Introduction
Rationale
Non-typhoidal Salmonella is one of the most common causes of human foodborne illness in the world (WHO, 2013). In the USA, an estimated 1.0–10.1% of human salmonellosis cases are attributed to the consumption of contaminated pork (Painter et al., Reference Painter, Hoekstra, Ayers, Tauxe, Braden, Angulo and Griffin2013). A computer-based benefit-cost analysis model of pre-harvest and processing interventions against Salmonella in the US pork production chain indicated that rinsing carcasses with water at different temperatures (with and without sanitizer) is a more cost-effective way of reducing the number of human salmonellosis cases than on-farm interventions, such as vaccination and meal feeding (Miller et al., Reference Miller, Liu, McNamara and Barber2005).
Spraying a carcass with water can help remove contaminants, but it may also act to spread bacteria across the carcass surface (Loretz et al., Reference Loretz, Stephan and Zweifel2011). Heating the water to a hot (75–85°C) as opposed to warm temperature may help to counteract this, but it may also impact the appearance (discoloration) and quality of the meat (Loretz et al., Reference Loretz, Stephan and Zweifel2011; Milios et al., Reference Milios, Drosinos and Zoiopoulos2014). Another disadvantage of hot water decontamination is that it requires extensive quantities of water, even if the water is recycled (Lawson et al., Reference Lawson, Jensen, Christiansen and Lund2009). Steam is more expensive to generate than hot water (Milios et al., Reference Milios, Drosinos and Zoiopoulos2014), and it may also cause carcass discoloration (Midgley and Small, Reference Midgley and Small2006). Steam ultrasound works by using steam to kill the bacteria, coupled with ultrasound to remove the heat-insulating air at the surface of the carcass (Lawson et al., Reference Lawson, Jensen, Christiansen and Lund2009). This system tends to use less energy than heated-water interventions (Lawson et al., Reference Lawson, Jensen, Christiansen and Lund2009). Steam vacuum is applied after the splitting of the carcass, with the vacuum helping to remove fecal contamination from the carcass surface (Lawson et al., Reference Lawson, Jensen, Christiansen and Lund2009); this treatment is designed for ‘spot’ treatment of parts of the carcass that are obviously contaminated, as it is impractical to use as a whole-carcass intervention (Midgley and Small, Reference Midgley and Small2006).
Organic acids kill or damage bacterial cells on the carcass surface by lowering the pH, but they may also cause carcass discoloration (Milios et al., Reference Milios, Drosinos and Zoiopoulos2014), and they can be corrosive to abattoir equipment (Midgley and Small, Reference Midgley and Small2006). Acetic acid may also cause eye and skin irritation to abattoir workers (Midgley and Small, Reference Midgley and Small2006).
Acidic electrolyzed oxidizing water (acidic EO water) is created by passing an electrical current through a saline solution (Midgley and Small, Reference Midgley and Small2006). Acidic EO water has a wide range of activity against many species of bacteria, attributed to its high-active chlorine (Cl2) content and oxidation-reduction potential; it does not cause discoloration of foodstuffs (Hricova et al., Reference Hricova, Stephan and Zweifel2008).
Trisodium phosphate (TSP), a household cleaning agent, works by disrupting the membranes of bacterial cells (Midgley and Small, Reference Midgley and Small2006). TSP is an environmental concern as it can promote algal blooms in ponds and lakes, so proper disposal after use is essential (Midgley and Small, Reference Midgley and Small2006).
While EU Regulation (EC) No 853/2004 of the European parliament and of the Council (http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:139:0055:0205:EN:PDF) allows only water to be used for the decontamination of pork carcasses, lactic acid is allowed for beef carcass decontamination (EFSA, 2011). In the USA, other substances, including organic acids, are permitted for decontaminating pork carcasses (United States Department of Agriculture Food Safety and Inspection Service (USDA/FSIS) Directive 7120.1 Revision 32, http://www.fsis.usda.gov/wps/wcm/connect/bab10e09-aefa-483b-8be8-809a1f051d4c/7120.1.pdf?MOD=AJPERES. Last accessed 1 March 2015). The approval process for chemical interventions in the USA is based on toxicology and efficacy. Prior to approval, the petitioner must provide data demonstrating that a proposed compound is safe for human consumption in the concentration and food matrix proposed. The petitioner must then provide data to demonstrate that the compound produces the desired effect on the target bacterium, based on the concentration and food matrix. However, we do not know the comparative effectiveness of these approaches; although a few narrative reviews have been published on the subject, at the time of our review, a systematic review of the effect of washes, rinses, and sprays on pork carcasses had not been conducted. Such a review could allow summarization of the magnitude of Salmonella enterica reduction associated with each product across several studies, with the expectation of providing information for better decision making by commercial abattoir operators and regulatory bodies.
Objective
The objective of this review was to describe the changes in the prevalence and/or quantity of Salmonella on pork carcasses or parts of pork carcasses after receiving pathogen reduction treatments during processing in field studies. The PICOS question was: What is the change in Salmonella prevalence or quantity (O = Outcome) associated with the use of pathogen reduction treatments applied as washes, rinses, or sprays (I = interventions) to pork carcasses or parts of pork carcasses (P = Population) in study designs (S = Study design) employing randomized or non-randomized comparative experiments with a parallel control group. An interim report of this review was presented at the 11th International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork (Totton et al., Reference Totton, Glanville, Dzikamunhenga, Dickson and O'Connor2015).
Materials and methods
Protocol and registration
A protocol documenting the research question, eligibility criteria, intended information sources, search strategy, study selection, data collection process (including a draft of the data extraction form), assessment of risk of bias (including a draft of the risk-of-bias form), and planned synthesis of results was written in advance. This protocol is not registered. It was decided after the review began to modify the protocol objective to include studies on parts of pork carcasses so that potentially relevant studies would not be excluded and data from laboratory-based studies would become eligible.
