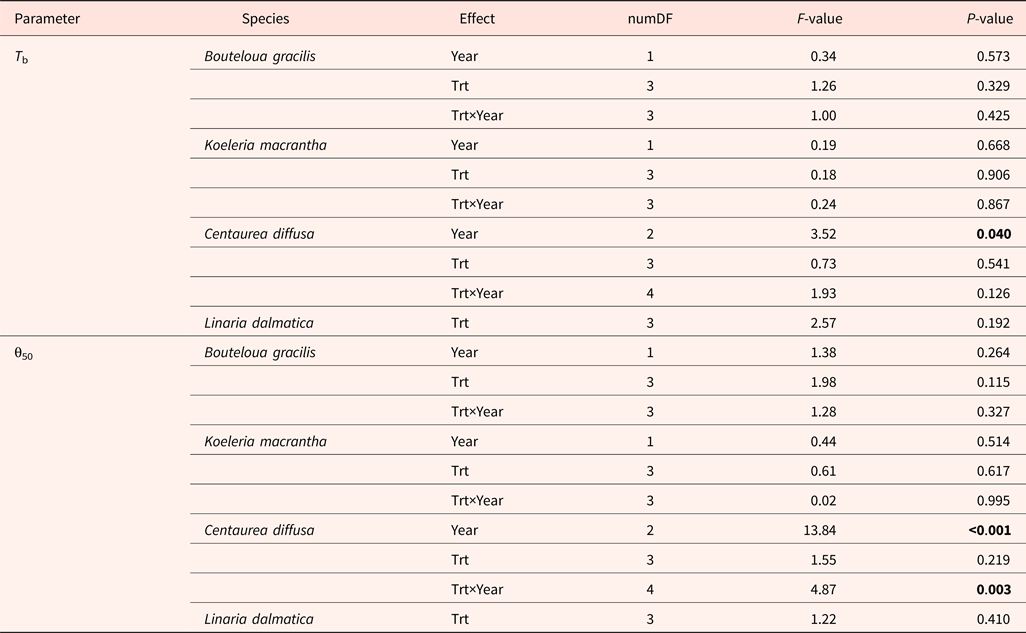
Treatments include ambient CO2, ambient temperature; ambient CO2, warming; elevated CO2, ambient temperature; and elevated CO2, warming. The bold font signify the P value that less than 0.05, indicating significant treatment effect.
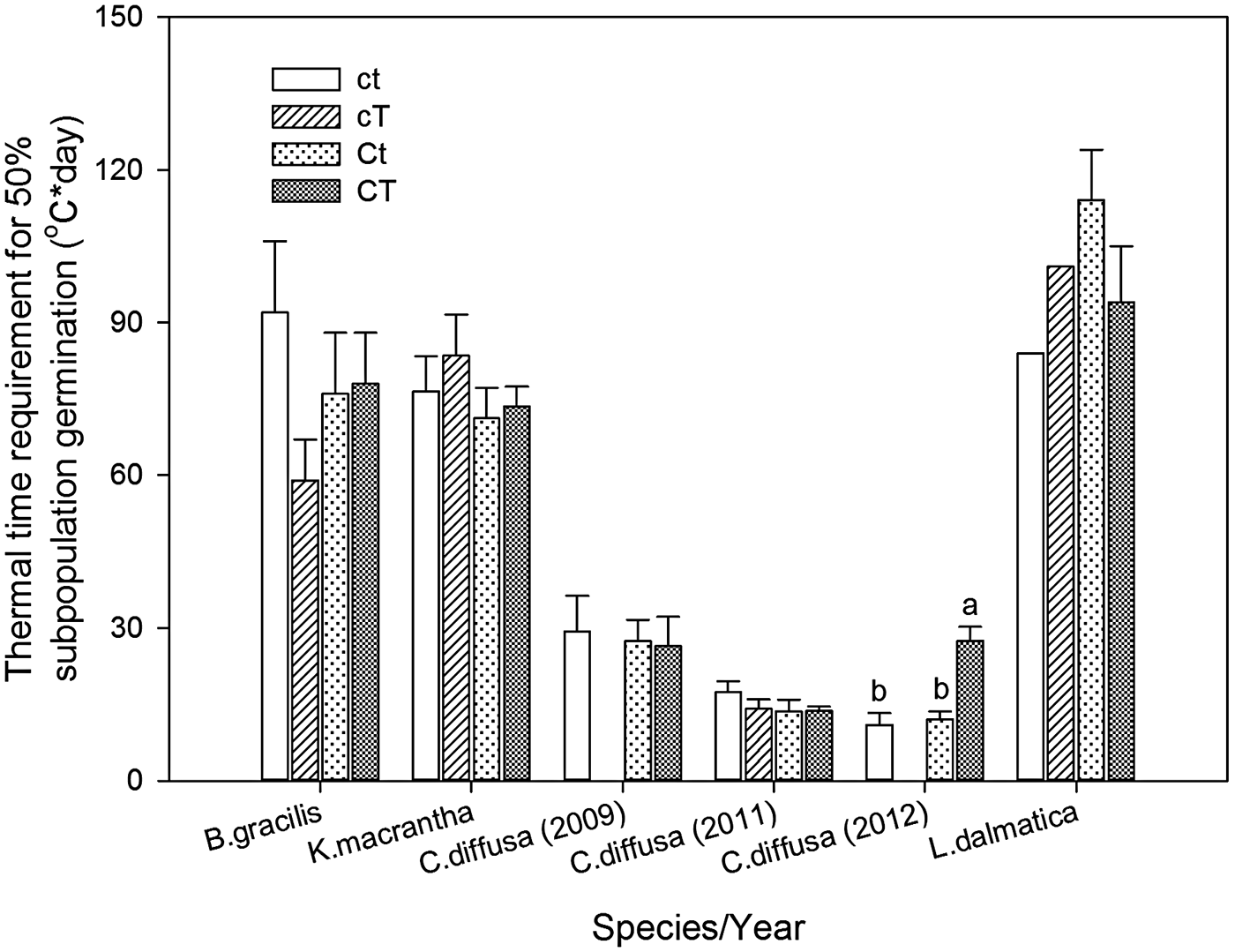
Centaurea diffusa had the smallest thermal time requirement to reach to 50% germination (θ50) (14.2°C day) among all species (Fig. 2). θ50 was not affected by the interaction of year and climate change treatments, except for that of C.diffusa (Table 6). Climate change treatments had a significant effect on θ50 of C. diffusa in 2012, where CT treatment significantly increased θ50 (Figs 1 and 2).
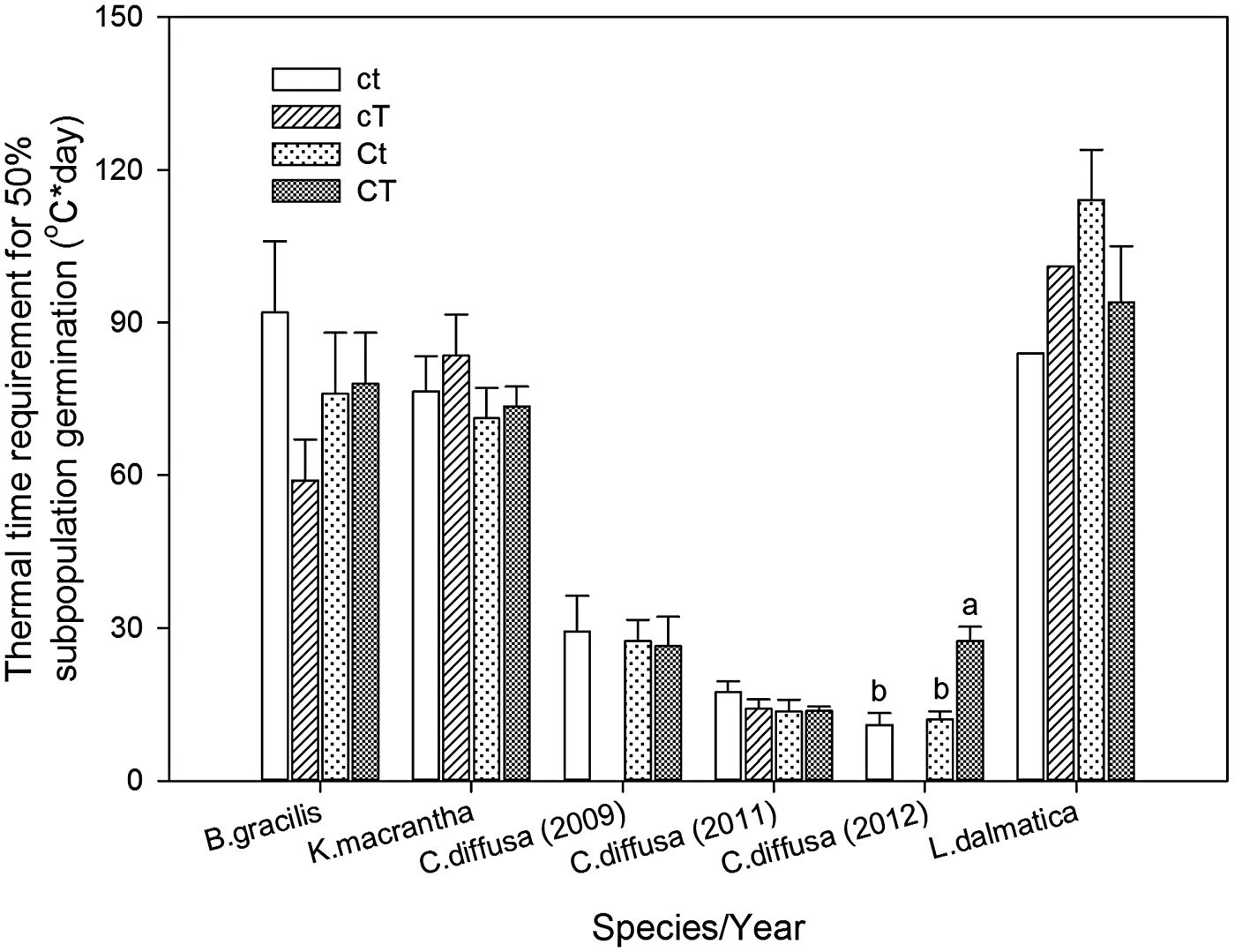
Figure 2. Thermal time requirement for 50% subpopulation germination (θ50, °C day) for Bouteloua gracilis (2007 and 2008), K. macrantha (2010 and 2011), Centaurea diffusa (2009, 2011 and 2012), and Linaria dalmatica (2009) from PHACE plots, located in Cheyenne, Wyoming. Treatments include ambient CO2, ambient temperature (ct); ambient