Introduction
Chagas disease is a parasitic infection caused by the protozoan Trypanosoma cruzi, which affects about 8 to 10 million people, mainly in Central and South America. The disease causes disability in infected individuals and more than 10,000 deaths per year. In Brazil, is estimated about 1.9 to 4.6 million infected people, with about 6,000 deaths annually. Changes in the epidemiological profile of the disease have occurred, with the emergence of cases and outbreaks in the Amazon region by oral and vector transmission (BRASIL, 2015; WHO, Reference World Health Organization2019).
The main complications of the disease are syndromes affecting the cardiac, digestive, neurological and endocrine systems. The cardiac system is the most compromised, due to the strong tropism of T. cruzi for cardiomyocytes (Andrade, Reference Andrade1999). Chronic chagasic cardiopathy (CCC) is the most frequent manifestation and affects about 30 to 40% of people infected by the protozoan (Andrade, Reference Andrade1999; Marin-Neto et al., Reference Marin-Neto, Rassi, Avezum, Mattos, Rassi, Morillo, Sosa-Estani and Yusuf2009; WHO, Reference World Health Organization2019). Death from chronic Chagas disease often results from congestive heart failure following the development of myocardial dysfunction, caused by chronic inflammation (Andrade, Reference Andrade1999; Teixeira et al., Reference Teixeira, Nascimento and Sturm2006).
The pathogenesis of CCC is related to myocardial changes, which are dependent on the parasite persistence and immune response (Marin-Neto et al., Reference Marin-Neto, Cunha-Neto, Maciel and Simões2007). Patients with CCC present several cardiovascular disorders, which are consequences of three main processes namely: inflammation, cell death and fibrosis (Marin-Neto et al., Reference Marin-Neto, Rassi, Avezum, Mattos, Rassi, Morillo, Sosa-Estani and Yusuf2009).
The cellular inflammatory response, the induction of a humoral autoimmune response (due to the ability of the parasite to mimic the host proteins) and the oxidative stress generated by T. cruzi infection, can lead to an increase in the parasite replication rate and consequent damage to the cardiac tissue (Paiva et al., Reference Paiva, Feijó, Dutra, Carneiro, Freitas, Alves, Mesquita, Fortes, Figueiredo, Souza, Fantappié, Lannes-Vieira and Bozza2012; Goes et al., Reference Goes, Rocha, Diniz, Aguiar, Machado and Vieira2016). Several autoantibodies have been described, among them anti-p2b and anti-B13, which play a pathogenic role in the production of lesions in cardiac tissue, both in animal model and humans (Yoshikawa et al., Reference Yoshikawa, Baba and Nagatomo2009; Cunha-Neto et al., Reference Cunha-Neto, Teixeira, Nogueira and Kalil2011).
While the main therapy for the treatment of Chagas disease is benznidazole (BZN), new triazole derivatives have emerged as promising therapies (Ribeiro et al., Reference Ribeiro, Nunes, Teixeira and Rocha2012; Campos-Estrada et al., Reference Campos-Estrada, Liempi, González-Herrera, Lapier, Kemmerling, Pesce, Ferreira, López-Muñoz and Maya2015). New therapies propose the reduction of the treatment regimen or the application of doses to slow the progress of the disease. Campos-Estrada et al. (Reference Campos-Estrada, Liempi, González-Herrera, Lapier, Kemmerling, Pesce, Ferreira, López-Muñoz and Maya2015) have demonstrated that the combination of simvastatin and BNZ prevents endothelial activation induced by T. cruzi infection. This study demonstrated that the anti-inflammatory effect of simvastatin is mediated by the inhibition of the nuclear kappa B factor, thereby improving endothelial function (Campos-Estrada et al., Reference Campos-Estrada, Liempi, González-Herrera, Lapier, Kemmerling, Pesce, Ferreira, López-Muñoz and Maya2015).