Eligibility criteria
No restrictions were placed on language of publication, publication date, or publication status. Relevant populations were pork carcasses or parts of pork carcasses from commercial swine in commercial abattoirs; however, after screening of titles and abstracts, it was decided to include laboratory-based studies. As the search and the screening would have captured laboratory-based studies, it was considered that no bias would occur due to this change. Traditional smallholder slaughter approaches were not applicable to the expected target population, who were commercial packers in the USA. Relevant interventions and comparators were pathogen reduction treatments applied as washes, rinses, and/or sprays including organic acids, aqueous ozone, EO water, potassium hydroxide (KOH), potassium sorbate, sodium hypochlorite (NaClO), TSP, Cl2, sodium chlorite (NaClO2), hot or cold water treatments, steam vacuuming and steam pasteurization or any combination of these treatments, to pork carcasses. The outcomes of interest were the presence and/or quantity of Salmonella on the carcass as measured by methods including bacterial culture, enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction, or other antigen-detecting methods. Relevant study designs were those employing comparative experiments on pork carcasses or carcass parts, whether naturally or artificially contaminated with Salmonella.
Information sources
The following information resources were searched in January 2015: Centre for Agricultural Biosciences International (CABI) Abstracts (1910–2015) (Web of Science interface), Science Citation Index (SCI) and Conference Proceedings Citation Index – Science (CPCI-S) (1900–2015) (Web of Science interface), Medline® and Medline® In-Process (1946–2015) (OVIDSP interface), Science.gov (via http://www.science.gov/scigov/), and International Conference on the Epidemiology and Control of Biological, Chemical and Physical Hazards in Pigs and Pork (Safe Pork) (1996–2012) (http://lib.dr.iastate.edu/safepork/). The last search was run on 31 January 2015. A limited updated literature search was performed in CABI Abstracts on 20 March 2015, which produced three new records. To find further relevant records that had not been captured by the information resources listed above, the reference lists of relevant review articles identified during the above search were scanned. The reference lists of all records that proceeded to the data extraction phase of the review were also checked for relevant references not captured by the database searches.
Search
A search strategy was developed to capture the concepts of pork carcass decontamination by an information specialist in consultation with food safety content experts. The concept of pork carcasses was combined with the second concept involving search terms for decontamination methods, using the AND operator. As well as the two concepts combined, there was also a general search for carcass decontamination (animal unspecified) to capture reports of the general issue. A more detailed search strategy was initially run in CABI Abstracts (Table S1). The final search strategy run in CABI Abstracts is shown in Table 1 (showing the number of records returned). Search strategies were adapted to run appropriately in other databases taking into account interface differences and database differences, such as the availability of subject indexing terms. The full search strategies for SCI and CPCI-S, Medline® and Medline® In-Process, Science.gov (via http://www.science.gov/scigov/), and Safe Pork are shown in Tables S2–S5, respectively. No restrictions were made with respect to language or document type for any of the searches.
Table 1. Search strategy run in CABI Abstracts on 21 January 2015 for a systematic review of pork carcass decontamination against Salmonella

Study selection
Records were imported into DistillerSR® (Evidence Partners, Ottawa, Ontario, Canada) and de-duplicated. Two reviewers working independently screened the records for eligibility. There were two levels of selection; the first level (Level 1) involved screening records for relevance, while the second level (Level 2) involved assessing records for eligibility. Records that passed both levels of selection advanced to the data extraction phase. Level 1 screening was based on the title and abstract alone, although the reviewers were not blinded to the author(s), title, abstract, and year of publication of each record during screening. The Level 1 form was pretested on 100 records before actual screening began. The Level 1 form consisted of a single question: ‘Does the study appear to be primary research on pathogen reduction washes/rinses/sprays for pork carcasses or parts of a pork carcass?’ The options for answering were: ‘Yes’, ‘No’, ‘Can't tell’, and ‘No, but this is a relevant review.’ If even one reviewer answered ‘Yes’ for a given record (even if the other reviewer disagreed) that record was passed to the second level of screening. For all the other answer combinations, records were excluded. Level 2 (assessment of eligibility) was based on the full text of the record. Disagreements between the reviewers at Level 2 were resolved by consensus. The Level 2 form consisted of the following questions and answer options:
-
Q1. Is the full text available in English?
-
Yes (Include)
-
No (Exclude)
-
-
Q2. Based on the full text, is the study about primary research on pathogen reduction washes/rinses/sprays for pork carcasses or parts of a pork carcass?
-
Yes (Include)
-
No (Exclude)
-
-
Q3. Was Salmonella found in any of the samples tested?
-
Yes (Include)
-
No, but the investigators did look at Escherichia coli, Enterobacteriaceae, coliforms, and/or
-
Total Plate Count (TPC) (Exclude)
-
No, the authors did not look at any of the above (Exclude)
-
-
Q4. Based on the full text does the study have a parallel comparative group?
-
Yes (Include)
-
No (Exclude)
-
-
Q5. Did the investigators measure the outcome only greater than 24 h after application of the intervention?
-
Yes (Exclude)
-
No (Include)
-
-
Q6. What type of study is this?
-
Challenge (artificial contamination)
-
Control (natural contamination)
-
Question 3 was revised during screening as the wording in the protocol (‘Q3. Did the investigators look at Salmonella?’) would otherwise have resulted in the inclusion of studies, in which the investigators looked for, but did not find, Salmonella in any of the tested samples. These studies would have had no relevant data to extract, so it was deemed best to exclude them at Level 2. The purpose of Question 4 was to eliminate studies, in which one intervention was tested by examining samples before and after intervention application; these studies had no control group and consequently did not elucidate the effects of not applying an intervention without being confounded by time. The purpose of Question 5 was to eliminate studies examining only the effects of storage on Salmonella.
Data collection process
All studies passing Level 2 screening underwent data extraction. Non-English language records were not translated; however, these were marked for reference so that if funds become available in the future, they can be translated and extracted. The data extraction forms for the study-level information and the intervention/outcome-level information were pilot-tested on two papers by both reviewers and revised for clarity and ease of use before the review began.