CO2, warming (cT); elevated CO2 concentrations, ambient temperature (Ct); and elevated CO2 concentrations, warming (CT). The bars are means ± standard error. Means comparisons were done using Tukey's test. Different lower case letters indicate significant differences at α = 0.05.
Discussion
Seed traits as affected by climate change
Contrary to our hypotheses, CO2 enrichment and warming exerted limited effects on seed fill and seed mass. The only significant response was that warming alone increase per-seed mass of C. diffusa, an invasive species (Table 5). Previous studies reported that increased temperature can disrupt normal seed development, increasing the proportion of shrivelled and abnormal seeds, and reducing seed fill (Spears et al., Reference Spears, Tekrony and Egli1997; Young et al., Reference Young, Wilen and Bonham-Smith2004). In addition, plants grown in warmer environments tended to produce smaller seeds due to quicker seed filling and faster ripening (Fenner, Reference Fenner1992), although positive effects of warming on seed mass have been reported in Beta vulgaris L. (Wood et al., Reference Wood, Scott and Longden1980) and Glycine max (L.) Merr. (Seddigh and Jolliff, Reference Seddigh and Jolliff1984). However, some previous experiments use extremely high temperatures to simulate heat stress, which is bound to harm seed yield. Our result agrees with Hovenden et al. (Reference Hovenden, Wills, Vander Schoor, Chaplin, Williams, Nolan and Newton2007), who reported that seed mass of most species was not affected in a global climate change experiment in a grassland in Tasmania. The 1.5/3.0°C (day/night) rise in temperature in the present study is within the range of prediction for temperature increase in the Great Plains by 2090 (Loehman, Reference Loehman2009). This study found no evidence for an effect of increased temperature, indicating that global warming might have little effect on seed fill and mass for native species in northern mixed-grass prairie. However, the data for C. diffusa may be an indication that some invasive species in these grasslands could benefit from higher temperature by producing heavier seeds.