Antioxidant therapy can be a promising approach for chronic chagasic patients once to oxidative stress apparently contributes to disease progression. Treatment with BZN involves the generation of reactive species and vitamin E depletion. Antioxidant therapy using supplementation with vitamin E after BZN treatment in patients with Chagas' heart disease reduced levels of thiobarbituric acid reactive substances (TBARS), carbonyl protein (PC), nitric oxide (NO) and reduced glutathione (GSH), inhibiting superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR) activities, as well as inflammatory markers, in stages of lower cardiac impairment (Ribeiro et al., Reference Ribeiro, Budni, Pedrosa, Farias, Parisotto, Dalmarco, Fröde, Oliveira-Silva, Colepicolo and Filho2010). On the other hand, in acute Chagas cardiomyopathy, studies in Swiss mice infected by T. cruzi and treated using vitamins C and E supplementation showed an irrelevant control of reactive tissue damage and myocarditis severity in infected animals (Novaes et al., Reference Novaes, Santos, Fialho, Gonçalves, Sequetto, Talvani and Gonçalves2017).
Vilar-Pereira et al. (Reference Vilar-Pereira, Carneiro, Mata-Santos, Vicentino, Ramos, Giarola, Feijó, Meyer-Fernandes, Paula-Neto, Medei, Bozza, Lannes-Vieira and Paiva2016) report that the treatment with resveratrol improved the cardiac function of mice in a chronic infection stage, activating the adenosine monophosphate-activated protein kinase (AMPK)-pathway and reducing reactive oxygen species (ROS) production, lipid peroxidation and heart parasite load, without altering the heart inflammatory infiltrates or vascularisation. When the authors used metformin (AMPK activator and a cardioprotective drug) and tempol (SOD-mimetic drug that efficiently neutralises ROS) to mimic the beneficial effects of resveratrol on heart function in the CCC mice model, they observe a better cardiac function but without altering heart parasite load. This study indicates that SOD mimetic activity and AMPK activation can be used as therapeutic strategies in Chagas heart disease and represents a starting point for the development of innovative therapies for CCC (Vilar-Pereira et al., Reference Vilar-Pereira, Carneiro, Mata-Santos, Vicentino, Ramos, Giarola, Feijó, Meyer-Fernandes, Paula-Neto, Medei, Bozza, Lannes-Vieira and Paiva2016).
Anti-inflammatory therapy using Ibuprofen to control oxidative/nitrosative stress and heart disease in mice reduced lipid and protein oxidation, antioxidant enzyme activity tissue, as well as levels of cytokines, PGF2α, NO and cardiac damage, without to alter cardiac parasitism. When used to control the inflammatory infiltrate, anti-inflammatory therapy was shown to be more rational than antioxidant therapy in the attenuation of oxidative/nitrosative stress and cardiac damage (Novaes et al., Reference Novaes, Santos, Fialho, Gonçalves, Sequetto, Talvani and Gonçalves2017).
Although there is evidence regarding the cellular and molecular mechanisms involved in the anti-inflammatory and antioxidant therapy and its benefits to treatment of CCC, there is not a standardisation of the use of these substances. Because of this, there is a need for a systematic approach to identify all the available research from clinical trials, for the purpose of summarising current knowledge and guiding a new path for research, such as to suggest an anti-inflammatory and/or antioxidant therapeutic strategy as part of the treatment of Chagas disease.
Methodology
A systematic literature review was performed based on the parameters of the Cochrane systematic review guide (Clarke and Horton, Reference Clarke and Horton2001; Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009; Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009).
Information sources and search strategy
A bibliographic search of articles published from 1970 to April 2018 was carried out in the following regional and international databases: MEDLINE, EMBASE, WEB OF SCIENCE, SCOPUS, LILACS and CENTRAL (Cochrane Library), without language restrictions. Reference lists were reviewed to identify possible additional studies. Abstracts were included if they provided complete information to allow their evaluation. The descriptors used were ‘Chagas cardiomyopathy’, ‘therapeutics’, ‘Chagas disease’, ‘anti-inflammatory agents’, ‘Trypanosoma cruzi’ and ‘antioxidants’. All possible combinations of these terms were applied.
Eligibility criteria and participants
Controlled, randomised or quasi-randomised trials were included, in the form of cohort studies, cases and controls, cross-sectional cut-off studies and case series. Only studies with populations aged >18 years and with at least 10 cases were included. The inclusion criteria were: clinical studies, treatment with anti-inflammatory agents, antioxidant therapy with vitamin C and E supplementation, patients with chagasic myocarditis. The anti-inflammatory substances were verified in PubMed Mesh and PubChem.