Two reviewers extracted intervention and outcome data independently from all relevant studies into DistillerSR®, with the exception of Epling (Reference Epling1987). Study-level and risk-of-bias data for Epling (Reference Epling1987) were extracted by two independent reviewers. Only one reviewer extracted the outcome data in Epling (Reference Epling1987), due to time constraints and the amount of data. A second reviewer then verified that this outcome data had been correctly extracted. All conflicts between reviewers at the data extraction stage were resolved by discussion and, as needed, by consulting a content expert. No investigators were contacted to confirm or obtain missing or unpublished data.
Data items
The final drafts of the study-level and intervention-outcome forms are presented in Tables S6 and S7, respectively. For the study-level information, we extracted the year, country, and the setting (e.g., laboratory, abattoir) in which the study was conducted, whether the experimental units were naturally or artificially contaminated (and in the latter case, the dose, route and serotype of Salmonella inoculated), and the laboratory methods used to measure the outcome. The intervention-outcome data extracted comprised characteristics (type, temperature, method of application) of the intervention, and for the outcome, the number of carcasses or samples that were positive for Salmonella or the Salmonella counts in each sample and the number of carcasses tested.
Risk of bias in individual studies
Risk of bias was assessed in a non-blinded manner by two reviewers. The risk-of-bias assessment form is reported in Table S8. The risk-of-bias form was based on The Cochrane Collaboration's Risk-of-Bias Tool (Table 8.5a in Higgins et al., Reference Higgins, Altman, Sterne, Higgins and Green2011), and was modified during the review process as follows: it was decided to remove the questions relating to the method used to conceal allocation of the experimental units to intervention groups, because none of the extracted papers discussed their allocation methods. Additionally, when assessing performance bias, the method used to collect samples to measure the outcome was considered. If swab samples were taken and the method of swabbing was not described in detail or was such that it might be subject to individual variation, performance bias was considered to be high. This was because many of the carcass interventions would have affected the experimental units in such a way (i.e. through smell, temperature, color change) that the person collecting the sample might have been able to determine that it had undergone an intervention (vs the control group) and might have expended more or less effort in swabbing the carcass, thus affecting the concentration or prevalence estimates for the experimental units in that group. If a more objective sampling method was used (excision of tissue of specified dimensions) or an objective swabbing technique was used (e.g. FSIS Method (FSIS/USDA, 1998)), the risk of performance bias was deemed to be low. Additionally, after consultation with our content expert (J. Dickson), outcome bias was considered to be low if the outcome was prevalence or concentration of Salmonella (reported as colony-forming units (CFU), Most Probable Number, or number of organisms).
Summary measures
The summary measures of interest specified in the protocol were mean differences for continuous outcomes (because the studies used different metrics) and summary risk ratios for categorical outcomes. The risk ratio was chosen because of its ease of interpretation, and as no summary effect was calculated, concerns about consistency of the effect measure did not need to be assessed (Egger et al., Reference Egger, Smith and Altman2008). In the final analysis, no summary effect measure was calculated for forest plot comparisons where control arm data were included more than once in pairwise comparisons because in this case sample size estimates would be inflated and variance underestimated if a summary effect were calculated. For example, if a three-arm trial existed, with two acid-based interventions and one control arm, these data would be presented as two pairwise comparisons with the control arm providing data twice, but no summary effect would be presented.
Synthesis of results
Depending on how comprehensively the results of the studies were reported, we proposed in the protocol to conduct a mixed-treatment comparison meta-analysis with ranking of the interventions. However, post hoc (evaluating the data available) it was decided to present the data by looking at specific pairwise comparisons of interest, in particular the comparison of acidic rinses to water, and heated-water treatments to cool-water treatments. All pairwise comparisons grouped in a given forest plot shared the same type of outcome measure (e.g. prevalence), the same type of intervention comparison (e.g. acidic rinses vs water) and the same study design (e.g. artificial inoculation of experimental units). Prior to conducting this analysis it was decided that calculation of a summary effect would likely not be conducted as few studies used the same concentration of an acid. When presenting the pairwise comparison, it was decided to use the risk ratio for each study with categorical outcomes and the standardized mean difference for the continuous outcomes because the metrics differed. Other interventions that were only tested in one study were summarized with text only. The forest plots used to present the data were created using the meta package (Schwarzer, Reference Schwarzer2015) in the software R (R Core Team, 2015).
Risk of bias across studies
We assessed studies to have an overall high risk of bias if they had at least one risk-of-bias domain with a high risk of bias. Presentation of risk-of-bias data was made using Review Manager (RevMan) software (version 5.3; Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, available online at http://tech.cochrane.org/revman/download). We originally planned to conduct an analysis for small-study effects, acknowledging that it might not be possible to detect small-study effects as most of the eligible studies were expected to be small. The criteria for defining a study as small were based on the number of experimental units, not on the number of pseudo-replicates, where pseudo-replicates were subsamples taken from each experimental unit.
Additional analysis
We proposed, a priori, that if the sample size was sufficient, we would conduct a meta-regression to determine what factors were associated with the magnitude of the effect size based on the demographic factors collected. However, meta-regression was not conducted due to the small number of relevant studies and the variety of interventions (type, concentration) among those studies. We did not propose, a priori, to do any other additional analyses.
Results
Study selection
Table 2 reports the number of records found before and after de-duplication for each database searched. The total number of records screened, assessed for eligibility, and included in the review, with reasons for exclusion at each stage, is shown in Fig. 1. Table S9 lists the citation information for each record excluded at the full-text stage (Level 2), along with the reason for its exclusion. Fourteen studies described in 17 publications were eligible for inclusion in the review.