Although CO2 enrichment did not affect seed mass in this study, greater individual seed mass under elevated CO2 concentrations has been observed in many species, including crops (Bai et al., Reference Bai, Tischler, Booth and Taylor2003), pasture and old-field species (Newton, Reference Newton1991), and grasses (Huxman et al., Reference Huxman, Hamerlynck, Jordan, Salsman and Smith1998). For 79 crops and wild species, individual seed mass increased an average of 4% under CO2 enrichment (Jablonski et al., Reference Jablonski, Wang and Curtis2002). This is probably due to increased photosynthesis (Sage and Kubien, Reference Sage and Kubien2003), water use efficiency (Morgan et al., Reference Morgan, LeCain and Pendall2011), and plant assimilate availability (Jablonski et al., Reference Jablonski, Wang and Curtis2002). It should be noted, however, that earlier climate change studies were often conducted in growth chambers or glasshouses (Huxman et al., Reference Huxman, Hamerlynck, Jordan, Salsman and Smith1998), within one growing season (Garbutt and Bazzaz, Reference Garbutt and Bazzaz1984), and with very high CO2 concentrations (Bai et al., Reference Bai, Tischler, Booth and Taylor2003). General conclusions or hypotheses based on responses observed in controlled environment conditions should be observed with caution until validated through field experiments (Drake et al., Reference Drake, Rogers, Allen, Strain and Cure1985). Climate change studies using Open-top Chambers (OTC) or Free Air CO2 Enrichment (FACE) systems enabled field studies under manipulated CO2 concentrations with relatively stable performance (Newton, Reference Newton1991), and have often yielded different results from previous studies, such as species-specific effects on individual seed mass, rather than a general pattern of enhanced mass (Thürig et al., Reference Thürig, Körner and Stöcklin2003; Stiling et al., Reference Stiling, Moon, Hymus and Drake2004; Hovenden et al., Reference Hovenden, Wills, Vander Schoor, Chaplin, Williams, Nolan and Newton2007; Williams et al., Reference Williams, Wills, Janes, Vander Schoor, Newton and Hovenden2007).
Surprisingly, seed viability was not affected by climate change treatments. C/N ratio in vegetative tissues and seed is believed to affect seed protein content for embryo growth, which in turn can influence seed viability (Andalo et al., Reference Andalo, Godelle, Lefranc, Mousseau and Till-Bottraud1996; Bai et al., Reference Bai, Tischler, Booth and Taylor2003). Previous PHACE reports have found elevated CO2 to decrease and warming to increase soil and plant N (Dijkstra et al., Reference Dijkstra, Blumenthal, Morgan, Pendall, Carrillo and Follett2010; Mueller et al., Reference Mueller, Blumenthal, Pendall, Carrillo, Dijkstra, Williams, Follett and Morgan2016). We did not test C/N ratios in the seeds in this study due to seed number limitation.
Impacts of CO2 enrichment, warming, and the combination of these two on seed germination were consistent across a wide range of temperature regimes (Table 4). Seed germination response to warming and/or elevated CO2 concentrations is species specific, agreeing with previous studies (Huxman et al., Reference Huxman, Hamerlynck, Jordan, Salsman and Smith1998; Williams et al., Reference Williams, Wills, Janes, Vander Schoor, Newton and Hovenden2007; Gao et al., Reference Gao, Wang, Zhang, Dong and Guo2012; Marty and BassiriRad, Reference Marty and BassiriRad2014). Future climate condition (CT) favoured seed germination of C4 grass B. gracilis but not the three C3 species in this study. In contrast, CT reduced seed germination of K. macrantha in 2011. C4 plants generally have a higher thermal optimum for photosynthesis (Ehleringer et al., Reference Ehleringer, Cerling and Helliker1997; Sage and Kubien, Reference Sage and Kubien2007). The promoting effects of warming on C4 plants could be augmented by CO2 by offsetting warming-induced desiccation (Morgan et al., Reference Morgan, LeCain and Pendall2011; Blumenthal et al., Reference Blumenthal, Resco, Morgan, Williams, Lecain, Hardy, Pendall and Bladyka2013). In the early years of the PHACE study, when our seeds were sampled, CT also stimulated the above-ground growth of B. gracilis (Morgan et al., Reference Morgan, LeCain and Pendall2011). Our results indicate that B. gracilis may benefit from combined warming and CO2 enrichment in terms of seed germination. However, it should be noted that seedlings of B. gracilis are very susceptible to drought (Briske and Wilson, Reference Briske and Wilson1980). The adventitious root growth for seedling establishment of this species is affected by the combination of humidity and temperature (Briske and Wilson, Reference Briske and Wilson1978). When the humidity requirement is met, higher temperature could favour the seedling growth of B. gracilis (Briske and Wilson, Reference Briske and Wilson1978). There are important regional differences in terms of precipitation in the USA. It is projected that the northern states would experience more precipitation in the winter and spring, except for the Northwest in the spring, while the Southwest is projected to experience less precipitation, particularly in the spring (National Climate Assessment; http://nca2014.globalchange.gov/report/our-changing-climate/precipitation-change#statement-16555). Hence, the impact of the higher seed germination will depend on these combined temperature/precipitation patterns in different regions.