Data collection, article selection and data extraction
All phases of the selection and processing of the study were carried out with the Cochrane Covidence software program. Before data extraction, peer reviewers selected the articles independently, evaluating their titles and abstracts according to pre-specified criteria. The discrepancies were resolved by consensus with the research team. When necessary, the authors were contacted for additional information.
Assessment of risk of bias
The risk of bias was assessed using the Cochrane Risk of Bias checklist. The items evaluated were: selection, allocation and obscuring of participants, blindness of outcome assessors, incomplete outcome data, control of confounding factors, measurement of exposure and results and conflicts of interest. Pairs of independent reviewers evaluated the methodological quality and the discrepancies were resolved through consensus among the team.
Results
A total of 4,138 articles was identified during the search, of which six were included in data extraction. Of these, four were related to antioxidant therapy with vitamin C and E supplementation and two articles addressed anti-inflammatory therapy (Fig. 1). Three randomised clinical trial protocols were also found, but the complete articles had not been published (Table 1).
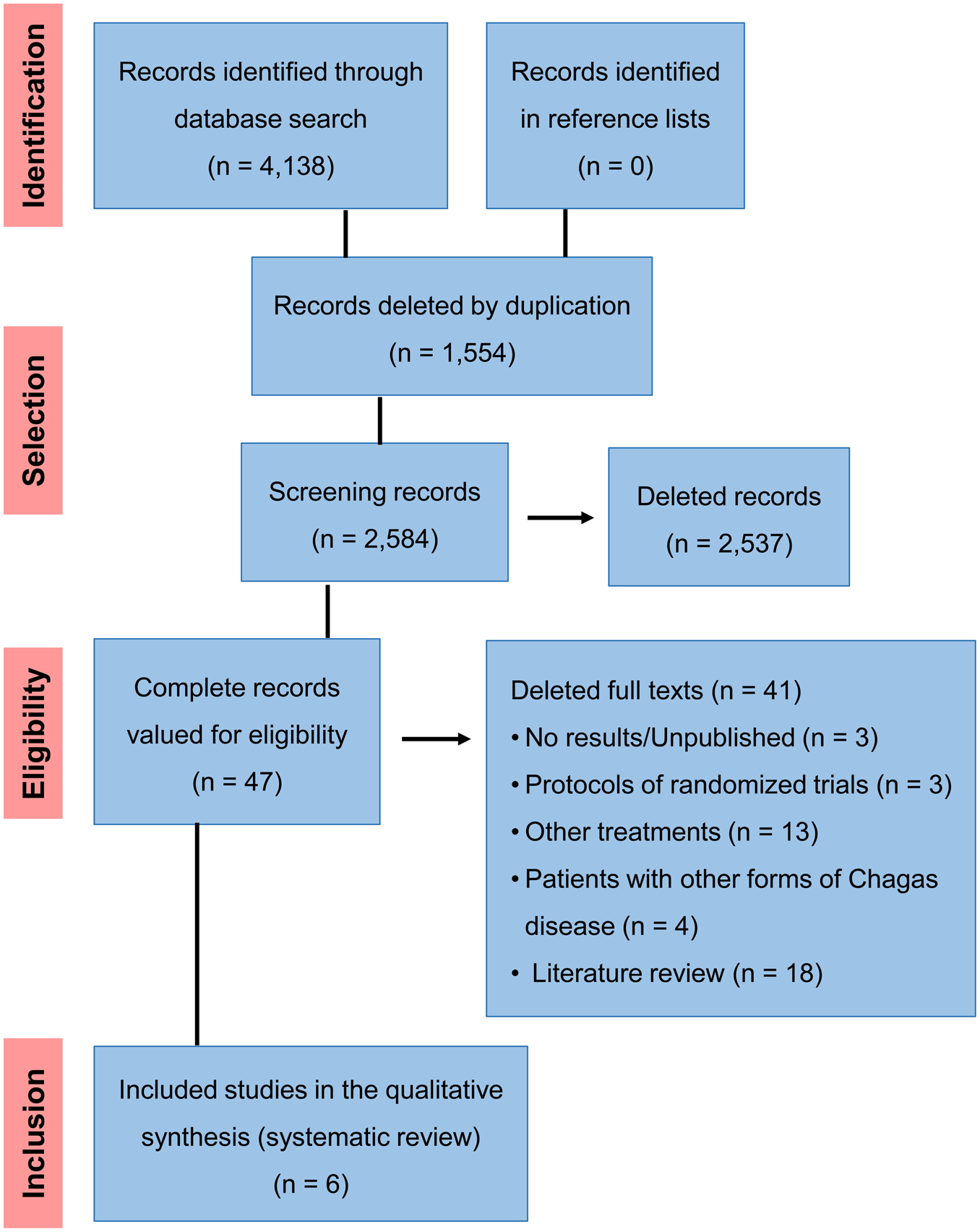
Fig. 1. Flow diagram of the studies.