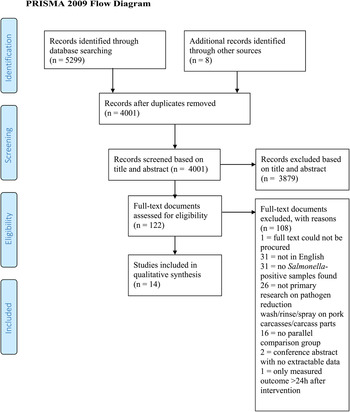
Fig. 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram for a systematic review of pathogen reduction sprays/rinses/washes for pork carcasses and carcass parts (template from Mohr et al., Reference Mohr, Liberati, Tetzlaff and Altman2009).
Table 2. The number of studies found for each electronic database before and after de-duplication in a systematic review of pathogen reduction treatments against Salmonella in pork carcasses

1 Includes three references found during an updated search conducted on 20 March 2015.
Study characteristics
The characteristics of the included studies are reported in Table 3. Of the included studies, only Machado et al. (Reference Machado, Gouveia, Picinin, Kich, de Cardoso and Ferraz2013) reported the year, in which their data were collected (in 2008). Of the included studies taking place at commercial abattoirs, the slaughter capacity was reported by Hamilton et al. (Reference Hamilton, Holds, Lorimer, Kiermeier, Kidd, Slade and Pointon2010) (greater than 500,000 y−1 and 5.5 carcasses min−1 during the study), Trivedi et al. (Reference Trivedi, Reynolds and Chen2007) (50–60 hogs day−1 twice each week at Plant A, and 24–40 hogs week−1 once each week at Plant C), and Epling et al. (Reference Epling, Carpenter and Blankenship1993) (700–800 hogs h−1), but not in the studies by Eggenberger-Solorzano et al. (Reference Eggenberger-Solorzano, Neibuhr, Acuff and Dickson2002) and van Netten et al. (Reference van Netten, Mossel and Huis In ‘t Veld1995). The methods used to inoculate the experimental units in the challenge studies are presented in Table 4. The methods used in each included study to measure the outcome of prevalence and/or quantity of Salmonella in the experimental units are presented in Table 5.
Table 3. Characteristics of included studies in a systematic review of pathogen reduction treatments against Salmonella on pork carcasses

CA, commercial abattoir; Lab, laboratory; URS, university/research slaughter plant; NR, not reported.
1 Citric acid was the main constituent (other constituents not reported).
2 Some samples were naturally infected.
Table 4. Inoculation methods used in challenge studies in a systematic review of pathogen reduction treatments against Salmonella on pork carcasses

NR, not reported; CFU, colony-forming units.
1 Salmonella selected had survived 120 s in 2% lactic acid (pH 2.3) at 21°C. Organisms were cultured in tryptic soy broth (TSB, pH 5.8) using 10% lactic acid at 30°C for 1 day then on fresh TSB-LA at 17°C for 2 days.
Table 5. Methods used to measure the prevalence and/or quantity of Salmonella in a systematic review of pathogen reduction treatments against Salmonella on pork carcasses

BPW, buffered peptone water; RV, rappaport-Vassiliadis; XLD, xylose lysine desoxycholate agar; MPN, most probable number; BGA, brilliant green agar; CLED, cystine–lactose–electrolyte deficient agar; LA, latex agglutination; ND, not done; XLT4, xylose lysine tergitol 4; TSI, triple sugar iron; LI, lysine iron; BTSBY, buffered trypticase soy broth with yeast extract; XLD-N, xylose lysine desoxycholate agar with novobiocin.
1 For this laboratory study, the authors tested for Enterobacteriaceae, but they knew that these were only Salmonella because they had previously killed off all the Enterobacteriacia already present via irradiation.
2 Tryptic soy agar.
3 The authors did not report what the ‘C’ stood for.
Risk of bias within studies
A graphical depiction of the risk-of-bias summary across and within studies is shown in Fig. 2. None of the studies provided a detailed description of the approach to allocating carcasses/skin/jowl to the interventions, and therefore for all studies the risk of bias due to confounding of the intervention effect by other factors was unclear. The risk of bias due to failure to blind the outcome assessor was considered low for all studies.

Fig. 2. Risk-of–bias-summary graph for a systematic review of pathogen reduction treatments against Salmonella in pork carcasses. Red circles refer to a high risk of bias, green circles to a low risk of bias, and yellow circles to an unclear risk of bias.
Results of individual studies
The results of included studies for all outcomes considered are presented in Table 6. Data from a thesis (Epling, Reference Epling1987) are not shown. Epling (Reference Epling1987) reported the reduction of Salmonella in experimentally inoculated skin samples after treatment with 1, 2, 5, and 10% lactic acid, a combination of 2% lactic acid and 2% acetic acid, 2% acetic acid alone, and water. Each of these interventions was applied at three different temperatures (25, 55, and 10°C) via immersion, no-charge sprayer (applied 40 cm away from the sample at a pressure of 137.9 kPa), or by an electrostatic sprayer (run at 1000 V) and tested for effect of the intervention at 10 min, 2.5 h, and 24 h (according to the Results section) or 25 h (according to the Methods section) after application. This resulted in 189 different intervention permutations, which would have been unwieldy to present in a table. Further, Epling (Reference Epling1987) reported neither the sample sizes for each intervention nor the precision estimates of the reported concentrations. Epling (Reference Epling1987) did report results (Table 6) of a separate study of the effect of 2% lactic acid on naturally contaminated pork carcasses, and these results appear to have been published in a peer-reviewed journal (Epling et al., Reference Epling, Carpenter and Blankenship1993).
Table 6. Outcomes of individual studies included in a systematic review of pathogen reduction treatments against Salmonella on pork carcasses or parts of pork carcasses

TSP, trisodium phosphate (AvGARDTM); LA, lactic acid; NR, not reported; AA, acetic acid.
1 As this study took place at a US commercial abattoir, the final wash probably consisted of water (J. Dickson, personal communication, 13 April 2015). Water temperature, pressure, and pH not reported.
2 This refers to the number of positive samples. The number of positive carcass halves was not reported.
3 72 carcass halves, 3 locations per carcass half (ham, belly, and jowl) = 216 samples.