After 3 years exposure to CT, C3 graminoids were favoured, mainly due to the vegetative regeneration of perennials, reversing the initial shift towards C4 grasses (Mueller et al., Reference Mueller, Blumenthal, Pendall, Carrillo, Dijkstra, Williams, Follett and Morgan2016). Shaw et al. (Reference Shaw, Zavaleta, Chiariello, Cleland, Mooney and Field2002) reported that no effect of CO2 on net primary production of an annual grassland was observed until the third year. Therefore, long-term studies are needed to determine the contribution of regeneration from seeds on community dynamics of grasslands.
Seed regeneration of invasive species in the mixed-grass prairie affected by global climate change
Climate change treatments had limited effects on seed characteristics including germination percentage of C. diffusa and L. dalmatica, the two invasive species in our study. The combination of the highest T b and the lowest θ50 enables C. diffusa to finish germination quickly once it starts at relatively higher temperature. In contrast, L. dalmatica (2009) had the highest θ50 but lowest T b, indicating that the initiation of germination for this species may occur early in the spring when temperatures are low, but it takes longer to germinate. T b and θ50 of B. gracilis were intermediate among species (Figs 1 and 2).
Blumenthal et al. (Reference Blumenthal, Resco, Morgan, Williams, Lecain, Hardy, Pendall and Bladyka2013) reported that L. dalmatica was favoured under elevated CO2 with a 13-fold increase in biomass and a 32-fold increase in seed production. Similarly, elevated CO2 increased number of open flowers, seed heads and seeds of C. diffusa 5-, 3- and 3-fold, respectively (Reeves et al., Reference Reeves, Blumenthal, Kray and Derner2015). Hence, elevated CO2, both with and without warming, seems to favour seed reproduction of C. diffusa and L. dalmatica quantitatively rather than qualitatively. Invasive species are often opportunistic and able to respond to environmental changes (Dukes and Mooney, Reference Dukes and Mooney1999; Bradley et al., Reference Bradley, Blumenthal, Wilcove and Ziska2010; Shine, Reference Shine2011; Walck et al., Reference Walck, Hidayati, Dixon, Thompson and Poschlod2011), but our results suggest that seed traits and germination play little role in this opportunism for these two grassland invaders.
Our results also indicated that C. diffusa and L. dalmatica had different recruitment strategies. Centaurea diffusa had lowest θ50 and L. dalmatica had lowest T b required for germination among the four species studied, respectively. Species with a lower T b can germinate early in spring, giving it competitive advantage during seedling establishment. Vujnovic and Wein (Reference Vujnovic and Wein1997) also reported that L. dalmatica can germinate at low temperatures. Species with a lower θ50 can finish seed germination more quickly, reducing the likelihood of predation or damage from adverse environmental conditions.
In conclusion, the combination of warming and CO2 enrichment enhanced seed germination of the C4 species B. gracilis which could promote its ability to regenerate over long timeframes in mixed-grass prairie under climate change. However, the timing of precipitation in conjunction with spring warming could determine in large part whether or not B. gracilis seedlings would be successful. Invasion by non-native species such as L. dalmatica and C. diffusa could increase under future climate conditions, possibly due to increased seed production but not seed regeneration per se, which might reduce biodiversity and reduce the ecosystem health potentially.
Acknowledgements
We thank D. Smith, E. Hardy, E. Pendall and D. Williams for installation and operation of the Prairie Heating and CO2 Enrichment (PHACE) experiment, and M. Parsons and J. Kray for seed collection. The summer students and laboratory technicians at the University of Saskatchewan are thanked for their dedicated support to the laboratory experiments. Dr Y. Wei and Dr J. Qiu are thanked for their useful comments on model construction and data analysis.
Financial support
This research is principally supported by the USDA-ARS Climate Change, Soils and Emissions Program, by the US Department of Energy's Office of Science (BER) through the Western Regional Center of the National Institute for Climatic Change Research at Northern Arizona University, and by NSF (DEB #1021559).