Table 1. Research protocols for anti-inflammatory and antioxidant therapy for chronic chagasic myocarditis

The studies included were conducted in Brazil and were published between 2002 and 2017. The risk of low bias according to modified Cochrane Risk of Bias Tool did not apply, as the studies in question were a quasi-experimental and prospective cohort study, and one study that included a case series (Table 2). Of the included studies, the mean follow-up time ranged from 2 to 65.5 months. Three studies did not describe the population, making it impossible to analyse the population as a whole (Table 3).
Table 2. Bias risk assessment by modified Cochrane risk of bias tool

NA, not applicable; NC, not clear.
Table 3. Main characteristics of the population

The studies on antioxidant therapy included were almost experimental and of the prospective cohort type. All used the same patients and type of intervention, namely 500 mg day−1 vitamin C and 800 IU day−1 vitamin E. Patients with Chagas cardiomyopathy were classified according to the modified Los Andes classification and reviewed by Xavier (Reference Xavier1999). They were classified into four groups: Group I (normal electrocardiogram and echocardiogram: no involvement of the heart); group II (normal/borderline electrocardiogram and abnormal echocardiogram: mild cardiac involvement), group III (abnormal electrocardiogram and echocardiogram without congestive heart failure: moderate cardiac involvement) and group IV (abnormal electrocardiogram and echocardiogram with congestive heart failure: severe cardiac functional class IV of the New York Heart Association).
The use of the carvedilol alone or combined with vitamins E and C supplementation was used to treat patients with CCC and investigate its antioxidant effect and consequent reduction of the oxidative stress. The authors showed that both treatments were effective in reducing systemic oxidative stress (reduction in TBARS and PC levels, as well as of most antioxidant enzymes activity) in the blood of CCC patients. However, the combined use was more significant in reducing the oxidative damage and indicates the possibility of synergism between these compounds. On the other hand, both treatments were apparently unable to contain the evolution of the inflammatory process, as indicated by the increase of adenosine deaminase (ADA) and myeloperoxidase (MPO), but attenuated systemic damage in patients with CCC, especially among those less cardiac involvement (Budni et al., Reference Budni, Pedrosa, Dalmarco, Dalmarco, Frode and Wilhelm Filho2013).
The study performed by Maçao et al. (Reference Maçao, Wilhelm Filho, Pedrosa, Pereira, Backes, Torres and Fröde2007) is a continuation of the work of Oliveira et al. (Reference Oliveira, Pedrosa and Filho2007) with the same patients, who exhibited an increase in oxidative stress associated with disease progression (Maçao et al., Reference Maçao, Wilhelm Filho, Pedrosa, Pereira, Backes, Torres and Fröde2007; Oliveira et al., Reference Oliveira, Pedrosa and Filho2007). The results indicate that antioxidant supplementation was able to neutralise or attenuate the progressive oxidative stress associated with the disease and BZN treatment. There were increased of vitamin E, PC and TBARS levels, as well as an inhibition of SOD, GPx and GR activities, and inflammatory markers in the plasma, mainly in the cardiac involvement stages. New treatment perspectives may include antioxidant therapy in order to mitigate the consequences of the oxidative stress in the CCC (Table 4).