4 Not applicable.
5 Not done.
6 Ultrasound.
7 Inoculum.
8 Number of replicates. Actual number of samples not reported.
9 Reduction in S. Typhimurium ± SEM log CFU cm−2.
10 Mean Salmonella counts (log10 cm−2). Measure of precision not reported.
11 Speed: 200 rev min−1.
12 Immediate lethality (mean reduction during the lactic acid treatment) ± SD (log10 CFU ml−1). A quenching solution was used to neutralize lactic acid at the end of treatment.
13 Number of replicates = 3. Number of samples not specified.
14 Mean reduction in S. Typhimurium ± SEM.
15 ww, since all carcasses (controls and treated carcasses) underwent conventional slaughter in a US commercial abattoir, all carcasses would have received a water wash (temperature, pressure, and pH of the water not reported) prior to any treatment (J. Dickson, personal communication, 13 April 2015).
16 Number of samples showing reduction in the count of the Most Probable Number of Salmonella following treatment (samples with counts below 3MPN were withdrawn).
17 Mean MPN of Salmonella sp. after treatment (It was not possible to extract the SEM from this paper as the data were presented in a figure and the error bars of the different treatments overlapped too much to distinguish.).
18 For the prevalence data, none of the treatments differed significantly from the control (physiological saline) group.
19 Citric acid was the main constituent (other constituents not reported).
20 SHS, standard hygienic slaughter. Although not specified in the paper, we assumed this entailed a water wash at the end of slaughter.
21 Both treatments were significantly different from the control group. Hot water and SANOVA™ were not significantly different from each other (P = 0.12).
22 Acidified NaClO2 (pH 2.4–2.6), ECOLAB Inc.
23 Mean log10 CFU cm−2 S. Typhimurium immediately following treatment ± mean square error (variance). Initial pathogen level for all treatments was approximately 6 log10 CFU cm−2.
24 19.9 ppm free Cl2.
25 EO water (68.25 ppm free Cl2).
26 66 ppm free Cl2.
27 Samples were placed in water in a centrifuge tube and vortexed for 0 s.
28 Log10 CFU cm−2 Salmonella present after treatment (measure of precision not reported).
29 The jowl was scalded prior to inoculation with the challenge organism.
30 The authors’ paper specifies 10 s in the Results section and 30 s in the Methods section.
31 Inoculated with Salmonella.
32 Outcome measured after 1 h.
33 Outcome measured after 24 h.
34 ww, the carcass was washed with water (duration, temperature and pressure not reported). If carcass was sprayed with acetic acid, the wash occurred afterwards.
35 Naturally infected.
36 Inoculated prior to treatment for a contamination level of 1.7 ± 0.2 (SD) log10 CFU cm−2.
37 Samples were contaminated using feces. Final level of Salmonella contamination before treatment not reported.
38 Five experiments were conducted. It is unclear if the three samples taken per carcass were pooled.
39 Mean rinse-off (log10 cm−2 ± SD).
40 Number of replicates.
41 Number of S. Typhimurium (log10 CFU cm−2) ± SEM remaining after treatment.
42 Each treatment was followed 2 min later by inoculation with bacteria followed 2 min later by a 15 s water rinse.
43 Six replicates, since the 5 s (three replicates) and 15 s (three replicates) treatment results were pooled because they were not significantly different.
Trivedi et al. (Reference Trivedi, Reynolds and Chen2007) examined the effects of a commercial household steam cleaner (Steam Fast SF 275, Top Innovation, Inc., Riverside, Missouri, USA, steam capacity 1500 W, water tank maximum capacity 1.44 L, steam chamber and hosepipe with nozzle) applied at 6–7 cm away from the ham, belly, and jowl of carcasses in 100 cm2 areas after the final carcass wash but before organic acid solutions were sprayed on the carcass. These experiments were performed in two slaughter plants (Plant A and Plant C); however, the Salmonella data for the two plants was combined, making it impossible to separate the data by plant. Statistical analysis of the Salmonella results was not undertaken. The results were reported as ‘Number of samples positive’ rather than number of carcasses positive, rendering interpretation of the results difficult. Three samples were taken per carcass, but it was not reported whether more than one of the four positive samples in the control group came from the same carcass or from different carcasses. The impact of the intervention would be considered very different, if for example three samples on one carcass were positive compared with one sample on three carcasses. This reporting approach made inclusion of the results into the conclusions of this review impossible.
Morild et al. (Reference Morild, Christiansen, Sørensen, Nonboe and Aabo2011a) examined the effect of steam ultrasound applied to a 10 × 10 cm2 piece of inoculated jowl skin. As this was the only study to examine this intervention it is not summarized in a forest plot. Steam ultrasound was applied in a test cabinet (85 × 79 × 57 cm3, Force Technology, Brøndby, Denmark) using steam (130°C) at 354.6–506.6 kPa applied through nine nozzles. Only the upper surface of the sample could be treated with this apparatus; the lower surface was therefore left untreated. Ultrasound (30–40 kHz) was generated through nozzles 10–12 cm from the surface of the sample, and kinetic energy was delivered by steam pressure. Results for the control groups were not reported. The authors reported that the reduction in S. enterica serovar Typhimurium was significantly different after 0.5 s vs 1.0 s of steam-ultrasound and after 1.0 s vs 2.0 s of steam-ultrasound. The mean reduction in S. Typhimurium between the two inoculation levels (104 vs 107) did not differ significantly for the 0.5 s (P = 0.073), 1.0 s (P = 0.095), 1.5 s (P = 0.084), and 2.0 s (P = 0.066) treatments.