Table 4. Summary of the main results of antioxidant therapy in patients with Chagas cardiomyopathy
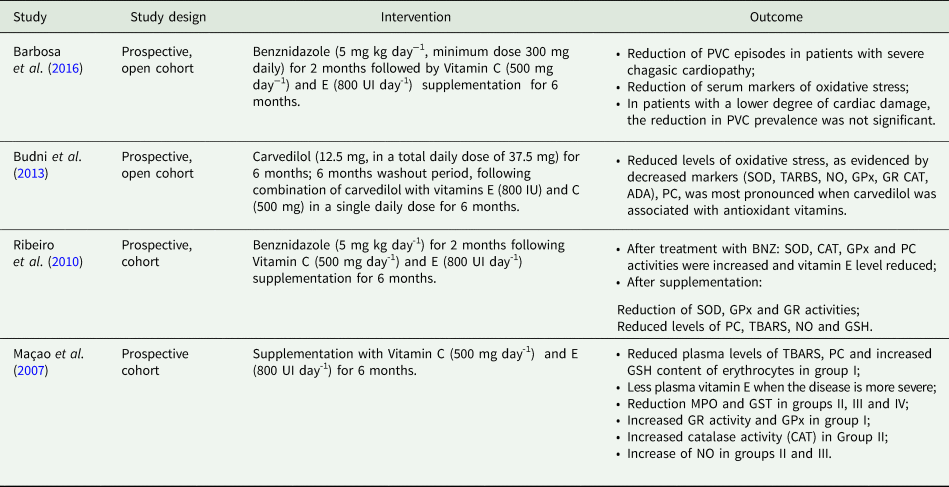
Plasma levels of TBARS, PC, glutathione S-transferase (GST) and NO were determined after combined vitamin supplementation, as well as SOD, GPx, GR and MPO. There was increased GSH in the erythrocytes. It was found that the lower the severity of the disease, the greater the amount of vitamin E in the plasma (Table 5).
Table 5. Comparison of the results according to the severity of the disease after vitamin supplementation

The anti-inflammatory agents used in the included studies were acetylsalicylic acid (ASA) and betamethasone (Rassi et al., Reference Rassi, Amato Neto, de Siqueira, Ferriolli Filho, Amato, Rassi and Rassi Junior2002; Sousa et al., Reference Sousa, Xavier, de Freitas and Moreno2008). There is a lack of evidence about the use of anti-inflammatory drugs in patients with CCC. In the study by Sousa et al. (Reference Sousa, Xavier, de Freitas and Moreno2008), ASA was used as an anticoagulant therapy for the prevention of cardioembolic vascular strokes in Chagas disease, while the study by Rassi et al. (Reference Rassi, Amato Neto, de Siqueira, Ferriolli Filho, Amato, Rassi and Rassi Junior2002) presents a risk of non-clear bias due to involving a series of cases (Tables 2 and 6).
Table 6. Summary of the main results regarding the use of anti-inflammatory agents in patients with Chagas cardiomyopathy

Discussion
The present study summarises the available evidence on the use of anti-inflammatory and antioxidant therapy with vitamin supplementation. The studies suggest that antioxidant therapy with vitamin C and E supplementation leads to the reduction of oxidative markers.
Vitamin E deficiency induces changes in leukocyte levels and exacerbates myocarditis and sympathetic denervation of ventricular hearts in infected rats, while significant leucopenia is evidenced by the decline in the number of B cells and T lymphocytes in the peripheral blood, and there is the induction of monocytosis, as well as an increased rate of the differentiation of monocytes into macrophages (Carvalho et al., Reference Carvalho, Camargos, Almeida, Peluzio Mdo, Alvarez-Leite, Chiari and Reis2006).
In experimental Chagas disease, animals treated with a dose of 500 mg of vitamin C exhibited greater tissue damage in the chronic phase of Chagas disease, possibly due to the paradoxical actions of the substance, which can act as a pro-oxidant or pro-inflammatory agent (Marim et al., Reference Marim, de Gusmão, Castanho, Deminice, Therezo, Jordão Júnior, de Assis, Taipeiro and Martins2015).