Morris et al. (Reference Morris, Lucia, Savell and Acuff1997) studied the effect of TSP (AvGARD™, Rhone-Poulenc Inc., Cranbury, New Jersey, USA) on samples of pork skin collected less than 45 min post-exsanguination and inoculated with rifampicin-resistant S. Typhimurium prior to treatment. As this intervention was only assessed in one study, it was not summarized in a forest plot. Results for the three untreated samples were not reported. For the samples dipped for any length of time (5, 10, or 15 s), the mean post-treatment concentration of Salmonella was lower for all samples dipped in actual TSP solutions compared with samples dipped for the same length of time in the corresponding 0% TSP control solution (P < 0.05). Within each concentration group (4, 8, or 12% TSP), samples dipped for different durations (5 or 10 or 15 s) did not have significantly different mean post-treatment concentrations of Salmonella (P > 0.05).
van Netten et al. (Reference van Netten, Huls in't Veld and Mossel1994) studied the effects of 2% lactic acid (pH 2.3; temperature 21 ± 2°C) vortexed (200 rev min−1 for 2 s) with inoculated skin cell suspensions (25 cm2 pork belly skin stomached in Seward Stomacher 400 and placed in 45 ml sterile peptone water (0.5% sodium chloride (NaCl), 1% peptone, pH 6.9)). Immediately after treatment the lactic acid activity was quenched using a 4 ml solution of 0.05 mol l−1 tripotassium phosphate (K3PO4), 3% (wt/vol) tryptic soy broth, and 0.3% (wt/vol) yeast extract to bring the pH of the skin suspension up to 7.4.
Christiansen et al. (Reference Christiansen, Krag and Aabo2009) studied the effect of 1 or 2.5% (vol/vol) lactic acid or hot sterile water on inoculated pork jowl skin samples (10 × 10 cm2). The samples were placed vertically, and the hot water or lactic acid was poured over them with a watering device to simulate in-line cabinet hot water carcass decontamination. For this experiment, 10 interventions were assessed. The comparisons relevant to this review are presented in Table 6.
Epling et al. (Reference Epling, Carpenter and Blankenship1993) used an electrostatic dispersion sprayer (air pressure 137.9 kPa; electrode potential 1000 V) to apply approximately 150 ml of 2% (v/v) L-lactic acid solution evenly over half of the carcass (n = 75 carcasses) from a distance of 40 cm. The lactic acid spray decreased the prevalence of Salmonella immediately after treatment (P < 0.05) and 24 h after treatment (P < 0.01) when applied to the ham or shoulder.
Fabrizio and Cutter (Reference Fabrizio and Cutter2004) examined the effect of spraying (using a food-grade hand-held garden sprayer (Hudson, Hastings, Minnesota, USA; Model 67220)) distilled water, NaClO, EO water, or aged EO water on inoculated pork bellies hung vertically on a stainless steel rack in a biological safety hood. The acidic EO water (1150 mV oxidation reduction potential; 50 ppm free Cl2) was produced by passing a 12% NaCl solution across a charged bipolar membrane (EO water generator, ROX Water Electrolyzer, Hoshizaki America, Inc., Peachtree City, Georgia, USA). Aged acidic EO water was created by storing acidic EO water for 24 h at 4°C in an airtight bottle. All the treatments (including the distilled water) were significantly different from the control (no-treatment) group (P < 0.05).
In their laboratory experiment, Eggenberger-Solorzano et al. (Reference Eggenberger-Solorzano, Neibuhr, Acuff and Dickson2002) examined pieces of skin (1 × 1 × 0.5 cm3), obtained from scalded hog carcasses, placed in 50 ml centrifuge tubes with water at different temperatures and vortexed for varying lengths of time. The authors found no significant difference (P > 0.05) between any of the water treatments in the level of Salmonella after treatment. In their commercial experiment, the authors studied the effects of acetic acid and/or hot water on carcasses. The hot water was applied using a low-pressure spray wash system at 172.4 kPa. Acetic acid (1.8% vol/vol) was applied at less than 172.4 kPa using the commercial acid rinse cabinet already installed at the abattoir so that the entire carcass was covered in approximately 3 s. When both treatments were used, hot water was applied first, followed, 7 s later, by acetic acid.
Biemuller et al. (Reference Biemuller, Carpenter and Reynolds1973) in their pilot plant studies sprayed acetic acid (pH 1.5 or 2.0), stannous chloride (SnCl2) or hydrogen peroxide (H2O2) onto inoculated pork carcasses from a distance of 8 cm from the carcass. To achieve pH 1.5 and 2.0 acetic acid, the authors added hydrochloric acid (HCl) to 0.1 N acetic acid solutions. As HCl is not approved for use in pork processing (FSIS/USDA, Directive 710.1 Revision 29, Available online at http://www.fsis.usda.gov/wps/wcm/connect/bab10e09-aefa-483b-8be8-809a1f051d4c/7120.1.pdf?MOD=AJPERES. Last accessed 20 September 2015), it was decided not to include this study in a forest plot summary. The authors also investigated the effect of steam applied for 10 s (according to their Results section) or 30 s (according to their Methods section) 2.5 cm from the carcasses (apparatus used to apply the steam not reported).
van Netten et al. (Reference van Netten, Mossel and Huis In ‘t Veld1995) examined the effect of water or lactic acid spray (400 ml min−1) at 11 or 55°C applied after the final carcass wash at a commercial abattoir. The lactic acid solutions were at a concentration of 2% (pH 2.3) or 5% (pH 1.9) and were made from a 50% L(+)-lactic acid stock solution (Chemie Combination, Amsterdam, The Netherlands). Spraying was carried out using a spray nozzle at a distance of 20 cm from the carcass with an electrostatic spray apparatus (Wezer, Assendelft, The Netherlands). After treatment, three samples were taken per carcass and if even one of these tested positive for Salmonella, the carcass was considered to be positive.
Morild et al. (Reference Morild, Olsen and Aabo2011b) examined the effects of hot water or 1% lactic acid rinses on Salmonella. They used swabbing to detect superficially attached bacteria and stomaching of tissue samples to detect firmly attached bacteria. The number of bacteria attached to surfaces did not differ significantly (P = 0.06) between 5-s vs 15-s treatments, so these data were pooled. The total number of superficially and firmly attached bacteria was higher after decontamination with both hot water and lactic acid compared with non-decontaminated (i.e. control) skin (P < 0.0001).