Other study using Swiss mice infected by T. cruzi and treated with vitamins C, E, or both (C/E) for 60 and 120 days showed an increase in the iron reduction capacity of plasma (FRAP) and GSH levels during the acute phase. In the chronic phase, a reduction of GSH levels was observed in the vitamin E treated group. Vitamin C/E treated group showed a reduction of TBARS. The antioxidant action of vitamins C and E reduced oxidative stress in the acute and chronic phases of Chagas disease with a marked effect following co-administration, indicating inherent synergism (Tieghi et al., Reference Tieghi, Manca, Garcia, Castanho, Therezo, Frei, Taipeiro and Martins2017).
Mimicking the effects of resveratrol on cardiac function with metformin and tempol, resulted in a reduction in lipid peroxidation but no changes in the intensity of parasite load, indicating that AMPK activation and neutralisation of ROS are key strategies for inducing tolerance to Chagas heart disease. Despite all the tissue damage observed in established Chagas heart disease, it was observed that physiological dysfunction can still be reversed by treatment with resveratrol, metformin and tempol, resulting in better cardiac function and representing a starting point for developing innovative therapies (Vilar et al., Reference Vilar-Pereira, Carneiro, Mata-Santos, Vicentino, Ramos, Giarola, Feijó, Meyer-Fernandes, Paula-Neto, Medei, Bozza, Lannes-Vieira and Paiva2016).
The progression of Chagas disease suggests an antioxidant depletion evidenced by reduced GSH concentrations in parallel with the progression of the disease and higher GPx activities in patients with moderate and severe disease involvement. In contrast, the antioxidant enzymes SOD, CAT and GR activities were not significantly different when compared with the chagasic grade of the patients (Oliveira et al., Reference Oliveira, Pedrosa and Filho2007). Increased ROS may compromise intracellular growth of T. cruzi. Studies have shown that oxidative stress generated by T. cruzi infection may lead to increased replication and may be due to thee bioavailability of iron (Paiva et al., Reference Paiva, Feijó, Dutra, Carneiro, Freitas, Alves, Mesquita, Fortes, Figueiredo, Souza, Fantappié, Lannes-Vieira and Bozza2012; Goes et al., Reference Goes, Rocha, Diniz, Aguiar, Machado and Vieira2016).
The behaviour of two clonal populations of T. cruzi (Col1.7G2 – T. cruzi I and JG – T. cruzi II) during infection of primary cultures of BALB/c embryonic cardiomyocytes showed that JG is a strain with strong tropism for cardiomyocytes presenting a higher rate of intracellular multiplication than Col1.7G2 parasites, which invade more heart cell cultures (Andrade et al., Reference Andrade, Machado, Chiari, Pena and Macedo1999, Reference Andrade, Galvao, Meirelles, Chiari, Pena and Macedo2010). After infection, cultured cardiomyocytes may increase the production of oxidative species, and their levels are higher in cultures infected with JG, which expresses lower levels of antioxidant enzymes. Inhibition of oxidative stress severely interferes with the intracellular multiplication rate of the JG population and suggests that JG and Col1.7G2 may sense extracellular oxidants in a distinct manner, which would then interfere differently with their development in cardiomyocytes (Dias et al., Reference Dias, Capila, Couto, Estrada, Gadelha, Radi, Piacenza and Andrade2017).
The role of ROS during T. cruzi infection remains contradictory. The antioxidant pathways observed during the intracellular infectious stages of protozoa of the genus Leishmania (family Trypanosomatidae) function as defence mechanisms against the respiratory burst produced by macrophages. It has been shown that ROS also play an important role in the development of the parasite and are important for its differentiation from promastigote to virulent amastigote forms (Mittra et al., Reference Mittra, Cortez, Haydock, Ramasamy, Myler and Andrews2013).
A similar mechanism occurs with T. cruzi, since the oxidative stress generated during the infection of human cardiomyocytes contributes to the intracellular development of the JG in the cells, and the inhibition of oxidative stress interferes with the intracellular multiplication rates (Dias et al., Reference Dias, Capila, Couto, Estrada, Gadelha, Radi, Piacenza and Andrade2017). Thus, although some studies have suggested that the ROS produced during the respiratory burst play an important role in the control of T. cruzi infection (Piacenza et al., Reference Piacenza, Alvarez, Peluffo and Radi2009; Alvarez et al., Reference Alvarez, Peluffo, Piacenza and Radi2011), other authors have demonstrated that ROS are important for the signalling and proliferation of the parasite (Andrews, Reference Andrews2012; Paiva et al., Reference Paiva, Feijó, Dutra, Carneiro, Freitas, Alves, Mesquita, Fortes, Figueiredo, Souza, Fantappié, Lannes-Vieira and Bozza2012).