Synthesis of results
We constructed three forest plots to help to identify patterns within the dataset. Fig. 3 presents a forest plot of the data from studies that assessed lactic acid-based interventions and reported measures of Salmonella that were concentration-based. Christiansen et al. (Reference Christiansen, Krag and Aabo2009) used the mean reduction in Salmonella between two time points, while Fabrizio and Cutter (Reference Fabrizio and Cutter2004) and Morild et al. (Reference Morild, Olsen and Aabo2011b) used the mean concentration post-treatment as the metric for comparison. In Fig. 3 there is little evidence of a consistent positive or negative effect of acid washes compared with water washes, as most estimates center around the null value of zero.

Fig. 3. Forest plot showing measures of Salmonella concentration from intervention studies that assessed lactic acid washes in commercial abattoirs. Standardized mean difference is used as the summary effect measure as the metrics for Salmonella were not consistent across studies. These data represent all possible comparisons, so control groups appear multiple times and summary effects are invalid. ’?C’ indicates that the temperature of the solution used to wash the pork was not reported.
In Fig. 4 we present data from studies that compared the prevalence of Salmonella after treatment with one form of water or steam to another treatment, which was usually a standard carcass treatment or cooler treatment. In Fig. 5 we present a forest plot for the Salmonella prevalence estimates for studies that used various acid treatments with some type of water treatment (either standard or warm/hot water). Again, it is important to note that a summary effect was not reported because the control arms were repeated. Fig. 5 provides a more consistent picture of a positive effect of acid-based treatments on Salmonella prevalence.

Fig. 4. Forest plot showing prevalence of Salmonella for interventions that compared variations of water/steam with standard/controls. These data represent all possible comparisons, so control groups appear multiple times and summary effects are invalid.

Fig. 5. Forest plot showing the prevalence of Salmonella for interventions that compared variations of acidic interventions with standard/controls. These data represent all possible comparisons, so control groups appear multiple times and summary effects are invalid (and therefore not shown in the figure). LA stands for lactic acid. The Machado et al. (Reference Machado, Gouveia, Picinin, Kich, de Cardoso and Ferraz2013) study reported the outcome as the percentage of samples that showed reduction in the count of the Most Probable Number of Salmonella after treatment.
Risk of bias across studies
As a meta-analysis was not conducted, we did not evaluate the effect of small-study effects on the outcome.
Additional analysis
No additional analysis was conducted.
Discussion
Summary of evidence
We used the GRADE (Grading of Recommendations Assessment, Development and Evaluation) evidence framework (Schünemann et al., Reference Schünemann, Brożek, Guyatt and Oxman2003) (strength of association, consistency, directness) as a basis for considering the conclusions; however, we did not conduct a formal GRADE panel meeting.
With respect to the strength of association, there does not seem to be strong evidence that one intervention protocol (e.g. acid temperature, acid concentration, hot water, cool water) is clearly superior to others for the control of Salmonella on pork carcasses. Overall, Fig. 4 shows a generally more favorable effect of warmer water over cooler or standard water washes for reducing the prevalence of Salmonella; however, only the Hamilton et al. (Reference Hamilton, Holds, Lorimer, Kiermeier, Kidd, Slade and Pointon2010) study showed a significant difference between the treatments. Further support for the use of water at temperatures higher than ambient comes from the data obtained from scalding operations. Although scalding is not considered a pathogen intervention in the traditional sense, the available data demonstrates a reduction in the incidence of Salmonella on carcasses after scalding (Davies et al., Reference Davies, McLaren and Bedford1999; Bolton et al., Reference Bolton, Pearce, Sheridan, Blair, McDowell and Harrington2002; Pearce et al., Reference Pearce, Bolton, Sheridan, McDowell, Blair and Harrington2004; Hernandez et al., Reference Hernandez, Gomez-Laguna, Luque, Herrera-Leon, Maldonado, Reguillo and Astorga2013). The FAO/WHO (2015) report on interventions noted that hot water washes ‘were recommended for consideration as a hazard-based intervention for the control of Salmonella’ on pork carcasses.
Fig. 5 shows a relatively consistent positive effect of acidic washes against Salmonella in studies, in which the outcome was categorical; however, in the majority of cases, this effect was not significant and in one comparison (Machado et al., Reference Machado, Gouveia, Picinin, Kich, de Cardoso and Ferraz2013) the effect of the acidic wash was negative. The EFSA (2011) evaluation of lactic acid concluded that the use of lactic acid in concentrations greater than 2% was effective in reducing the prevalence of Salmonella on beef carcasses. This data cannot be directly extrapolated to pork carcasses. However, the FAO/WHO (2015) report on interventions also concluded that organic acid rinses were ‘recommended for consideration as a hazard-based intervention for the control of Salmonella’ for reducing the incidence of non-Typhoidal Salmonella on pork carcasses.
The directness of the findings to pork production is mixed; some studies were conducted in abattoir settings, which are clearly relevant to the target population. Laboratory-based challenge studies predominate in this review for obvious reasons (i.e. it is an unacceptable public health risk to introduce Salmonella into an abattoir). The validity of findings from challenge studies in our opinion should be viewed as such: the results were probably optimal because the laboratory studies occurred in controlled settings, and we would expect the same interventions to have smaller or more variable effects when applied in commercial settings. The lack of data within the public domain makes it difficult to draw scientifically defensible conclusions.
This review was focused on studies of Salmonella on pork skin because of the issue of directness. The use of other organisms (E. coli, etc.), other animal species (beef or poultry), or tissue type (muscle) were considered too indirect to be useful for answering the review question. Note that we use the term directness because it matches the GRADE evidence framework, however ‘applicability’ would be a suitable synonym.
A few narrative reviews on the efficacy of various pathogen reduction treatments on carcasses have been conducted. Caution must be used in comparing findings and conclusions across reviews because different studies included in these reviews may show variable results and the reviewers draw different conclusions due to differences in types of carcasses examined (lamb, beef, pork), the initial microbial load on the carcasses, the species of micro-organisms studied and type of tissue sampled (skin, muscle, connective tissue) (Midgley and Small, Reference Midgley and Small2006).
Loretz et al. (Reference Loretz, Stephan and Zweifel2011) conducted a narrative review of bacterial reduction treatments (against Salmonella and other bacteria) for pork carcasses and, like us, found that most studies focused on water and/or steam with most chemical interventions focused on organic acids. Midgley and Small (Reference Midgley and Small2006) conducted a comprehensive review; however, the majority of studies cited were on beef and lamb carcasses and included many microorganisms besides Salmonella. They found that with water interventions, higher temperatures and pressures were more successful at removing bacteria than lower temperatures and pressures; however, these findings were based on lamb and beef carcasses. Midgley and Small (Reference Midgley and Small2006) found that with organic acid interventions, as for water interventions, warmer temperatures (in this case 50–55°C) were more effective against microorganisms than cooler temperatures of organic acids.
Midgley and Small (Reference Midgley and Small2006) concluded that Cl2 is effective against microorganisms at high concentrations, but its effects are diminished in the presence of large quantities of organic matter; unfortunately, the high concentrations needed for Cl2 to be most effective are not permitted in the food industry.
Our recommendation is that more studies be conducted in commercial abattoirs comparing organic acids with water rinses. Based on the low number of included studies in this review, the high heterogeneity between studies and the overall quality of the evidence in this review, we cannot recommend changes in industry practice regarding use of one specific intervention over any others. We are aware that it is likely many studies (experimental or observational) have been conducted that are not publically available. This practice perhaps helps companies maintain competitive advantages. However, when regulatory decisions are being made, then government officials are often required to ‘fall back’ on what is publically available because consumers and stakeholders want to know the basis for any decisions and that means ‘publically available data’. As this review documents, publically available data is often limited and incompletely reported. We would strongly encourage the open and transparent publication of data that either supports of refutes the use of pathogen reduction treatments. Further, those studies should be designed and reported in a manner that enables end-users to assess biases and extract data for decision making.
Of the records assessed for eligibility in our review that were excluded because the authors did not investigate Salmonella, we did flag all records where the authors examined E. coli, Enterobacteriaceae, coliforms, and/or TPC as outcomes (Table S9). These records may be extracted at a later date, should funds become available.
Limitations
The conduct of this review is consistent with current standards for systematic reviews. Steps were taken to ensure that an a priori protocol was developed and made available (Protocol S1). An extensive database search was conducted along with reference checking. Record screening, eligibility assessment and data extraction were undertaken by two independent reviewers. The data were reported comprehensively, and conservative and thoughtful analysis (within the limitations of the data available) was provided to the end-user of the review. Our ability to explicitly address the review question about the magnitude of reduction we would expect based on pathogen reduction treatments was limited by the approach to reporting the underlying data and the absence of repeated protocols assessed by independent groups. The most common issue was the failure of authors of the included studies to clarify the unit of concern and the units for measures of variation. This, along with the heterogeneity of the included studies (natural vs artificial contamination, prevalence- vs concentration-based outcomes, and the limited number of similar interventions tested) and low number of included studies precluded our ability to perform a meta-analysis. We did not follow-up with the authors of these papers regarding missing data because our experience with previous reviews indicated that this could have potentially taken months to hear back from these authors and would have extended the time required to complete the review.
In addition, 31 non-English-language records were excluded at the eligibility assessment phase of this review. Some of these may have contained extractable data, but were not available to us because they were not translated. Investigators could improve their data collection and reporting in manuscript by making use of the REFLECT Guidelines (O'Connor et al., Reference O'Connor, Sargeant, Gardner, Dickson, Torrence, Dewey, Dohoo, Evans, Gray, Greiner, Keefe, Lefebvre, Morley, Ramirez, Sischo, Smith, Snedeker, Sofos, Ward and Wills2010) to foster future systematic comparisons with other studies.
It would have been preferable to have been able to assess a specific concentration and duration of acid or a specific temperature of water (i.e. a quantitative assessment would have been preferable). However, as is evidenced from the information about the study characteristics, interventions, methods of detection, and outcomes, few studies were directly comparable (Tables 3–6).
One of the limiting factors of this review is the lack of data within the public domain. There are far more published reports on the effect of pathogen interventions on beef carcasses than on pork carcasses. It is common practice in the meat and poultry industry to extrapolate data from one species to another, although differences in processing systems and carcass surfaces may make extrapolation difficult at best. This lack of published data, and the resulting difficulty in drawing valid conclusions, has been previously noted (FAO/WHO, 2015).
After the cut-off date for consideration for the review, two additional review documents (Midgley and Small, Reference Midgley and Small2006; Young et al., Reference Young, Wilhelm, Cahill, Nakagawa, Desmarchelier and Rajić2015) were brought to our attention. We evaluated the bibliographic lists of these reviews and identified three potentially relevant studies (Table S10), which were not evaluated as part of this review, but which can be considered for an update of this review in the future.
Conclusion
The conclusion we reached from these data is that there is no strong evidence for the efficacy of one particular intervention. With respect to consistency, the most consistently observed association is a positive effect of acid washes on measures of Salmonella that were categorical; however, this is based on individual results and not a summary result, which was not calculated for reasons already discussed.
Supplementary Material
The supplementary material for this article can be found at http://dx.doi.org/10.1017/S1466252316000025.
Acknowledgments
This review was funded by the National Pork Board Grant # 14-287. We thank Chase Prouty, who procured some of the papers. The National Pork Board consults J. Dickson as a subject matter expert regarding scientific information, not to endorse anything that they do. The remaining co-authors of this manuscript have no conflicts of interest to disclose.