The included clinical studies revealed that the strategy of antioxidant therapy effectively attenuates cardiac damage. However, there is a need for further studies with patients from different regions. In relation to anti-inflammatory therapy, the studies used betamethasone and AAS as anti-inflammatory agents. It is therefore impossible to reach a conclusion on recommendations regarding the use of these drugs, due to the lack of studies with methodological quality.
Animal experimental studies demonstrated that vitamin C and E supplementation was irrelevant in neutralising reactive tissue damage and myocarditis in infected animals. In contrast, ibuprofen treatment reduced tissue levels of cytokines, PGF2α and NO, as well as lipid and protein oxidation, antioxidant enzyme activity and cardiac damage, without interfering with cardiac parasitism (Novaes et al., Reference Novaes, Santos, Fialho, Gonçalves, Sequetto, Talvani and Gonçalves2017).
However, studies have shown that the administration of ASA prior to infection appeared to prevent the behavioural changes induced by acute infection, but led to accelerated mortality (Silvero-Isidre et al., Reference Silvero-Isidre, Morínigo-Guayuán, Meza-Ojeda, Mongelós-Cardozo, Centurión-Wenninger, Figueredo-Thiel, Sanchez and Acosta2018). Molina-Berríos et al. (Reference Molina-Berríos, Campos-Estrada, Lapier, Duaso, Kemmerling, Galanti, Ferreira, Morello, López-Muñoz and Maya2013) found that aspirin does not affect parasitaemia or mortality in infected mice, but reduces both cardiac inflammatory infiltrates and thromboxane levels. In addition, at 90 days post-infection, aspirin normalised the levels of soluble intercellular adhesion molecule-1 and sE-selectin, indicating that this drug may be included in the clinical treatment of chronic Chagas disease.
Limitations and potentialities
The present study has some limitations. It was not possible to draw conclusions about the contribution of vitamin supplementation or the possible additive or synergistic effect of its combined use. Despite the search for evidence published in electronic databases, there are omissions of unpublished, non-indexed or private records.
The lack of clinical studies with accuracy and with populations from different regions reduces the possibility of the inclusion of anti-inflammatory therapy. The main limitation of the present study is therefore the scarcity of randomised clinical studies on anti-inflammatory therapy in patients with CCC. In addition, most of the information is taken from Brazil, a country with relatively high rates of Chagas disease.
Despite these limitations, our study has several strengths. One of them is the methodological analysis, including a highly sensitive search strategy. Consequently, our systematic review exceeds the scope of previous narrative reviews by including a much larger number of references.
Final considerations
There are no conclusive data to support the use of vitamin supplementation and anti-inflammatory agents for the treatment of chagasic cardiomyopathy. Unless randomised clinical trials provide evidence of a treatment effect, and the risks and potential benefits are established, policy makers, clinicians and academics should be cautious in recommending and administering vitamin C and E supplementation for the treatment of heart failure in patients with Chagas disease. The efficacy and safety of pharmacological anti-inflammatory interventions in the treatment of chagasic myocarditis in patients are unknown.
There is a lack of randomised clinical trials, especially for anti-inflammatory therapy, indicating caution when interpreting results. The included studies indicate the possibility of vitamin supplementation as a complementary treatment of Chagas disease and also the use of other medicinal products that may possess an antioxidant action, whether combined or not with this supplementation. Antioxidant therapy has proven to be a viable alternative for attenuating the oxidative stress of CCC, aiming to achieve a protective profile resulting in better patient prognosis.
The present research indicates the need for studies of adequate methodological quality with representative samples from different regions, which describe the studied population and other relevant prognostic factors, such as cardiac electrical function analysis, to establish the most effective therapeutic strategy.
Financial support
This research received no specific grant from any funding agency, the commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.










